Abstract
This study investigated the effects of different celery (Apium graveolens) seed extracts on blood pressure (BP) in normotensive and deoxycorticosterone acetate–induced hypertensive rats. The hexanic, methanolic, and aqueous-ethanolic extracts were administered intraperitoneally and their effects on BP and heart rate (HR) were evaluated in comparison with spirnolactone as a diuretic and positive control. Also, the amount of n-butylphthalide (NBP), as an antihypertensive constituent, in each extract was determined by HPLC. The results indicated that all extracts decreased BP and increased the HR in hypertensive rats, but had no effect on normotensive rats. The data showed that administration of 300 mg/kg of hexanic, methanolic, and aqueous-ethanolic (20/80, v/v) extracts of the celery seed caused 38, 24, and 23 mmHg reduction in BP and 60, 25, and 27 beats per minute increase in the HR, respectively. Also, the HPLC analysis data revealed that the content of NBP in the hexanic extract was 3.7 and 4 times greater than methanolic and aqueous-ethanolic extracts. It can be concluded that celery seed extracts have antihypertensive properties, which appears to be attributable to the actions of its active hydrophobic constitutes such as NBP and can be considered as an antihypertensive agent in chronic treatment of elevated BP.
Key Words: blood pressure, celery seed, chronic administration, tail cuff
Introduction
Increased blood pressure (BP) is one of the important risk factors for coronary heart disease, which is the largest cause of mortality in industrial countries.1 Hypertension (HTN) has been termed the silent killer, an asymptomatic chronic disorder that, if undetected and untreated, silently damages the blood vessels, heart, brain, and kidneys.2 Thus, hypertensive patients have increased risk of silent ischemia and unrecognized myocardial infarction (MI). Due to this fact, patients with acute MI often have preexisting HTN that has been undetected or untreated.3 Preexisting HTN increases the case-fatality rate associated with an acute MI and substantially increases the risk of hemorrhagic stroke during thrombolytic therapy, especially when systolic BP exceeds 175 mmHg.4 Controlling HTN can also improve exertional dyspnea, nocturia and possibly even erectile dysfunction caused by endothelial dysfunction.5 A health-promoting lifestyle, weight loss, and decreased dietary NaCl have been shown to lower the risk of developing HTN. Drug therapy will be necessary if these interventions are not efficient in decreasing BP.6 Medications are recommended for hypertensives with BP more than 140/90 mmHg.7 To achieve ideal BP, the majority of hypertensives will require treatment with more than one drug.8 Appropriate combinations of these drugs at lower doses may have additive effects on BP with a lower incidence of side effects. Taking BP medications can cause side effects such as headache, dizziness, tachycardia, feeling tired, and sexual dysfunction.9
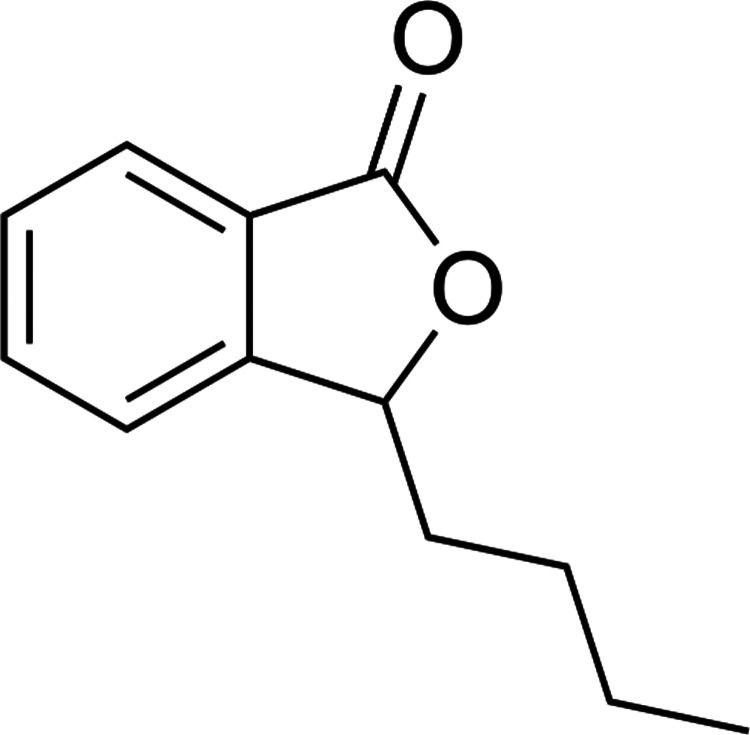
Nowadays, there are many herbal medicines for management of HTN, for example, garlic, hawthorn, and cayenne pepper.10–13 There are some ingredients in the herbs that synergistically produce beneficial effects.14,15 Apium graveolens, commonly known as celery, is a plant species in the family Apiaceae. Celery grows up to 1-m height and has odd-pinnate compound leaves with dentate leaflets on a central stem.16,17 In recent pharmacological studies, celery has demonstrated antioxidant, hypolipidemic and anti-inflammatory activities.18,19 It was also administered as an antihypertensive agent in folk medicine.20 n-Butylphthalide (NBP) (Fig. 1) along with sedanolide, is one of the chemical constituents in celery oil, which is primarily responsible for the aroma and taste of celery.21 Previous studies in animal models suggested that NBP, extracted from other herbs, may be useful for the treatment of HTN.21–23 In this study, we investigated the antihypertensive effect of chronic administration of hexanic, aqueous-ethanolic and methanolic extracts of A. graveolens in rats. We also determined the amount of NBP in the above-mentioned extracts by HPLC. The data suggested that celery extracts possess hypotensive properties in rats and should be further investigated as a potential intervention for HTN in humans.
FIG. 1.
The chemical structure of n-butylphthalide (NBP).
Materials and Methods
Chemicals
Methanol, ethanol (80%), liquid paraffin, and n-hexane were obtained from Merck (Darmstadt, Germany). Spirnolactone was obtained from Pars Darou Pharmaceutical Corporation (Tehran, Iran). NBP was purchased from Langchem, Inc. (Shanghai, China). Deoxycorticosterone acetate (DOCA) and normal saline (NS) (0.9%) were obtained from Hakim Pharmaceutical Corporation (Tehran, Iran) and Samen Corporation (Mashhad, Iran), respectively.
Extraction and HPLC analysis
The celery extracts were isolated from celery seeds (obtained from Imam Pharmacy, Mashhad, Iran, and the identity was confirmed by the herbarium of school of pharmacy). Briefly, celery seeds were ground and powdered and the dry powder (50 g) was suspended in 250 mL of solvent (n-hexane, methanol, or aqueous-ethanol [20/80, v/v]) at room temperature and shaken for 48 h in the darkness. Then, the suspension was centrifuged (2594 g for 10 min) and the supernatant was separated. The solution was allowed to dry in darkness at room temperature. The hexanic extract was dissolved in liquid paraffin and the other extracts were dissolved in NS (0.9%) before injection.
Chromatographic determination of NBP was carried out using a Younglin Acme 9000 system (Gyeonggi-do, South Korea), consisting of an SP930D solvent delivery module, SDV50A solvent mixing vacuum degasser, column oven CTS30, UV730 dual wavelength UV/VIS detector, and ODSA C18 (4.6 mm×150 mm, 5-μm) column. The data analysis was performed by Autochro 3000 software. The injection volume was 20 μL, the flow rate was 1 mL/min, and the column temperature was fixed at 30°C. The UV detector was set to 230 nm. A gradient method was applied in which the mobile-phase composition was changed from 20% methanol in water to 80% in 20 min run time. The same concentration of all extracts (100 μg/mL) were prepared in methanol and injected into the HPLC. The concentrations of NBP were calculated from the area under curve by comparing them to an NBP standard solution.
Animals and drugs
Male Wistar rats (250–320 g) were obtained from the animal facilities of the Pharmaceutical Research Center, BuAli Research Institute, Mashhad University of Medical Sciences. The animals were housed six per cage with a 12-h light/12-h dark cycle at 21°C±2°C and had free access to food and 1% saline solution.
About 54 rats were randomly divided into nine groups: All groups except group 9 (normotensive rats), received DOCA for 7 weeks (20 mg/kg, twice weekly, s.c.). From week 4 to 7, drugs were injected daily intraperitoneally (i.p.) as mentioned below.
Hexanic extracts (100, 200, and 300 mg/kg) were administered to groups 1, 2, and 3, methanolic (300 mg/kg) and aqueous-ethanolic extracts (300 mg/kg) for groups 4 and 5, and spirnolactone (50 mg/kg) for the positive control group (group 6). Negative control groups (group 7 and 8) received NS 0.9% (solvent of methanolic and aqueous-ethanolic extracts) and liquid paraffin (solvent of the hexanic extract) (0.5 mL). The normotensive group (group 9) received NS 0.9% (0.5 mL, i.p. twice weekly, n=6) for 7 weeks and the hexanic extract (300 mg/kg, i.p. every day) from week 4 to 7.
For evaluation of the persistent effect of celery and spirnolacton, groups 3 and 6 received DOCA, for two more weeks after stopping the administration of drugs at week 7.
Handling and experimental procedures for all animals were in accordance with the Mashhad University of Medical Sciences Ethics Committee Acts.
Measurement of BP and heart rate
The BP and heart rate (HR) were determined at the end of every week using the tail-cuff method. Before measurement, the animal was placed and relaxed in a rat holder at room temperature (25°C) about 15 to 20 min for acclimation. The results of BP were significantly more reliable under these conditions. At lower temperatures, tail blood flow was reduced and determination of BP was very difficult. Systolic blood pressure was measured by means of the tail-cuff method using a SP844 MLT844 physiological pressure transducer. Acquisition of data was performed by a computerized system Power Lab (AD Instruments, v5.4.2). For each animal, the BP and HR were measured 3 times, in a period of 30 min, and the average of data was reported.
Statistical analysis
Data are expressed as mean±SEM. The data were assessed by one-way repeated measure analysis of variance followed by the Tukey's post hoc test. A value of P<.05 was considered statistically significant.
Results
Amounts of NBP in celery extracts
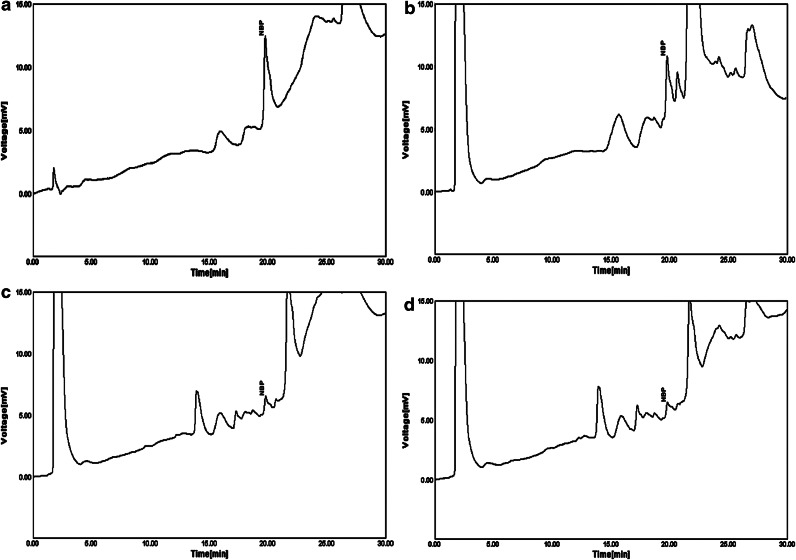
The HPLC analysis showed that the amounts of NBP in hexanic, methanolic, and aqueous-ethanolic (20/80, v/v) were 3.46, 0.93, and 0.85 mg/g of extracts, respectively (Fig. 2). The NBP is an oily compound with higher solubility in nonpolar solvents compared to water and other polar media. Also, the concentration of other nonpolar compounds of celery seeds would be higher in hexanic extracts.
FIG. 2.
Chromatograms of a standard methanolic solution of NBP (1 μg/mL) (a), hexanic extract (100 μg/mL) (b), methanolic extract (100 μg/mL) (c), and aqueous-ethanolic extract (100 μg/mL) (d) of celery seeds.
Systolic BP
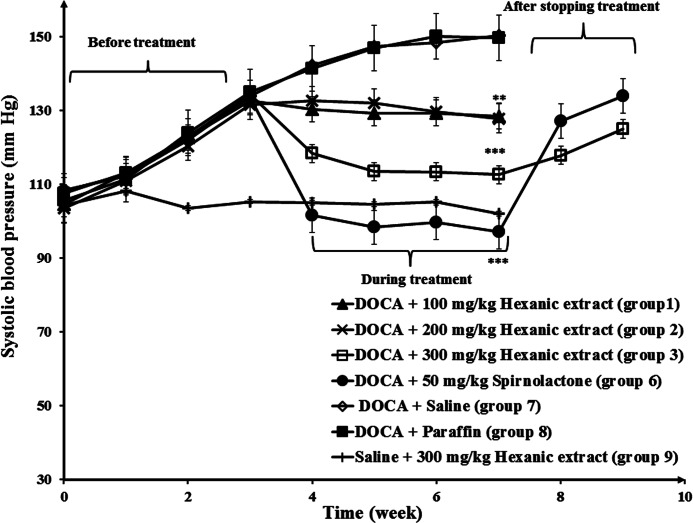
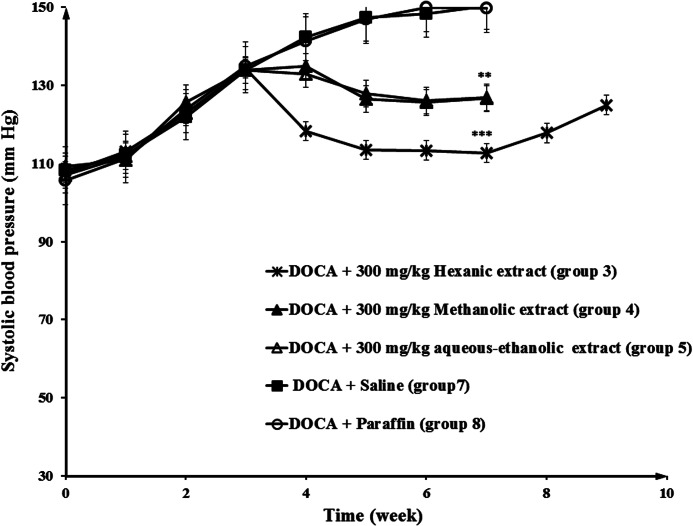
Administration of DOCA to the negative control groups (groups 7 and 8) increased the BP from 10.5 to 15 mmHg in a period of 7 weeks, but BP in the normotensive group (group 9) remained unchanged during this time (Fig. 3). Also, the data showed that BP was decreased in the spirnolactone (group 6) and hexanic celery seed extract groups (groups 1, 2, and 3), in comparison with negative control groups, in hypertensive rats. At the end of week 7, the BP values in group 3 (300 mg/kg hexanic extract) and 6 (50 mg/kg spirnolactone) were 112 and 97 mmHg, respectively. After stopping the treatment, the BP increased to 125 and 134 mmHg, in group 3 and 6 at the end of week 9. As shown in Figure 4, the antihypertensive effect of hexanic extracts was significantly higher than methanolic and aqueous-ethanolic extracts of celery seeds in rats.
FIG. 3.
Decrease in blood pressure (BP) in response to various doses of hexanic extracts in normotensive and hypertensive rats. Negative control groups received vehicle (saline or paraffin). Statistical analysis showed a significant difference between all treatment groups (hexanic extracts and spirnolactone) and negative control groups. **P<.01, ***P<.001.
FIG. 4.
Decrease in BP in response to 300 mg/kg dose of hexanic, methanolic, and aqueous-ethanolic extracts in hypertensive rats. Negative control group received vehicle (saline or paraffin). Statistical analysis showed a significant difference between all treatment groups (hexanic, methanolic, and aqueous-ethanolic extract) and negative control groups. **P<.01, ***P<.001.
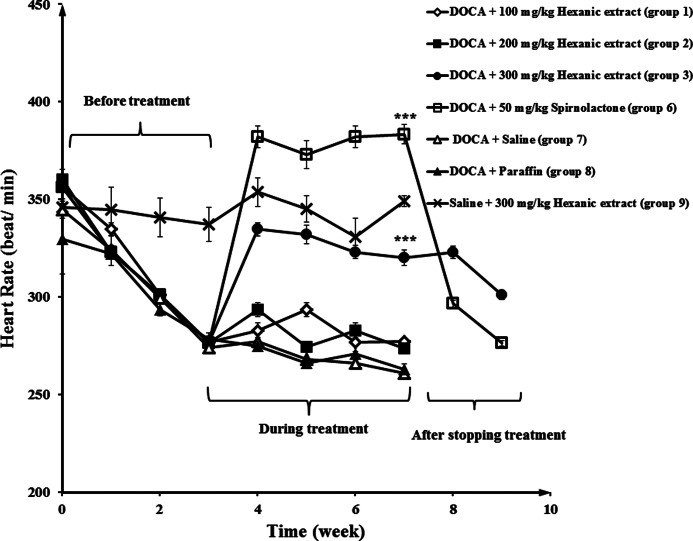
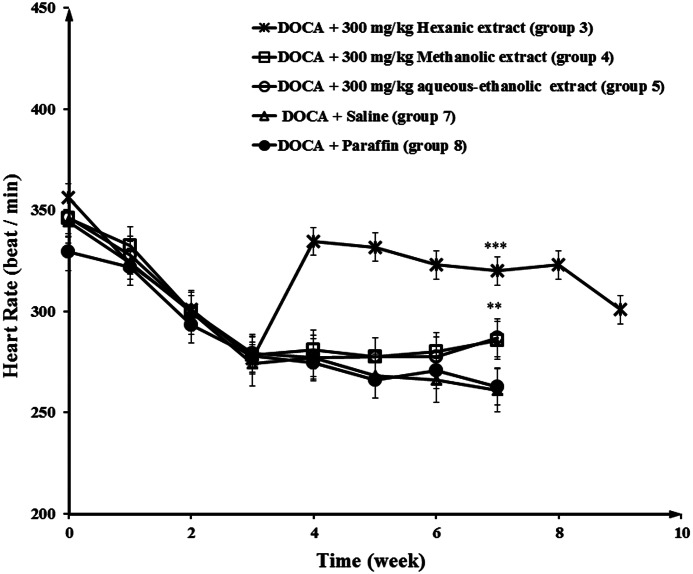
Administration of DOCA in negative control groups (groups 7 and 8) decreased the HR from 350 to 260 beats per minute (BPM) in a period of 7 weeks, while the HR in the normotensive group (group 9) remained unchanged (Fig. 5). Also, the data showed that spirnolactone (group 6) and hexanic celery seed extract groups (groups 1, 2, and 3) experienced an increased HR, in comparison with negative control groups, in hypertensive rats. At the end of week 7, the HR values in group 3 (300 mg/kg hexanic extract) and 6 (50 mg/kg spirnolactone) were 330 and 380 BPM, respectively. After stopping the treatment, the HR changed to 310 and 290 BPM, in group 3 and 6 at the end of week 9. As shown in Figure 6, the effect of hexanic extracts on HR was significantly greater than those of methanolic and aqueous-ethanolic celery seed extracts.
FIG. 5.
Increase in the heart rate (HR) in response to various doses of hexanic extracts in normotensive and hypertensive rats. Negative control group received vehicle (saline or paraffin). Statistical analysis showed a significant difference between 300 mg/kg dose of hexanic extracts and 50 mg/kg dose of spironolactone in hypertensive rats compared to the negative control group. ***P<.001.
FIG. 6.
Increase in the HR in response to 300 mg/kg dose of hexanic, methanolic, and aqueous-ethanolic extracts in hypertensive rats. Negative control group received vehicle (saline or paraffin). Statistical analysis showed a significant difference between all treatment groups (hexanic, methanolic, and aqueous-ethanolic extracts) and negative control groups. **P<.01, ***P<.001.
Discussion
All extracts of celery seeds reduced the BP and increased the HR in hypertensive rats. Also, the extracts had no effect on BP and HR in normotensive groups. Persistence of the antihypertensive effect, after stopping the treatment, was also significantly greater in group 3 (received 300 mg/kg hexanic extract) in comparison with the spironolactone administration group, possibly due to a lower elimination. NBP is one of the active constituents in celery.24–26 Some researchers have reported antihypertensive effects of some other herbs, for example, Solanaceae, in which NBP is one of the main fractions.27 It is an oily and colorless compound in celery. The solubility of NBP in nonpolar solvents is significantly higher than in aqueous or polar media.23 Based on HPLC analysis, the amount of NBP in hexanic extracts is 3.7 and 4 times greater than in methanolic and aqueous-ethanolic extracts, respectively. It can be suggested that the amounts of other hydrophobic active compounds in hexanic extracts are also higher than in the other extracts, and that the oily fraction of the celery seed plays an important role in antihypertensive effects of this herb, and not just NBP. The hypotensive effects of two other extracts could be due to the presence of other active polar or hydrophilic compounds with higher solubility in aqueous media. Other constitutes of celery seeds are falcariondiol, bergapten, oplopandiol, trans-cinnamic acid, caffeoylquinic acid, benzolic acid, and minerals.28 In addition to NBP, some of these compounds may be involved in its hypotensive activity. Some studies have reported that NBP has a diuretic effect in rats.29,30 The urine volume of rats in group 3 (receiving 300 mg/kg hexanic extract) was significantly greater than other extract groups (methanolic and aqueous-ethanolic groups) and the spirnolactone-positive control group, as the animal cage in group 3 (300 mg/kg) was usually wet, compared to other groups (data was not shown). Thus, its diuretic effect could be one of the possible antihypertensive mechanisms of celery seeds. Also, chronic administration of extracts significantly increased the HR in the extract groups, whereas the HR was decreased in negative control groups due to elevation of BP. In fact, if the BP falls, the baroreceptor firing rate decreases and baroreceptor reflexes act to help restore the BP by increasing the HR. Thus, the vasodilatory effect of components in celery extracts maybe involved in hypotensive and HR-elevating effects of this herb. The safety of drug administration should be always considered in all therapeutic interventions. According to the previous researches, no toxicity has been reported in the administration of different doses of celery seeds. In a study by Powanda et al. (2010), no toxicity was seen after 28 days administration of 150 and 5000 mg/kg per day of celery seeds.31 Also, Al-Howiriny et al. did not observe any toxic symptoms or mortality in IP administration of doses 250 and 500 mg/kg per day.32 Therefore, the present work provides evidence of a hypotensive effect of celery seeds, which appears to be attributable to the action of some hydrophobic constituents, for example, NBP in this plant. However, more studies should be done to clarify the mechanisms of this effect.
Acknowledgment
The authors gratefully acknowledge the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial support through grant number 900763.
Author Disclosure Statement
The authors have declared no conflict of interests.
References
- 1.Iyer A. Chan V. Brown L. The DOCA-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev. 2010;6:291–297. doi: 10.2174/157340310793566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminzadeh MA. Azizi ZA. Hamidi A. Monjazeb M. Omrani GR. Association of mean arterial blood pressure with plasma total homocysteine level, but not with the common C677T MTHFR gene mutation in postmenopausal Iranian women. Int J Endocrinol Metabol. 2006;4:88–95. [Google Scholar]
- 3.Huetteman DA. Bogie H. Direct blood pressure monitoring in laboratory rodents via implantable radio telemetry. Methods Mol Biol. 2009;573:57–73. doi: 10.1007/978-1-60761-247-6_4. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank JM. Thorp JM. Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;329:581–584. doi: 10.1016/s0140-6736(87)90231-5. [DOI] [PubMed] [Google Scholar]
- 5.Tanner GA. Tanner JA. Chronic caffeine consumption exacerbates hypertension in rats with polycystic kidney disease. Am J Kidney Dis. 2001;38:1089–1095. doi: 10.1053/ajkd.2001.28614. [DOI] [PubMed] [Google Scholar]
- 6.Prisant LM. Weir MR. Papademetriou V, et al. Low-dose drugcombination therapy: an alternative first-line approach to hypertension treatment. Am Heart J. 1995;130:359–366. doi: 10.1016/0002-8703(95)90454-9. [DOI] [PubMed] [Google Scholar]
- 7.Borghi C. Cicero AF. Hypertension: management perspectives. Expert Opin Pharmacother. 2005;13:1999–2003. doi: 10.1517/14656566.2012.708733. [DOI] [PubMed] [Google Scholar]
- 8.Derer W. Muller DN. Dechend R. New antihypertensive drugs. MMW Fortschr Med. 2012;154:72–73. doi: 10.1007/s15006-012-0253-6. [DOI] [PubMed] [Google Scholar]
- 9.Girerd X. Laroche P. Hanon O, et al. Use of antihypertensive drugs in France and relationship with cardiovascular disease. Ann Cardiol Angeiol (Paris) 2012;61:213–217. doi: 10.1016/j.ancard.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Chang WT. Dao J. Shao ZH. Hawthorn: potential roles in cardiovascular disease. Am J Chin Med. 2005;33:1–10. doi: 10.1142/S0192415X05002606. [DOI] [PubMed] [Google Scholar]
- 11.Foushee DB. Ruffin J. Banerjee U. Garlic as a natural agent for the treatment of hypertension: a preliminary report. Cytobios. 1982;34:145–152. [PubMed] [Google Scholar]
- 12.Pharand C. Ackman ML. Jackevicius CA. Paradiso-Hardy FL. Pearson GJ. Use of OTC and herbal products in patients with cardiovascular disease. Ann Pharmacother. 2003;37:899–904. doi: 10.1345/aph.1C163. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee SK. Maulik SK. Effect of garlic on cardiovascular disorders. Nutr J. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai K. Yokoyama Y. Kokuryo T, et al. Inchinkoto, an herbal medicine, exerts beneficial effects in the rat liver under stress with hepatic ischemia-reperfusion and subsequent hepatectomy. Ann Surg. 2012;251:692–700. doi: 10.1097/SLA.0b013e3181d50299. [DOI] [PubMed] [Google Scholar]
- 15.Stout CW. Weinstock J. Homoud MK, et al. Herbal medicine: beneficial effects, side effects, and promising new research in the treatment of arrhythmias. Curr Cardiol Rep. 2003;5:395–401. doi: 10.1007/s11886-003-0097-x. [DOI] [PubMed] [Google Scholar]
- 16.Sheng JP. Liu C. Shen L. Comparative study of minerals and some nutrients in organic celery and traditional celery. Guang Pu Xue Yu Guang Pu Fen Xi. 2009;29:247–249. [PubMed] [Google Scholar]
- 17.Zhou K. Zhao F. Liu Z, et al. Triterpenoids and flavonoids from celery (Apium graveolens) J Nat Prod. 2009;72:1563–1567. doi: 10.1021/np900117v. [DOI] [PubMed] [Google Scholar]
- 18.Iyer D. Patil UK. Effect of chloroform and aqueous basic fraction of ethanolic extract from Apium graveolens L. in experimentally-induced hyperlipidemia in rats. J Complement Integr Med. 2011;8:ii. doi: 10.2202/1553-3840.1529. [DOI] [PubMed] [Google Scholar]
- 19.Ninfali P. Bacchiocca M. Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. J Agric Food Chem. 2003;51:2222–2226. doi: 10.1021/jf020936m. [DOI] [PubMed] [Google Scholar]
- 20.Gharouni M. Sarkati A. Application of apium graveolens in treatment of hypertension. J Tehran Univ Med Sci. 2000;3:67–69. [Google Scholar]
- 21.Houston MC. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog Cardiovasc Dis. 2005;47:396–449. doi: 10.1016/j.pcad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Tsi D. Tan BKH. Cardiovascular Pharmacology of 3-n-butylphthalide in Spontaneously Hypertensive Rats. Phytother Res. 1997;11:576–582. [Google Scholar]
- 23.Wilson CW., III Relative recovery and identification of carbonyl compounds from celery essential oil. J Food Sci. 1970;35:766–768. [Google Scholar]
- 24.Kurobayashi Y. Katsumi Y. Fujita A. Morimitsu Y. Kubota K. Flavor enhancement of chicken broth from boiled celery constituents. J Agric Food Chem. 2008;56:512–516. doi: 10.1021/jf072242p. [DOI] [PubMed] [Google Scholar]
- 25.Tuetun B. Choochote W. Pongpaibul Y, et al. Celery-based topical repellents as a potential natural alternative for personal protection against mosquitoes. Parasitol Res. 2008;104:107–115. doi: 10.1007/s00436-008-1167-1. [DOI] [PubMed] [Google Scholar]
- 26.Woods JA. Jewell C. O'Brien NM. Sedanolide, a natural phthalide from celery seed oil: effect on hydrogen peroxide and tert-butyl hydroperoxide-induced toxicity in HepG2 and CaCo-2 human cell lines. In Vitr Mol Toxicol. 2001;14:233–240. doi: 10.1089/109793301753407984. [DOI] [PubMed] [Google Scholar]
- 27.Ibarrola DA. Hellion-Ibarrola MC. Montalbetti Y, et al. Antihypertensive effect of nuatigenin-3-O-beta-chacotriose from Solanum sisymbriifolium Lam. (Solanaceae) (nuati pyta) in experimentally hypertensive (ARH+DOCA) rats under chronic administration. Phytomedicine. 2011;18:634–640. doi: 10.1016/j.phymed.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Zhou K. Wu B. Zhuang Y, et al. [Chemical constituents of fresh celery] Zhongguo Zhong Yao Za Zhi. 2009;34:1512–1515. [PubMed] [Google Scholar]
- 29.Li L. Zhang B. Tao Y, et al. DL-3-n-butylphthalide protects endothelial cells against oxidative/nitrosative stress, mitochondrial damage and subsequent cell death after oxygen glucose deprivation in vitro. Brain Res. 2009;1290:91–101. doi: 10.1016/j.brainres.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Wu LR. Luo Y. Mechanism of action of butylphalide against the injury following oxygen glucose deprivation/reoxygenation in rat cortical neurons. Yao Xue Xue Bao. 2008;43:366–370. [PubMed] [Google Scholar]
- 31.Powanda MC. Rainsford KD. A toxicological investigation of a celery seed extract having anti-inflammatory activity. Inflammopharmacology. 2010;19:227–233. doi: 10.1007/s10787-010-0049-1. [DOI] [PubMed] [Google Scholar]
- 32.Al-Howiriny T. Alsheikh A. Alqasoumi S, et al. Gastric antiulcer, antisecretory and cytoprotective properties of celery (Apium graveolens) in rats. Pharm Biol. 2010;48:786–793. doi: 10.3109/13880200903280026. [DOI] [PubMed] [Google Scholar]