Abstract
In 1996, Bt-cotton (cotton expressing a Bacillus thuringiensis toxin gene) expressing the Cry1Ac protein was commercially introduced to control cotton pests. A threat to this first generation of transgenic cotton is the evolution of resistance by the insects. Second-generation Bt-cotton has been developed with either new B. thuringiensis genes or with a combination of cry genes. However, one requirement for the “stacked” gene strategy to work is that the stacked toxins bind to different binding sites. In the present study, the binding of 125I-labeled Cry1Ab protein (125I-Cry1Ab) and 125I-Cry1Ac to brush border membrane vesicles (BBMV) of Helicoverpa armigera was analyzed in competition experiments with 11 nonlabeled Cry proteins. The results indicate that Cry1Aa, Cry1Ab, and Cry1Ac competed for common binding sites. No other Cry proteins tested competed for either 125I-Cry1Ab or 125I-Cry1Ac binding, except Cry1Ja, which competed only at the highest concentrations used. Furthermore, BBMV from four H. armigera populations were also tested with 125I-Cry1Ac and Cry1Ab to check the influence of the insect population on the binding results. Finally, the inhibitory effect of selected sugars and lectins was also determined. 125I-Cry1Ac binding was strongly inhibited by N-acetylgalactosamine, sialic acid, and concanavalin A and moderately inhibited by soybean agglutinin. In contrast, 125I-Cry1Ab binding was only significantly inhibited by concanavalin A. These results show that Cry1Ac and Cry1Ab use different epitopes for binding to BBMV.
Helicoverpa armigera (Hübner) is one of the most important insect pests in many cotton-producing countries, including Australia, India, and China. Larvae cause important economical losses to fiber and vegetable crops and have proven to be difficult to control by conventional means, in part because many pest populations have already evolved resistance to chemical insecticides (20, 21, 27). A rather recent alternative has been the introduction of cotton expressing a Bacillus thuringiensis insecticidal protein (Bt-cotton). B. thuringiensis is a gram-positive bacterium that produces crystalline proteins (delta-endotoxins, Cry toxins, or Cry proteins) during its sporulation phase of growth and has been used as a microbial insecticide for many years. Cry proteins have a narrow and specific spectrum of action against different pests, including coleopteran, dipteran, and lepidopteran species (34). Among the advantages of Bt crops are increased crop productivity, reduced production costs, selectivity and specificity against the target pests, conservation of biodiversity, and no demonstrated threat to the environment and human health (15, 29).
Heliothine species are rather tolerant to Cry toxins compared to many other lepidopteran pests (http://www.glfc.forestry.ca/bacillus). Despite the high number of Cry proteins discovered to date (>100) (5) (http://www.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/index.html), just a few have proven to be effective for their control. Cry1Ab, Cry1Ac, Cry2Aa, and Cry2Ab are the most toxic Cry proteins against H. armigera and H. zea and, along with Cry1Fa, against Heliothis virescens and H. punctigera (reviewed in reference 24). The introduction of the cry1Ac gene in selected lines of cotton has increased the productivity and has reduced the number of conventional insecticide applications (14). Bollgard cotton (Monsanto Co., Chesterfield, Mo.), a first-generation Bt-cotton that expresses Cry1Ac, has been commercialized since 1996 (32). In 2002 there were about 4.6 million Ha planted with Bt-cotton worldwide, and this figure is expected to increase significantly in the coming years as the adoption of this technology continues to expand in large established markets (such as China and Australia) and is commercially approved in new countries (such as India) (15).
A potential problem associated with this first generation of transgenic cotton expressing Cry1Ac is the possibility that the insect populations may evolve resistance to this toxin. In contrast to applications with chemical insecticides or with B. thuringiensis conventional products, the constitutive expression of the toxin in Bt-cotton allows very few escapes and thus exerts a strong selection pressure on the target population. For this reason, alternatives to Cry1Ac-cotton have been developed, such as Bt-cotton expressing other B. thuringiensis genes (a hybrid cry1Ab/cry1Ac gene, a vip3 gene) or a combination of the cry1Ac gene with other genes (either cry2Ab or cry1F) (14). Starting in 2002, the first of such second-generation Bt-cotton, producing the Cry1Ac and Cry2Ab toxins, has been approved for commercial planting in Australia (15). The combined expression of these two toxins not only aims at preserving the effectiveness of Bt cotton in terms of delaying the evolution of resistance but also renders a more effective product against the major pests of this crop by combining the action of the two toxins.
One requirement for the “stacked” gene strategy to work is that the “stacked” toxins have a different mode of action (9, 10). Some cases of multiple Cry toxin resistance and cross-resistance have been shown to be due to alteration of a common binding site (17, 23, 36). Altered binding is the best-characterized mechanism of resistance to Cry toxins and generally confers high resistance levels (10). By means of the study of the interaction of Cry proteins with the larval midgut, the aim of the present study was to determine the adequacy of the second generation Bt-cotton varieties expressing cry genes and to recommend or discourage new combinations of cry genes based on their predicted usefulness for insect resistance management. We have used 125I-Cry1Ab and 125I-Cry1Ac toxins and brush border membrane vesicles (BBMV) to propose a general model for Cry1Ab and Cry1Ac binding to specific sites in the larval midgut of H. armigera. We have determined the inhibitory effect of selected sugars and lectins on Cry1Ab and Cry1Ac binding and whether midgut binding sites are shared between these toxins or by any of nine other Cry proteins selected for being among the most active toxins against lepidopterans. Furthermore, we have tested BBMV from four different H. armigera populations to check the influence of the insect population on the binding site model.
MATERIALS AND METHODS
Production and purification of B. thuringiensis Cry proteins. The recombinant B. thuringiensis strains used in the present study (Cry protein they produced) were as follows: EG1273 (Cry1Aa), EG7077 (Cry1Ab), EG11070 (Cry1Ac), EG11916 (Cry1Ba), EG1081 (Cry1Ca), EG7300 (Cry1Da), EG11069 (Cry1Fa), and EG7279 (Cry1Ja) (Ecogen, Inc., Langhorne, Pa.) and EG7543 (Cry2Aa1) and EG7699 (Cry2Ab2) (Monsanto Co., Chesterfield, Mo.). Purified and trypsin activated Cry9Ca (the Lys mutant) (22) was kindly provided by Jeroen Van Rie (Bayer BioScience N.V., Ghent, Belgium).
Recombinant B. thuringiensis strains were grown in CCY medium (35) supplemented with the appropriated antibiotic for 48 h at 29°C. Spores and crystals were separated by centrifugation at 9,700 × g for 12 min and then washed four times with 1 M NaCl-10 mM EDTA. The pellet was finally suspended in 10 mM KCl. Crystal solubilization was carried out in carbonate buffer (50 mM Na2CO3; 0.1 M NaCl; 10 mM dithiothreitol; pH 10.5) for 1 h with constant shaking. After centrifugation to eliminate insoluble material, protoxin activation was carried out with trypsin (type I from bovine pancreas; Sigma Chemical Co., St. Louis, Mo.), and the completion of the reaction was checked by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Trypsin activated Cry proteins were purified by anion-exchange chromatography with the MonoQ HR5/5 column by fast-protein liquid chromatography (Pharmacia, Uppsala, Sweden). Protein was quantified by the Bradford assay (3) with bovine serum albumin (BSA) as a standard.
Iodination of Cry1A toxins.
Purified Cry1Ab and Cry1Ac toxins were labeled with 125I by the method of chloramine-T (37). Specific activities of the labeled proteins were analyzed by an enzyme-linked immunosorbent sandwich assay (37). The specific activities for 125I-Cry1Ab and 125I-Cry1Ac were 2.9 and 24 mCi/mg, respectively.
Insect populations, midgut isolation, and BBMV preparation.
One laboratory H. armigera population and three field populations were used. The laboratory population had been maintained in the laboratory for several years on an artificial diet. The field populations were from the provinces of Barcelona (Catalonia region, Northeastern Spain) and Seville and Córdoba (both from the Andalusia region, Southern Spain). The Andalusian populations were from the cotton growing area of Spain but not the Catalan population. Insects were reared at 25°C, in 60% relative humidity, and with a 16-8 (light-dark) photoperiod. Midguts were dissected from last-instar (L5) larvae, washed in ice-cold MET buffer (250 mM mannitol, 17 mM Tris-HCl, 5 mM EGTA [pH 7.5]), frozen in liquid nitrogen, and kept at −80°C until required. BBMV were prepared by the MgCl2 precipitation method (39), and the protein concentration was determined by the method of Bradford (3) with BSA as a standard.
Binding assays.
Binding assays were performed at room temperature in a final volume of 0.1 ml of binding buffer (1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl [pH 7.4], 0.1% BSA). Bound ligand was separated from free ligand by centrifugation at 11,000 × g for 10 min, followed by two washes with 0.5 ml of cold binding buffer (12). Radioactivity in the pellet was measured directly in the microtubes in a gamma counter (Compugamma 1282; LKB). Appropriate conditions for carrying out the binding assays were determined in preliminary experiments. The incubation times were 1 h for 125I-Cry1Ab and 30 min for 125I-Cry1Ac, and the amount of labeled toxin in the 0.1-ml assay mixture was 1 ng for 125I-Cry1Ab and 0.14 ng for 125I-Cry1Ac. The appropriate BBMV concentration to be used in the competition assays was determined for each BBMV preparation.
Homologous and heterologous competition experiments were done by incubating the labeled Cry toxin in the presence of increasing amounts of nonlabeled competitor and the appropriate BBMV concentration. For the 125I-Cry1Ab assays, the BBMV protein concentration was 0.04 mg/ml for the laboratory population. For the 125I-Cry1Ac assays, the BBMV protein concentration was 0.045 mg/ml for the laboratory population, 0.02 mg/ml for the Barcelona population, and 0.03 mg/ml for the Seville and Córdoba populations. All binding experiments were repeated two to three times.
Inhibition of 125I-Cry1Ab and 125I-Cry1Ac binding by sugars and lectins.
All sugars and lectins were from Sigma. The sugars tested were N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), N-acetylneuraminic acid (sialic acid), and α-d-mannose. The lectins tested were soybean agglutinin (SBA, which binds GalNAc), wheat germ agglutinin (WGA, which binds, from higher to lower affinity, GlcNAc, sialic acid, and GalNAc), and concanavalin A (ConA, which binds α-d-mannose and, with lower affinity, α-d-glucose). Inhibition experiments with sugars were performed as in the binding assays described above but with a preincubation of the labeled toxin with the sugar for 45 min at room temperature, prior to the start of the assay with the addition of the BBMV. With lectins, the same protocol was used except that the preincubation step was done with BBMV and the assay was started with the addition of the labeled toxin. Inhibition experiments were replicated two to three times.
Data analysis.
The analyses of binding data to obtain the dissociation constants and the concentration of binding sites was performed by using the LIGAND program (28). Graphic representations and curve fittings were done with the Graphpad Prism version 3.02 for windows (Graphpad Software, San Diego, Calif. [www.graphpad.com]).
RESULTS
Binding of 125I-Cry1Ac to BBMV from different populations of H. armigera.
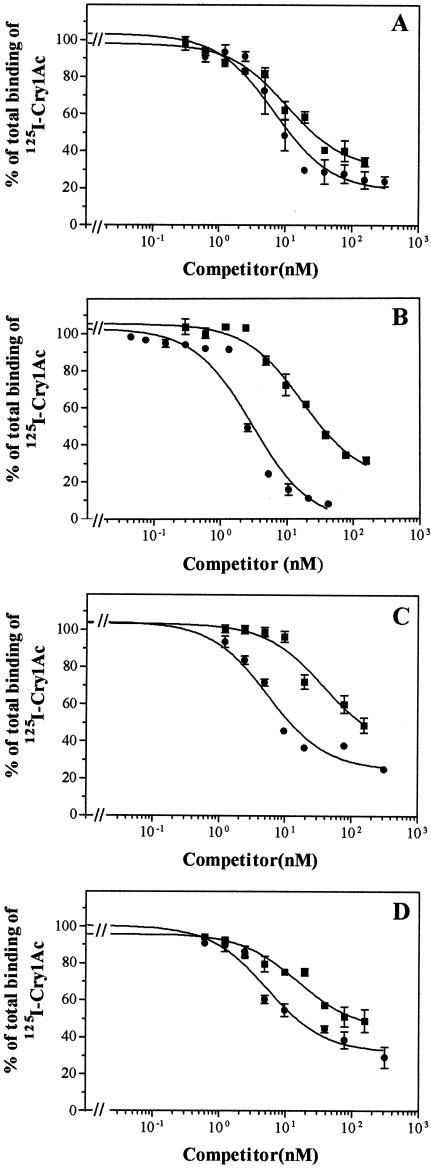
BBMV from a laboratory population and three field populations were tested for their binding characteristics to 125I-Cry1Ac. The results of competition experiments were qualitatively similar in all four cases, either with Cry1Ac as a homologous competitor or with Cry1Ab as a heterologous competitor (Fig. 1). The homologous competition assay indicated saturable binding of 125I-Cry1Ac, since increasing concentrations of nonlabeled Cry1Ac decreased the fraction of 125I-Cry1Ac binding to the BBMV. The heterologous competition assay showed that Cry1Ab competed for 125I-Cry1Ac binding, indicating that these two toxins share the same binding sites. However, there was always some 125I-Cry1Ac binding that could not be displaced by Cry1Ab even at high concentrations.
FIG. 1.
Binding of 125I-Cry1Ac at increasing concentrations of Cry1Ac (•) and Cry1Ab (▪) to BBMV from four different populations of H. armigera: laboratory (A), Barcelona (B), Seville (C), and Córdoba (D).
Quantitative analysis of the competition curves showed that both curves could only be fitted to a single-site model equation. The estimated dissociation constants (Kd) and concentrations of binding sites (Rt) for the four populations are shown in Table 1. Kd values were in the ranges of 0.92 to 4.2 nM for 125I-Cry1Ac and 10 to 43 nM for Cry1Ab. Rt values ranged from 19 to 59 pmol/mg of BBMV protein for 125I-Cry1Ac and from 13 to 23 pmol/mg of BBMV protein for Cry1Ab. The Kd values for 125I-Cry1Ac were always lower than the Kd values estimated for Cry1Ab, indicating a lower affinity of Cry1Ab for Cry1Ac binding sites. The Rt values for 125I-Cry1Ac were either equal to or higher than their respective Rt values for Cry1Ab.
TABLE 1.
Dissociation constants (Kd) and concentration of binding sites (Rt) for Cry1Ab and Cry1Ac binding to BBMV from different populations of H. armigeraa
| Population | Mean binding parameters of Cry toxins ± SEM for: |
|||
|---|---|---|---|---|
| Cry1Ac |
Cry1Ab |
|||
| Kd (nM) | Rt (pmol/mg) | Kd (nM) | Rt (pmol/mg) | |
| Laboratory | 1.5 ± 0.4 | 22 ± 4 | 10 ± 2 | 15 ± 3 |
| Barcelona | 0.92 ± 0.12 | 59 ± 4 | 17 ± 4 | 23 ± 5 |
| Seville | 2.9 ± 0.7 | 53 ± 9 | 43 ± 8 | 13 ± 9 |
| Córdoba | 4.2 ± 0.9 | 19 ± 4 | 34 ± 8 | 19 ± 4 |
Binding parameters for Cry1Ac were obtained from homologous competition data. Binding parameters for Cry1Ab were obtained from heterologous competition data by using 125I-Cry1Ac as the labeled ligand. The results are the means of two experiments.
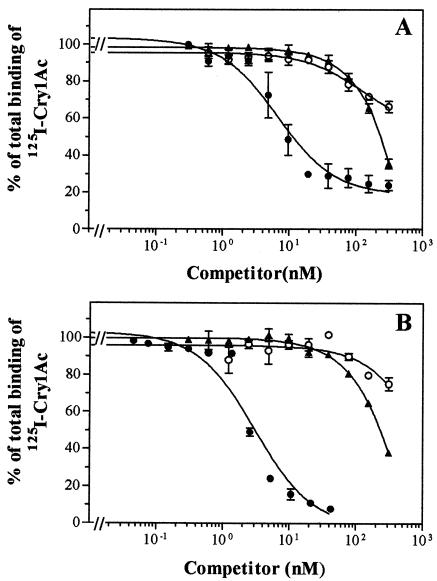
The effect of other Cry proteins as heterologous competitors on 125I-Cry1Ac binding was tested with BBMV from the laboratory population and a field population (Barcelona). Cry1Aa and Cry1Ja were tested with both BBMV, and the results were essentially identical (Fig. 2), with both proteins competing for 125I-Cry1Ac binding sites only at relatively high concentrations. The rest of the proteins (Cry1Ba, Cry1Ca, Cry1Da, Cry1Fa, Cry2Aa, Cry2Ab, and Cry9Ca) did not compete for 125I-Cry1Ac binding sites (Fig. 3).
FIG. 2.
Binding of 125I-Cry1Ac at increasing concentrations of Cry1Ac (•), Cry1Aa (▴), and Cry1Ja (○) to BBMV from the laboratory (A) and Barcelona (B) populations. The homologous competition curve is the same as in Fig. 1 and is displayed here as a reference.
FIG. 3.
Binding of 125I-Cry1Ac at increasing concentrations of Cry1Ac (•), Cry1Fa (▵), Cry2Ab (✽), and Cry9Ca (⧫) to BBMV from the laboratory population. The homologous competition curve is the same as in Fig. 1 and 2 and is displayed here as a reference. Heterologous data with Cry1Ba, Cry1Ca, Cry1Da, and Cry2Aa are not shown, but they were essentially similar to those displayed.
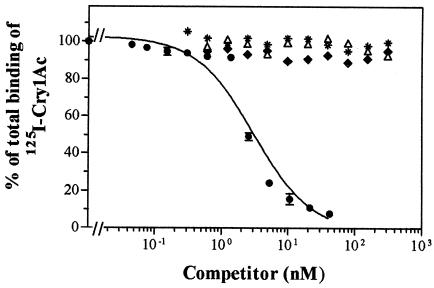
Binding of 125I-Cry1Ab to H. armigera BBMV.
Homologous and heterologous competition experiments with 125I-Cry1Ab were performed with BBMV from the laboratory population (Fig. 4). The homologous competition curve indicated saturable binding, and its quantitative analysis estimated a Kd = 26 ± 5 nM and an Rt = 62 ± 11 pmol/mg of BBMV protein. From all of the heterologous Cry proteins tested (Cry1Aa, Cry1Ac, Cry1Ba, Cry1Ca, Cry1Da, Cry1Fa, Cry1Ja, Cry2Aa, Cry2Ab, and Cry9Ca), only Cry1Aa, Cry1Ac, and Cry1Ja competed for 125I-Cry1Ab binding, although the latter only did it at very high concentrations (Fig. 4). Cry1Ac completely displaced 125I-Cry1Ab binding, indicating that all 125I-Cry1Ab binding sites are recognized by Cry1Ac. In contrast, at the highest concentration tested, Cry1Aa was not able to completely displace 125I-Cry1Ab binding.
FIG. 4.
Binding of 125I-Cry1Ab to BBMV from the laboratory population at increasing concentrations of competitor. (A) Cry1Ab (▪, solid line), Cry1Aa (▴, broken line), Cry1Ac (•, dotted line), and Cry1Ja (○, dashed-dotted line). (B) Cry1Ab (▪, solid line), Cry1Fa (▵), Cry2Ab (✽), and Cry9Ca (⧫). Heterologous data with Cry1Ba, Cry1Ca, Cry1Da, and Cry2Aa are not shown, but they were essentially similar to those displayed.
125I-Cry1Ab and 125I-Cry1Ac binding inhibition by sugars and lectins.
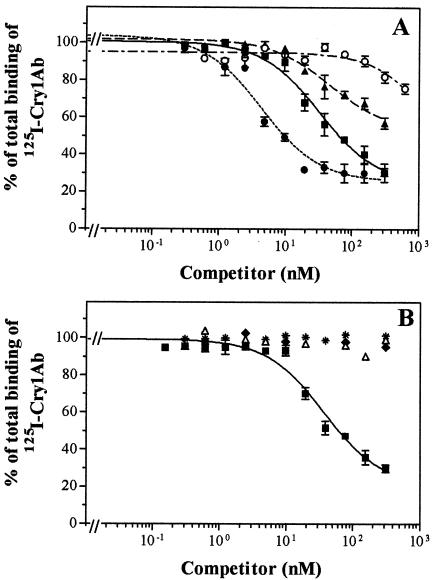
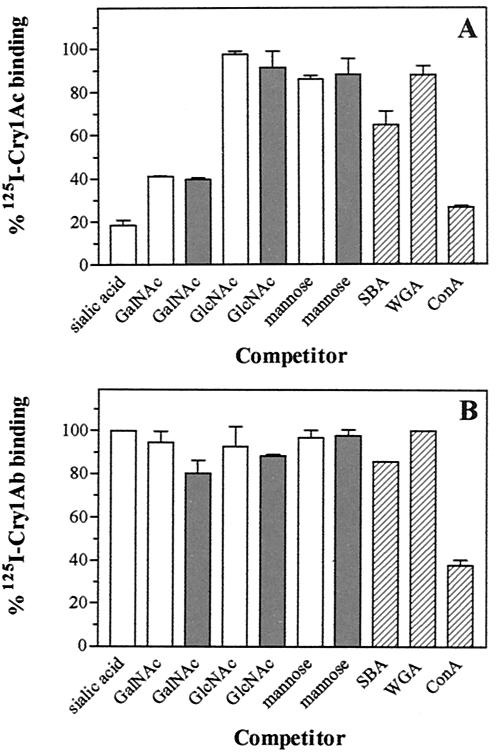
Different sugars and lectins were tested for their ability to inhibit 125I-Cry1Ab and 125I-Cry1Ac binding to BBMV. The results indicated a different binding inhibition pattern of these toxins (Fig. 5). ConA was the only product that strongly inhibited 125I-Cry1Ab binding (62%). SBA and high concentrations of GalNAc and GlcNAc (250 mM) had a minor influence on this toxin binding (from 11 to 19% inhibition). WGA, sialic acid, and mannose had no significant effect. However, 125I-Cry1Ac binding was strongly inhibited by ConA, GalNAc, and sialic acid (from 60 to 81%) and moderately inhibited by SBA (35%). WGA, GlcNAc, and mannose had only a minor influence on the binding inhibition of this toxin.
FIG. 5.
Effect of sugars and lectins on 125I-Cry1Ac (A) and 125I-Cry1Ab (B) binding to H. armigera BBMV. Inhibition experiments with sugars were performed with a preincubation of the labeled protein with the sugar at 25 mM (□) or 250 mM (░⃞) for 45 min at room temperature prior to the start of the assay with the addition of the BBMV. With lectins (at 50 μg/ml [▨]), the preincubation step was done with BBMV and the assay was started with the addition of the labeled protein.
DISCUSSION
Reported data on the susceptibility of H. armigera to individual Cry proteins generally agree on Cry1Ab and Cry1Ac being among the most toxic ones (along with Cry2Aa and Cry2Ab), although their relative potencies varied among the different studies (4, 24). Because one of the aims of the present study was to propose a general model for Cry1Ab and Cry1Ac binding to specific sites in the larval midgut of this species, we first checked the influence of working with different populations on the binding results. The qualitative results obtained with BBMV from the four populations were essentially identical, although quantitative estimates gave Kd and Rt values slightly different between populations (the greatest difference was found in the Kd for 125I-Cry1Ac). In all populations Cry1Ac bound with higher affinity (lower Kd) than Cry1Ab did. The Kd value for Cry1Ab obtained from homologous competition data confirmed its significantly lower affinity than Cry1Ac (∼20-fold). Therefore, our binding results would agree with Cry1Ac being more toxic than Cry1Ab to H. armigera larvae, although it is well known that there is not always a direct relationship between binding affinity and toxicity (7, 38).
To propose a general model for binding of Cry proteins to specific sites in the larval midgut of H. armigera, we have performed heterologous competition experiments with 125I-Cry1Ab and 125I-Cry1Ac labeled toxins and a set of 11 nonlabeled Cry proteins. These Cry proteins were selected for being among the most active against lepidopterans, although only Cry1Aa, Cry1Ab, Cry1Ac, Cry2Aa, and Cry2Ab have been reported to be toxic for H. armigera (4, 24) (to our knowledge, Cry1Ja has never been tested). Only four of them competed for Cry1Ab and Cry1Ac binding sites: Cry1Aa, Cry1Ab, Cry1Ac, and Cry1Ja. The simplest model that fits these results would be a single binding site shared by Cry1Ab and Cry1Ac, to which Cry1Aa and Cry1Ja also bind but not the other toxins. However, this model cannot explain why Cry1Ab does not completely compete for binding of 125I-Cry1Ac (Fig. 1) or why the 125I-Cry1Ab versus Cry1Aa heterologous curve was far from reaching the bottom plateau of the homologous curve at the highest concentration tested (Fig. 4A). It is worth noting the striking similarities of our heterologous competition results with those previously published for H. virescens with the Cry1A proteins (37). In this species, a three-site model was proposed based on the qualitative data from the competition curves. In this model, one population of binding sites (population A) is shared by the three toxins, a second population (B) binds Cry1Ab and Cry1Ac but not Cry1Aa, and a third population (C) has only affinity for Cry1Ac. The fact that the quantitative analysis of the data fitted a one-site model could be explained, assuming that the affinity of Cry1Ab and Cry1Ac for their respective populations of binding sites was similar and, thus, the occurrence of more than one binding site could not be inferred by the quantitative treatment of the data. Our results with H. armigera could also be explained by a three-site model, which we cannot discard as a plausible model for our data. The fact that neither Cry1Ab nor Cry1Aa could completely compete for binding of 125I-Cry1Ac (Fig. 1 and 2) suggests that Cry1Ac has at least two different binding sites, one of them not being recognized by the heterologous proteins (equivalent to H. virescens population of binding sites C). In the reciprocal experiment, Cry1Ac completely competed for binding of 125I-Cry1Ab (Fig. 4A), which would indicate that all binding sites of the latter are recognized by Cry1Ac. The fact that Cry1Aa did not completely compete for binding of 125I-Cry1Ab (Fig. 4A) suggests two different binding sites for Cry1Ab (equivalent to H. virescens binding site populations A and B). We are aware that this three-site model could still be a simplification of the actual toxin-binding interactions that take place in vivo.
It is interesting that in all lepidopteran species tested so far, Cry1Ac and Cry1Ab always share binding sites in radioligand competition experiments, sometimes even an apparently single binding site (2, 6, 7, 8, 18, 37, 38). It has been known for some time that Cry1Ac binding to BBMV is inhibited by GalNAc (19, 25, 26); however, Cry1Ab binding has been found not to be inhibited by this sugar (25, 26). Our results show that this is also the case with H. armigera, since 125I-Cry1Ac binding was strongly inhibited by GalNAc, whereas 125I-Cry1Ab was minimally affected. This same pattern of inhibition was also found with sialic acid, indicating that Cry1Ac, but not Cry1Ab, requires sugar residues to bind to BBMV in H. armigera. This binding inhibition is specific, since other sugars, such as GlcNAc and mannose, had little or no effect on the binding of either toxin. SBA, a lectin that has affinity for GalNAc, produced more inhibition of 125I-Cry1Ac than 125I-Cry1Ab binding. In contrast, ConA, which binds specifically to mannose and glucose residues, inhibited 125I-Cry1Ac and 125I-Cry1Ab binding similarly, suggesting that these sugar residues are close to the epitopes to which these toxins bind, although mannose residues themselves would not be used for binding.
The fact that Cry1Ab and Cry1Ac share binding sites in radioligand competition experiments seems to be in contradiction to the binding inhibition results with sugars. Furthermore, results from ligand blots have generally shown that, under denaturing conditions, Cry1Ac and Cry1Ab bind to different BBMV proteins (16, 30, 31). This paradox can be explained by proposing that binding sites in BBMV are oligomeric complexes of glycosylated membrane proteins (30). Binding of Cry1Ac and Cry1Ab could take place through different epitopes of the multimeric receptor and at the same time hinder binding of the heterologuos toxin by impeding access to a nearby site. This does not exclude the possibility that both toxins could also share identical epitopes, but in this case full binding might require anchorage of the Cry protein to both the shared and the nonshared epitopes. Support for the latter situation can be found in the biochemical analysis of resistant strains of Plutella xylostella for which, for the common Cry1A binding site, some mutations have been found to preclude binding of only Cry1Ab and others to preclude binding of more than one Cry1A toxin (2, 33, 36, 40).
Although Cry1Fa is not very toxic to H. armigera (24) and there are as yet no data reported for the toxicity of Cry1Ja, from an academic standpoint it is interesting to discuss the results obtained with these two proteins in competition experiments with the Cry1A proteins. In the seven lepidopteran species tested thus far, Cry1Ja competes for binding with Cry1Ac (13, 16). Similarly, in two species tested, Cry1Fa competes for binding with Cry1A proteins (11, 16). Based on these results, the alteration of a common receptor has been proposed to be the main mechanism of cross-resistance to these toxins in strains of H. virescens (16) and P. xylostella (2, 11, 13) that had been selected with Cry1A mixtures. Our results with these two toxins and BBMV from H. armigera contrast with those published for other species. Cry1Fa does not compete at all (this may contribute to its low toxicity to this species) and Cry1Ja only competes at very high concentrations, indicating very low affinity for the Cry1A binding sites and little or no biological significance of this competition in H. armigera.
The lack of competition of Cry2Ab protein for the Cry1Ac binding sites is relevant since the cry2Ab gene has been introduced, in combination with the cry1Ac gene, in the so-called second generation of Bt cotton (14). The purpose of using “stacked” cry genes in Bt plants is not only to broaden the pest spectrum but also to be used as a strategy for resistance management whenever the Cry proteins they produce do not share the same mechanism of action (9, 10). According to our results, Cry2Ab does not share binding sites with Cry1Ac. Since binding site alteration is generally the mechanism that confers higher levels of resistance and cross-resistance to Cry toxins (10), it is unlikely that single mutations in field populations will be found that, by affecting the binding site for one of the “stacked” toxins, would confer resistance to the second Cry toxin in these plants. A result that supports our prediction is the one reported recently for an Australian population of H. armigera, which was selected for Cry1Ac resistance (1). The population was cross-resistant to Cry1Ab but not to Cry2Aa or Cry2Ab, and the resistant insects had lost the capacity of binding Cry1Ac. This result also supports the biological relevance of the binding data obtained in our study.
The model for Cry toxin binding proposed for H. armigera strongly discourages combination of cry1A genes in plants to be protected against this pest because of the possibility of selecting for alleles that could confer resistance to more than one Cry1A toxin by the alteration of the common binding site. According to the model, other Cry proteins active against this pest, such as Cry2Aa or Cry2Ab, can be used in combination with either Cry1Ac or Cry1Ab without the threat of selecting for resistance alleles conferring major protection to these two families of toxins. Since H. armigera is a polyphagous pest, this recommendation not only applies to “stacked” genes in Bt-cotton but also to other crops that could be developed in the future either with a combination of cry genes or simply with just one cry gene targeting insect populations with a previous history of exposure to B. thuringiensis sprays.
Acknowledgments
We thank Jim Baum for kindly providing the B. thuringiensis strains producing Cry2A toxins and Jeroen Van Rie for useful comments on the manuscript. We also thank Primitivo Caballero (Universidad Pública de Navarra, Pamplona, Spain), Rosa Gabarra (IRTA, Cambrils, Barcelona, Spain), Enrique Vargas (Universidad de Córdoba, Córdoba, Spain), and Carlos Avilla (Universidad de Sevilla, Seville, Spain) for kindly providing H. armigera larvae.
This study was supported by the Spanish Ministry of Science and Technology with a competitive research grant (reference no. AGL2000-0840-C03-01), a fellowship for A.E. (FP2000-5497), and a research contract for B.E. from the “Ramón y Cajal” program.
REFERENCES
- 1.Akhurst, R. J., W. James, L. J. Bird, and C. Beard. 2003. Resistance to the Cry1Ac δ-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 96:1290-1299. [DOI] [PubMed] [Google Scholar]
- 2.Ballester, V., F. Granero, B. E. Tabashnik, T. Malvar, and J. Ferré. 1999. Integrative model for binding sites of Bacillus thuringiensis toxins in susceptible and resistance larvae of the diamondback moth (Plutella xylostella). Appl. Environ. Micobiol. 65:1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of dye-binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti, S. K., A. Mandaokar, P. A. Kumar, and R. P. Sharma. 1998. Efficacy of lepidopteran specific δ-endotoxins of Bacillus thuringiensis against Helicoverpa armigera. J. Invertebr. Pathol. 72:336-337. [DOI] [PubMed] [Google Scholar]
- 5.Crickmore, N., D. R. Zeigler, J. Feitelson, E. Schnepf, J. Van Rie, D. Lereclus, J. Baum, and D. H. Dean. 1998. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denolf, P., S. Jansens, M. Peferoen, D. Degheele, and J. Van Rie. 1993. Two different Bacillus thuringiensis delta-endotoxin receptors in the midgut brush border membrane of the European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae). Appl. Environ. Microbiol. 59:1828-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escriche, B., J. Ferré, and F. J. Silva. 1997. Occurrence of a common binding site in Mamestra brassicae, Phthorimaea operculella, and Spodoptera exigua for the insecticidal crystal proteins CryIA from Bacillus thuringensis. Insect Biochem. Mol. Biol. 27:651-656. [DOI] [PubMed] [Google Scholar]
- 8.Estada, U., and J. Ferré. 1994. Binding of insectidical crystal proteins of Bacillus thuringiensis to the midgut brush border of the cabbage looper, Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae), and selection for resistance to one of the crystal proteins. Appl. Environ. Microbiol. 60:3840-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferré, J. 2003. Insect resistance to Bacillus thuringiensis toxins, p. 141-155. In T. Lelley, E. Balázs, and M. Tepfer (ed.), Ecological impact of GMO dissemination in agro-ecosystems. Facultas Verlags- und Buchhandels AG, Vienna, Austria.
- 10.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 11.Granero, F., V. Ballester, and J. Ferré. 1996. Bacillus thuringiensis crystal proteins Cry1Ab and Cry1Fa share a high-affinity binding site in Plutella xylostella (L.). Biochem. Biophys. Res. Commun. 224:779-783. [DOI] [PubMed] [Google Scholar]
- 12.Herrero, S., and J. Ferré. 2001. Comparison of different methodologies for binding assays of Bacillus thuringiensis toxins to membrane vesicles from insect midguts. J. Invertebr. Pathol. 78:275-277. [DOI] [PubMed] [Google Scholar]
- 13.Herrero, S., J. González-Cabrera, B. Tabashnik, and J. Ferré. 2001. Shared binding sites in Lepidoptera for Bacillus thuringiensis Cry1Ja and Cry1A toxins. Appl. Environ. Microbiol. 67:5729-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James, C. 2002. Global review of commercialized transgenic crops: 2001 feature: Bt cotton. International Service for the Acquisition of Agri-Biotech Applications, Ithaca, N.Y.
- 15.James, C. 2002. Global status of commercialized transgenic crops: 2002. Preview. International Service for the Acquisition of Agri-Biotech Applications, Ithaca, N.Y.
- 16.Jurat-Fuentes, J. L., and M. J. Adang. 2001. Importance of Cry1 δ-endotoxin domain II loops for binding specificity in Heliothis virescens (L.). Appl. Environ. Microbiol. 41:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurat-Fuentes, J. L., F. L. Gould, and M. J. Adang. 2002. Altered glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Appl. Environ. Microbiol. 68:5711-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karim, S., S. Riazulddin, F. Gould, and D. H. Dean. 2000. Determination of receptor binding properties of Bacillus thuringiensis δ-endotoxins to cotton bollworm (Helicoverpa zea) and pink bollworm (Pectinophora gossypiella) midgut brush border membrane vesicles. Pest. Biochem. Physiol. 67:198-216. [Google Scholar]
- 19.Knowles, B. H., P. J. Knight, and D. J. Ellar. 1991. N-Acetylgalactosamine is a part of the receptor in the insect gut epithelia that recognizes an insecticidal protein from Bacillus thuringiensis. Proc. R. Soc. Lond. B 245:31-35. [DOI] [PubMed] [Google Scholar]
- 20.Krathi, K. R., D. R. Jadhav, R. R. Wanjari, S. S. Ali, and D. Russel. 2001. Carbamate and organophosphate resistance in cotton pests in India, 1995 to 1999. Bull. Entomol. Res. 91:37-46. [PubMed] [Google Scholar]
- 21.Krathi, K. R., D. R. Jadhav, R. R. Wanjari, S. S. Ali, and D. Russel. 2001. Pyrethroid resistance and mechanisms of resistance in field strains of Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 94:253-263. [DOI] [PubMed] [Google Scholar]
- 22.Lambert, B., L. Buysse, C. Decock, S. Jansens, C. Piens, B. Saey, J. Seurinck, K. Van Audenhove, J. Van Rie, A. Van Vliet, and M. Peferoen. 1996. A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family Noctuidae. Appl. Environ. Microbiol. 62:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, M. K., F. Rajamohan, F. Gould, and D. H. Dean. 1995. Resistance to Bacillus thuringiensis CryIA δ-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl. Environ. Microbiol. 61:3836-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao, C., D. G. Heckel, and R. Akhurst. 2002. Toxicity of Bacillus thuringiensis insecticidal proteins for Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae), major pests of cotton. J. Invertebr. Pathol. 80:55-66. [DOI] [PubMed] [Google Scholar]
- 25.Luo, K., S. Sandagala, L. Masson, A. Mazza, R. Brousseau, and M. J. Adang. 1997. The Heliothis virescens 170-kDa aminopeptidase functions as “receptor A” by mediating specific Bacillus thuringiensis Cry1A δ-endotoxin binding pore formation. Insect. Biochem. Mol. Biol. 27:735-743. [DOI] [PubMed] [Google Scholar]
- 26.Masson, L., Y. Lu, A. Mazza, R. Brousseau, and M. J. Adang. 1995. The CryIA(c) receptor purified from Manduca sexta displays multiple specificities. J. Biol. Chem. 270:20309-20315. [DOI] [PubMed] [Google Scholar]
- 27.McCaffery, R. A. 1998. Resistance to insecticides in heliothine Lepidoptera: a global view. Phil. Trans. R. Soc. Lond. B 353:1735-1750. [Google Scholar]
- 28.Munson, P. J., and D. Rodbard. 1980. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107:220-239. [DOI] [PubMed] [Google Scholar]
- 29.Nester, E. W., L. S. Thomashow, M. Metz, and M. Gordon. 2002. 100 years of Bacillus thuringiensis: a critical scientific assessment. American Academy of Microbiology, Washington, D.C. [PubMed]
- 30.Oddou, P., H. Hartmann, and M. Geiser. 1991. Identification and characterization of Heliothis virescens midgut membrane proteins binding Bacillus thuringiensis δ-endotoxins. Eur. J. Biochem. 202:673-680. [DOI] [PubMed] [Google Scholar]
- 31.Oddou, P., H. Hartmann, F. Radecke, and M. Geiser. 1993. Immunologically unrelated Heliothis sp. and Spodoptera sp. midgut membrane-proteins bind Bacillus thuringiensis CryIA(b) δ-endotoxin. Eur. J. Biochem. 212:145-150. [DOI] [PubMed] [Google Scholar]
- 32.Perlak, F. J., M. Oppenhuizen, K. Gustafson, R. Voth, S. Sivasupramaniam, D. Heering, B. Carey, R. A. Ihrig, and K. Roberts. 2001. Development and commercial use of Bollgard® cotton in the USA: early promises versus today's reality. Plant J. 27:489-501. [DOI] [PubMed] [Google Scholar]
- 33.Sayyed, A. H., R. Haward, S. Herrero, J. Ferré, and D. J. Wright. 2000. Genetic and biochemical approach characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth. Appl. Environ. Microbiol. 66:1509-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart, G. S. A. B., K. Johnstone, E. Hagelberg, and D. J. Ellar. 1981. Commitment of bacterial spores to germinate. Biochem. J. 198:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabashnik, B. E., Y. B. Liu, T. Malvar, D. G. Heckel, L. Masson, V. Ballester, F. Granero, J. L. Ménsua, and J. Ferré. 1997. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 94:12780-12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rie, J. S. Jansens, H. Höfte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis delta-endotoxins: importance of specific receptors on brush border membrane of the midgut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 38.Wolfersberger, M. G. 1990. The toxicity of two Bacillus thuringiensis δ-endotoxins to gypsy moth larvae is inversely related to affinity to binding sites on midgut brush border membranes for the toxins. Experientia 46:475-477. [DOI] [PubMed] [Google Scholar]
- 39.Wolfersberger, M. G., P. Luthy, A. Maurer, P. Parenti, V. F. Sacchi, B. Giordana, G., and M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86:301-308. [Google Scholar]
- 40.Wright, D. J., M. Iqbal, F. Granero, and J. Ferré. 1997. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for field resistance to Bacillus thuringiensis subsp. kurstaki and Bacillus thuringiensis subsp. aizawai. Appl. Environ. Microbiol. 63:1814-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]