Abstract
Skin biopsy is a valuable diagnostic tool for small-fiber-predominant neuropathy by the quantification of intra-epidermal nerve fiber density (IENFD). It has the unique advantage of being a minimally invasive procedure with the potential for longitudinal evaluation of both sensory and autonomic fibers. Unmyelinated small fibers are not otherwise quantified objectively with such a level of sensitivity as has been reported with IENFD. Recent advances include an expansion of the skin punch biopsy technique to evaluate larger myelinated fibers and mechanoreceptors, and recent work has also focused on additional methods of quantifying dermal fibers and densely innervated autonomic structures. This review discusses current work using skin biopsy for the pathologic analysis of peripheral nerve fibers in neuropathy of various causes as well as its use in clinical trials.
Keywords: Skin biopsy, Small-fiber sensory neuropathy, Polyneuropathy, Dermal nerve fibers, Epidermal nerve fibers, Intraepidermal nerve fibers, Unmyelinated nerve fibers, Myelinated nerve fibers, Cutaneous disease, Mechanoreceptors, Meissner corpuscles
Introduction
The discovery of protein gene product (PGP) 9.5 as a ubiquitin hydrolase component of axons [1] provided unequivocal evidence of the presence of unmyelinated nerve fibers in the epidermis and its detection has since become a vital tool for qualitative and quantitative studies of cutaneous nerve fiber densities and morphology [2]. Skin punch biopsy is a minimally invasive procedure and is particularly suitable for detecting nociceptive small fibers, a population of fibers largely undetectable though nerve conduction studies and sural nerve biopsies [3].
Skin punches are typically between 2 and 5 mm in diameter and extend to a depth of approximately 4 mm, where larger myelinated fibers and autonomic structures also reside. Glabrous, nonhairy skin contains a dense population of mechanoreceptors and large myelinated afferent fibers that can reveal additional pathologic changes in myelinating Schwann cells and nodal and internodal architecture [4].
Both less invasive and noninvasive methods of sampling and imaging cutaneous structures have also been developed to evaluate small fibers and mechanoreceptors. Small-fiber density in the epidermis may be evaluated by the skin blister technique, in which a negative-pressure vacuum is applied to a region of the skin so that the epidermis may be separated from the dermis for epidermal fiber quantification [5]. Recently, in vivo reflectance confocal microscopy has been developed as a noninvasive technique to evaluate mechanoreceptor Meissner corpuscle density [6, 7].
Since the advent of the panaxonal PGP 9.5 antibody and the pioneering publications reporting the use of skin punches and immunohistochemistry for the analysis of intraepidermal nerve fiber density (IENFD) [8], there has been an expansion of the technique to expound on sensory and autonomic fibers as well as mechanoreceptors and large dermal fibers. This review provides a summary of recent work using glabrous or hairy skin punch biopsy to reveal sensory and autonomic nerve fiber pathologic changes.
Methods of Processing
Tissue processing methods of skin punches depend on subsequent visualization techniques. For immunohistochemistry, fixation is typically done with paraformaldehyde. Immunoperoxidase staining allows bright-field quantification, and immunofluorescent staining can be coupled with either epifluorescence microscopy or confocal microscopy. Species-specific secondary antibodies may visualize multiple antigen-bound primary antibodies selected in a number of combinations to investigate neuronal structures, cutaneous structures, and neurotransmitters relevant to the sensory system as well as the autonomic system (Table 1). In clinical practice, bright-field immunohistochemistry is most commonly used [9]. Although confocal microscopy is more technically difficult and time-consuming, it allows the acquisition of images with greater optical z resolution than is possible through widefield microscopy. Combining a z stack of cutaneous structures taken throughout thick sections into a three-dimensional reconstruction allows the visualization of large structures wholly in focus (Fig. 1).
Table 1.
Antibodies and their targeted structures in skin biopsies
| Antibody | Target | Immunoreactive structures | |
|---|---|---|---|
| Anti-PGP 9.5 | Protein gene product 9.5 | Axons | |
| Anti-MBP | Myelin basic protein | Compact myelin | |
| Anti-Col IV | Collagen type IV | Basal membrane, blood vessels | |
| Anti-Nava | Voltage-gated sodium channels | Node of Ranvier | |
| Anti-Caspr | Contactin-associated protein | Paranodes | |
| Anti-VIP | Vasoactive intestinal peptide | Autonomic cholinergic and adrenergic fibers (i.e., innervating sweat glands, hair follicles, AVAs, Merkel complexes) | |
| Anti-DβH | Dopamine β-hydroxylase |
|
Autonomic noradrenergic fibers (i.e., innervating arrector pili, AVAs) |
| Anti-sub P | Substance P | Peptidergic C fibers associated with Meissner corpuscles, NGF-dependent axons | |
| Anti-CGRP | Calcitonin gene related peptide | ||
| Anti-S100 | S100 protein | Schwann cells, myelinating or nonmyelinating; Meissner corpuscle capsule | |
| Anti-GAP43 | Growth-associated protein 43 | Primarily C fibers that are constantly remodeling | |
| Anti-NF | Neurofilaments | Larger-diameter fibers (e.g., Aδ and Aβ) | |
| Anti-TH | Tyrosine hydroxylase | Sympathetic C fibers innervating blood vessels and pilomotor muscles | |
| Anti-p75 | Low-affinity nerve growth factor receptor | Schwann cells |
AVA arteriovenous anastomoses, NGF nerve growth factor
Pan sodium channel antibody stains all subtypes (e.g., 1.2 and 1.6–1.8 are found in peripheral nerves)
Fig. 1.
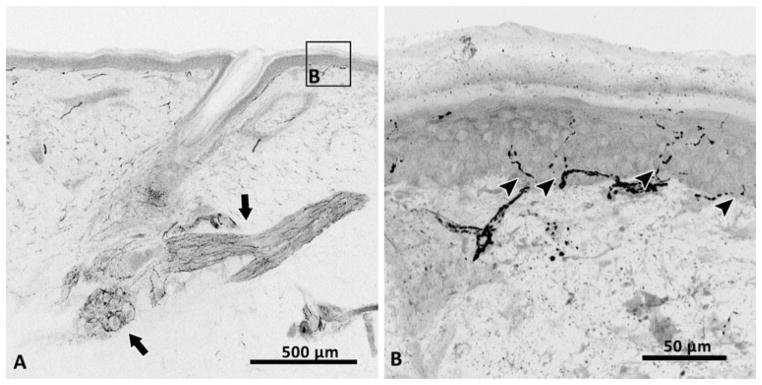
Protein gene product 9.5 staining of axons (black) in a 50-μm-thick section of skin depicting the rich sensory and autonomic innervation of hairy skin (a). Autonomic fibers innervate an arrector pili muscle and a sweat gland (arrows). Sensory fibers are seen at the base of the hair follicle and also branching from the subepidermal neural plexus, crossing the dermal–epidermal basement membrane (arrowheads) to innervate the epidermis (b)
Cutaneous ultrastructure may be visualized by electron microscopy (EM), which requires fixation with glutaraldehyde followed by osmification and Epon embedding. Thin and ultrathin sections may be cut with a microtome and then contrasted by a standard EM protocol [10]. This method makes possible the quantification of myelin thickness (G ratio) and organelle localization. For example, skin biopsy has been used for the localization of mitochondria in axonal swellings of patients with diabetes [11] and also the accumulation of intra-axonal mitochondria in Charcot–Marie–Tooth disease type 1A [10].
Immuno-EM has also been used in skin biopsy studies to quantify protein expression [10]. Protein content may also be quantified by Western blot; however, the relatively small amount of neuronal tissue in the skin requires larger or multiple biopsies to be taken solely for this technique. Immuno-EM has an advantage over Western blotting in that protein expression can be localized to specific cellular structures. In short, conjugation of antibodies to electron-dense gold particles may be used to calculate the percent expression according to the number of conjugated particles divided by the total area examined. For example, peripheral myelin protein 22 expression was calculated as a percent composition of the compact myelin of a dermal nerve [10]. Various techniques and some of their applications to skin biopsy are summarized in Table 2.
Table 2.
Techniques applied to skin biopsies with their relevant applications
| Technique | Applications | References |
|---|---|---|
| Immunohistochemistry | IENF density | Kennedy et al. [85] |
| Pilomotor innervation | Nolano et al. [20•] | |
| Sudomotor innervation | Gibbons et al. [18] | |
| Internodal length | Li et al. [86] | |
| Nodal width | Nolano et al. [23], Provitera et al. [87] | |
| Myelin G ratio | Nolano et al. [23] | |
| Axon diameter | Nolano et al. [23], Provitera et al. [87] | |
| Immuno-EM | Percent protein expression | Katona et al. [88] |
| EM | Myelin periodicity | Li et al. [86] |
| Myelin decompaction | Ceuterick-deGroote et al. [56] | |
| Localization of mitochondria | Saporta et al. [10] | |
| Tracking of IENF regeneration | Polydefkis et al. [71] | |
| Organelle accumulation in axonal swellings | Ebenezer et al. [11] | |
| PCR | mRNA expression | Li et al. [86] |
EM electron microscopy, IENF intraepidermal nerve fiber, mRNA messenger RNA
Quantification
Standard procedures of intraepidermal nerve fiber counting have been thoroughly reviewed [12]. In short, previous methods were adapted to include only fibers that intersect the dermal–epidermal basement membrane, excluding more superficial fragments. This method has been well documented to have high interobserver reliability and reproducibility between laboratories [13]. Although the skin blister method is less invasive than punch biopsies, it has been less widely used because it is a longer procedure, requiring 30–90 min for blister formation. Even so, good correlations have been reported between both sampling methods in regard to epidermal fiber density [14].
The subepidermal neural plexus runs parallel to the skin’s surface and is a dense nerve bundle from which individual cutaneous nerves branch; it, like many densely innervated structures, has proven difficult to quantify, although marked disease-related reductions have been noted [15, 16]. For evaluation of innervation of the subepidermal neural plexus, semi-quantitative methods may be used that are based on assigning numerical categories corresponding to degrees of innervation ranging, for example, from hyperinnervation (+1) to none whatsoever (−4) [17]. Recently, a novel quantification method of “manual morphometry” as well as unbiased stereologic techniques were applied to quantification of the similarly dense (autonomic) innervation of sweat glands, suggesting an alternative to quantifying such densely innervated dermal structures [18]. Furthermore, in a comparative study, semiquantitative methods had poor reliability and low correlation to neuropathy scores, whereas both automated application and manual application of stereology-based techniques were reliable and correlated well with IENFD and examination findings [19•].
Other recent quantification methods include the analysis of a single frame of a confocal z stack, thereby selecting a manageable sample of autonomic fibers of the arrector pili muscle [20•]. Quantification of fluorescence intensity can also be used for such densely innervated structures [21], although this method is generally accepted to be at high risk of bias owing to differing experimental conditions even when experiments are carefully run in parallel.
Hair follicles serve as the primary mode of mechanosensation in hairy skin and, like other mechanoreceptive structures, are innervated by large myelinated Aβ fibers. Glabrous skin contains a dense population of Meissner corpuscles that detect touch and low-frequency vibration [22]. The myelinated fibers supplying Meissner corpuscles in glabrous skin have been quantified as the density of fibers per square millimeter of skin analyzed and were termed intrapapillary myelinated endings [23]. The density of Meissner corpuscles has also been suggested as a potential biomarker of neuropathy [24]. Marked reductions have been reported in several disorders, including HIV [6], spinobulbar muscular atrophy [15], Friedreich’s ataxia [16], experimental diabetes [25], systemic sclerosis [26], Charcot–Marie–Tooth disease [7, 10], chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), and Guillain–Barré syndrome (GBS) [27••].
Utility of Skin Biopsy in Detecting Neuropathy
Skin biopsy has become a common method to evaluate patients presenting with small-fiber-predominant polyneuropathy. It first became widely used in HIV-associated polyneuropathy, which frequently does not exhibit nerve conduction study abnormalities until later in the course of the disease [28]. IENFD was used successfully as an end-point measure in several prospective cohorts and clinical trials evaluating HIV neuropathy [29–31]. McArthur et al. pioneered both normative data references [32] and reliability studies [13]. A worldwide normative study was also recently published [33].
Diabetes is the leading cause of peripheral neuropathy and typically presents as distal, symmetric small-fiber-predominant neuropathy [34]. Early work using skin biopsy established the length-dependent nature of diabetic poly-neuropathy by biopsying distal and proximal sites in parallel [32]. The axonal regeneration marker growth-associated protein 43 has also been suggested as a biomarker of the deficiency of regeneration found in the peripheral nerves of individuals with type II diabetes [35].
There is a known involvement of large fibers in diabetic neuropathy as well, although work by Singleton and Smith [36] has suggested that C-fiber involvement may occur earlier than myelinated fiber involvement on the basis of several studies of asymptomatic diabetic patients and patients with impaired glucose tolerance and neuropathy. It is clear that histological studies uniformly show greater neuropathic changes than electrophysiologic measures, as nerve biopsy also has greater sensitivity than nerve conduction studies for detecting peripheral nerve abnormalities in diabetes [37]. This may be true for medication-induced neuropathy as well. The findings of nerve conduction studies were shown to change minimally even over relatively long follow-up times (more than 5 years) [38, 39]. Owing to the risk of morbidity, sural nerve biopsies are rarely done diagnostically, which has limited pathologic assessment of myelinated fibers. Nerve fiber density was more sensitive than nerve conduction in detecting neuropathy from nitrofurantoin, flecainide, and oxaliplatin [40–42] and is being used as an end point for several studies of chemotherapy-induced neuropathy [43, 44]. Glabrous skin, in particular, has a high density of dermal myelinated fibers, and could be developed as an alternative for pathologic evaluation of large myelinated fibers in diabetic patients [45••, 46, 47•].
Despite a practice parameter published in 2009 by the American Academy of Neurology citing only a level C recommendation [48], skin biopsy is widely used to evaluate patients for both small-fiber-predominant polyneuropathy and distal symmetric neuropathy in which the findings of nerve conduction studies are normal. The decreased sensitivities cited by England et al. [48] may be partially due to using only one site for IENFD [49], or the inclusion of heterogeneous groups of patients. In addition, previous studies have not included patients with neuropathic pain due to other conditions as controls [48].
The advantages of skin biopsy include its wide availability (multiple laboratories offer IENFD evaluation), potential for longitudinal analysis of nerve fibers, and the objective nature of quantifying sensory fibers. Quantitative sensory testing has been demonstrated to be highly subjective [50] and also much less sensitive than IENFD quantification, with most measures detecting only relatively large changes in fiber densities (more than 12 fibers per millimeter) [51]. In another recent study [52], significant reductions in the numbers of PGP 9.5-immunoreactive fibers as well as TRPV1-immunoreacive fibers were reported in diabetic patients after a relatively short 6-month skin biopsy follow-up, providing further support for the sensitivity of IENFD in tracking neuropathy.
Until recently, skin biopsies have not been performed in disorders affecting mainly myelinated nerves. Recent efforts have been made to quantify myelinated fibers and identify segmental demyelination [10], inflammation [53], and anti-myelin-associated glycoprotein [54]. GBS has traditionally been viewed as a largely myelinated fiber disorder, yet dysautonomia has long been recognized as a common feature. Skin biopsy in GBS patients revealed decreased IENFD and that sudomotor density was decreased in five of 17 patients [55]. Hypomyelination and onion bulb formation were identified in skin biopsies of Charcot–Marie–Tooth neuropathy patients [10, 56]. Also, hallmark segmental demyelination was identified in patients with CIDP compared with controls [10]. Real-time PCR can also be used to demonstrate upregulation of inflammatory markers in CIDP [53]. Perivascular infiltration in vasculitic neuropathy has also been appreciated in skin biopsies [57].
Use of Skin Biopsy in Detecting Voltage-Gated Channels
Skin biopsy has recently been used to detect changes in voltage-gated sodium channel expression in patients with small-fiber neuropathy. There are nine different subtypes of sodium channels, several of which (1.2, 1.6, 1.7, 1.8) are found in peripheral nerves [58]. Mutations in subtype 1.7 have been associated with erythromelalgia and insensitivity to pain [59, 60]. SCN9A codes for Nav1.7 and has conclusively been shown to have a crucial role in pain [61]. Patients screened for small-fiber neuropathy by skin biopsy and quantitative sensory testing underwent genetic testing confirming SCN9A mutations [62, 63]. Cell culture studies (from dorsal root ganglion cells with identical Nav1.7 mutations as in patients) demonstrate increased excitability with current clamp models [64]. Several patients also had autonomic symptoms [62, 63]. Nav1.8 upregulation has been observed in animal models of neuropathic pain [65]. Furthermore, dysregulation of Nav1.6 and Nav1.8 has been observed in diabetic rat models [66]. In short, known disruptions of voltage-gated channels have been reported in humans and in animal models of painful neuropathy. Skin biopsy could play an important role in further investigations.
Applications of Skin biopsy in Clinical Research
There are several advantages to studying cutaneous nerves. First, skin biopsies are repeatable, unlike nerve biopsies, which allow the investigator multiple time points over the course of a study to quantify innervation. Skin biopsy with nerve fiber density measurements has been used as a secondary endpoint for several studies, including lifestyle intervention in prediabetic neuropathy [67], HIV peripheral neuropathy [68, 69], and Fabry disease [70]. Secondly, axonal regeneration is also possible to observe within reasonable time periods (months) in human participants [71]; which may represent a more appropriate clinical endpoint than current density measures or neurophysiologic measures. The two axotomy models used currently have been chemical denervation using capsaicin [71] and excision axotomy in which a small punch is performed first followed by a larger, encompassing punch taken at a later time point [72•]. Chemical axotomy using capsaicin can also be used to evaluate regeneration of autonomic fibers [73••].
Applications of Skin Biopsy in Autonomic Disorders
The use of skin biopsy has been expanded to multiple autonomic disorders as the quantification of autonomic fibers has become more specific; however, it may still be underused in these disorders. The commonest autonomic disorder in which skin biopsy has been used to quantify autonomic innervation is diabetic neuropathy. Correlation of the loss of sudomotor fibers with hyperglycemia has been reported [74]. Initial quantification of both pilomotor and sudomotor denervation was performed in diabetic patients [18, 20•]. It may also be helpful in chemotherapy-induced autonomic neuropathy. A case report of toxic neuropathy with bortezomib therapy in three patients showed not only loss of IENFD but also decreased numbers of adrenergic fibers and sudomotor fibers [75].
Parkinson’s disease (PD) is a common cause of autonomic failure, which paradoxically affects peripheral autonomic fibers in addition to possible central fibers in the locus ceruleus and nucleus of the vagus [76]. Decreased sudomotor and pilomotor muscle innervation has been documented in patients with less than 15 years of PD [77] and may precede the development of autonomic symptoms. Increased level of α-synuclein has been identified in chest skin but not distal skin from postmortem samples of PD patients compared with control patients [78]. IENFD (somatic C fibers) is reduced in PD patients compared with age-matched controls, suggesting additional sensory involvement as well [79].
Multiple system atrophy (MSA), a severe neurodegenerative disease presenting with autonomic failure coupled with parkinsonism or cerebellar degeneration, has a mean life expectancy of 5–9 years [80]. MSA has been shown to have relatively normal skin autonomic innervation compared with pure autonomic failure [81], another cause of severe autonomic dysfunction. Pure autonomic failure is an idiopathic disorder with restricted accumulation of α-synuclein in peripheral autonomic neurons not associated with other movement disorders [82]. It is tantalizing to suggest that skin biopsy may be a possible tool to differentiate pure autonomic failure and PD with autonomic symptoms from MSA, which has a mostly central or preganglionic autonomic involvement.
Postural tachycardia syndrome is a syndrome most likely including a heterogeneous group of disorders with a common occurrence of significant orthostatic tachycardia without concurrent drop in blood pressure. Skin biopsy in patients with postural tachycardia syndrome showed normal skin norepinephrine concentration but with some morphologic abnormalities in the somatic C fibers in three of the eight patients studied [83]. No further studies have been performed in this population.
Limitations of the Procedure
Several limitations of skin biopsy have been secondary to multiple factors. One is the heterogeneous nature of peripheral neuropathy—it is possible that some disorders have greater C-fiber degeneration than others, which may bias findings. Another limitation has been the lack of uniform correlation of skin biopsy results with other neuropathy end points [43, 49, 84]. The greatest limitation has been the lack of specific identifying characteristics for the cause of neuropathy. Histological evaluation has not provided a way to differentiate between the causes of disease. This may be due to a “final common pathway” shared by all neuropathies, or may be due to a lack of specific neuronal markers. Except in clinical trial settings, the findings of skin biopsy rarely change clinical management, especially in disorders such as diabetes where the cause of neuropathy is known, even if the pathogenesis is not.
The skin has a rich innervation of sensory and autonomic fibers, but the relatively high ratio of connective tissue to neural tissue limits some methods of analysis. For example, protein quantification by Western blot requires a larger skin biopsy that what is typically sampled for immunohistochemistry alone. Immuno-EM is an alternative method; however, there is a high degree of uncertainty in ultrathin sectioning as to whether neural tissue is being targeted. Also, large samples of nerve likely have better sensitivity for conditions also affecting vessels and connective tissue such as amyloidosis, vasculitis, leprosy, etc.
Conclusions
In summary, skin biopsy has a unique role in identifying sensory and autonomic neuropathy given its greater sensitivity for identifying pathologic changes in unmyelinated fibers. Further use of skin biopsy in clinical trial end points is expected. The advantage of using skin biopsy continues to be its ease of sampling, repeatability, and possibilities for investigating nerve regeneration. Advances in specific biomarkers would be ideal for discriminating between various causes of peripheral and autonomic neuropathies. Evaluation of autonomic fiber and large-fiber innervation in addition to small sensory fibers will provide more comprehensive descriptions of neuropathic changes and potentially broaden the use skin biopsy as a biomarker of peripheral neuropathy.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wang L, Hilliges M, Jernberg T, et al. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res. 1990;261:25–33. doi: 10.1007/BF00329435. [DOI] [PubMed] [Google Scholar]
- 2.Lauria G. Innervation of the human epidermis. A historical review. Ital J Neurol Sci. 1999;20:63–70. doi: 10.1007/s100720050013. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann DN, Griffin JW, Hauer P, et al. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–40. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 4.Myers MI, Peltier AC, Li J. Evaluating dermal myelinated nerve fibers in skin biopsy. Muscle Nerve. 2012 doi: 10.1002/mus.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy WR, Nolano M, Wendelschafer-Crabb G, et al. A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve. 1999;22:360–71. doi: 10.1002/(sici)1097-4598(199903)22:3<360::aid-mus9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann DN, Boger JN, Jansen C, Alessi-Fox C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2007;69:2121–7. doi: 10.1212/01.wnl.0000282762.34274.94. [DOI] [PubMed] [Google Scholar]
- 7.Almodovar JL, Ferguson M, McDermott MP, et al. In vivo confocal microscopy of Meissner corpuscles as a novel sensory measure in CMT1A. J Peripher Nerv Syst. 2011;16:169–74. doi: 10.1111/j.1529-8027.2011.00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy WR, Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci. 1993;115:184–90. doi: 10.1016/0022-510x(93)90223-l. [DOI] [PubMed] [Google Scholar]
- 9.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903–9. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 10.Saporta MA, Katona I, Lewis RA, et al. Shortened internodal length of dermal myelinated nerve fibres in Charcot-Marie-Tooth disease type 1A. Brain. 2009;132:3263–73. doi: 10.1093/brain/awp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebenezer GJ, McArthur JC, Thomas D, et al. Denervation of skin in neuropathies: the sequence of axonal and Schwann cell changes in skin biopsies. Brain. 2007;130:2703–14. doi: 10.1093/brain/awm199. [DOI] [PubMed] [Google Scholar]
- 12.Sommer C, Lauria G. Skin biopsy in the management of peripheral neuropathy. Lancet Neurol. 2007;6:632–42. doi: 10.1016/S1474-4422(07)70172-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith AG, Howard JR, Kroll R, et al. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228:65–9. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Panoutsopoulou IG, Wendelschafer-Crabb G, Hodges JS, Kennedy WR. Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology. 2009;72:1205–10. doi: 10.1212/01.wnl.0000340984.74563.1c. [DOI] [PubMed] [Google Scholar]
- 15.Manganelli F, Iodice V, Provitera V, et al. Small-fiber involvement in spinobulbar muscular atrophy (Kennedy’s disease) Muscle Nerve. 2007;36:816–20. doi: 10.1002/mus.20872. [DOI] [PubMed] [Google Scholar]
- 16.Nolano M, Provitera V, Crisci C, et al. Small fibers involvement in Friedreich’s ataxia. Ann Neurol. 2001;50:17–25. doi: 10.1002/ana.1283. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur JC. Pathology and quantitation of cutaneous innervation. In: Dyck PJ, Thomas PK, editors. Peripheral meuropathy. Philadelphia: Elsevier; 2005. pp. 869–95. [Google Scholar]
- 18.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology. 2009;72:1479–86. doi: 10.1212/WNL.0b013e3181a2e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sudomotor innervation: a comparison of three methods. Muscle Nerve. 2010;42:112–19. doi: 10.1002/mus.21626. This study compared unbiased stereologic techniques, a recently published novel sudomotor quantification method, and semiquantitative methods of ranking the degree of sweat gland innervation. The authors reported a fast and reliable automated method of quantification and caution against semi-quantitative methods shown to have low reliability and little clinical correlation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Nolano M, Provitera V, Caporaso G, et al. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology. 2010;75:1089–97. doi: 10.1212/WNL.0b013e3181f39cf4. Nolano et al. recently proposed a new method of quantification of pilomotor nerves by counting fibers from a single frame of a confocal z stack. They reported high interobserver and intraobserver reliability with the technique. They described a reduction in the autonomic innervation of the pilomotor nerves of diabetic patients, with the severest depletion of noradrenergic dopamine β-hydroxylase immunoreactive fibers. [DOI] [PubMed] [Google Scholar]
- 21.Casanova-Molla J, Morales M, Sola-Valls N, et al. Axonal fluorescence quantitation provides a new approach to assess cutaneous innervation. J Neurosci Methods. 2011;200:190–8. doi: 10.1016/j.jneumeth.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Vega JA, Garcia-Suarez O, Montano JA, et al. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc Res Tech. 2009;72:299–309. doi: 10.1002/jemt.20651. [DOI] [PubMed] [Google Scholar]
- 23.Nolano M, Provitera V, Crisci C, et al. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol. 2003;54:197–205. doi: 10.1002/ana.10615. [DOI] [PubMed] [Google Scholar]
- 24.Dyck PJ. Enumerating Meissner corpuscles: future gold standard of large fiber sensorimotor polyneuropathy? Neurology. 2007;69:2116–8. doi: 10.1212/01.wnl.0000286934.55620.96. [DOI] [PubMed] [Google Scholar]
- 25.Pare M, Albrecht PJ, Noto CJ, et al. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007;501:543–67. doi: 10.1002/cne.21262. [DOI] [PubMed] [Google Scholar]
- 26.Provitera V, Nolano M, Pappone N, di GC, et al. Distal degeneration of sensory and autonomic cutaneous nerve fibres in systemic sclerosis. Ann Rheum Dis. 2005;64:1524–6. doi: 10.1136/ard.2005.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Hutton EJ, Carty L, Laura M, et al. c-Jun expression in human neuropathies: a pilot study. J Peripher Nerv Syst. 2011;16:295–303. doi: 10.1111/j.1529-8027.2011.00360.x. In an interesting recent study, glabrous skin biopsies and sural nerve biopsies were examined for an upregulation of c-Jun expression as a marker of Schwann cell plasticity. A clear sign of nuclear Schwann cell expression of c-Jun was seen in glabrous skin biopsies from patients with Charcot–Marie–Tooth disease type 1A, GBS, and CIDP, suggesting c-Jun as a marker of dedifferentiation and demyelination. [DOI] [PubMed] [Google Scholar]
- 28.Verma A. Epidemiology and clinical features of HIV-1 associated neuropathies. J Peripher Nerv Syst. 2001;6:8–13. doi: 10.1046/j.1529-8027.2001.006001008.x. [DOI] [PubMed] [Google Scholar]
- 29.Polydefkis M, Yiannoutsos CT, Cohen BA, et al. Reduced intra-epidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–9. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Simpson DM, Katzenstein D, Haidich B, et al. Plasma carnitine in HIV-associated neuropathy. AIDS. 2001;15:2207–8. doi: 10.1097/00002030-200111090-00025. [DOI] [PubMed] [Google Scholar]
- 31.Simpson DM, Kitch D, Evans SR, et al. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–87. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 32.McArthur JC, Stocks EA, Hauer P, et al. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–20. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 33.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15:202–7. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 34.Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–34. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bursova S, Dubovy P, Vlckova-Moravcova E, et al. Expression of growth-associated protein 43 in the skin nerve fibers of patients with type 2 diabetes mellitus. J Neurol Sci. 2012;315:60–3. doi: 10.1016/j.jns.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 36.Singleton JR, Smith AG. Therapy insight: neurological complications of prediabetes. Nat Clin Pract Neurol. 2006;2:276–82. doi: 10.1038/ncpneuro0172. [DOI] [PubMed] [Google Scholar]
- 37.Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48:578–85. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- 38.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 39.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 40.Burakgazi AZ, Messersmith W, Vaidya D, et al. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. 2011;77:980–6. doi: 10.1212/WNL.0b013e31822cfc59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burakgazi AZ, Polydefkis M, Hoke A. Skin biopsy-proven flecainide-induced neuropathy. Muscle Nerve. 2012;45:144–6. doi: 10.1002/mus.22239. [DOI] [PubMed] [Google Scholar]
- 42.Tan IL, Polydefkis MJ, Ebenezer GJ, et al. Peripheral nerve toxic effects of nitrofurantoin. Arch Neurol. 2012;69:265–8. doi: 10.1001/archneurol.2011.1120. [DOI] [PubMed] [Google Scholar]
- 43.Koskinen MJ, Kautio AL, Haanpaa ML, et al. Intraepidermal nerve fibre density in cancer patients receiving adjuvant chemotherapy. Anticancer Res. 2011;31:4413–6. [PubMed] [Google Scholar]
- 44.Erasmus Medical Center. [Accessed Sep 2012];Skin biopsies in chemotherapy-induced neuropathy. 2011 http://clinicaltrials.gov/ct2/show/NCT00956033?term=skin+biopsy&rank=12.
- 45••.Doppler K, Werner C, Henneges C, Sommer C. Analysis of myelinated fibers in human skin biopsies of patients with neuropathies. J Neurol. 2012;259:1879–87. doi: 10.1007/s00415-012-6432-7. This study evaluated both unmyelinated epidermal fibers and myelinated dermal fibers in proximal thigh biopsies from a large cohort of patients with neuropathy of various causes, revealing abnormalities in both fiber populations. This study demonstrates the valuable potential to investigate both large-fiber and small-fiber innervation in skin biopsy. [DOI] [PubMed] [Google Scholar]
- 46.Myers MI, Li J, Artibee K, Peltier AC. Evaluation of dermal myelinated fibers in diabetic polyneuropathy. Paper presented at the American Academy of Neurology annual meeting; New Orleans. 21–28 April, 2012. [Google Scholar]
- 47•.Lauria G, Cazzato D, Porretta-Serapiglia C, et al. Morphometry of dermal nerve fibers in human skin. Neurology. 2011;77:242–9. doi: 10.1212/WNL.0b013e318225ab51. The dermis is densely innervated by nerves typically arranged in large bundles that have proven difficult to quantify. Lauria et al. proposed a new method of quantifying dermal nerve fiber density by delineating a manageable portion of the dermis (200 μm below the basement membrane) and measuring the length of dermal nerves. Reported values demonstrated reliability and reproducibility, indicating that the method could potentially be used in clinical practice. [DOI] [PubMed] [Google Scholar]
- 48.England JD, Gronseth GS, Franklin G, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review) Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation PM R. 2009;1:5–13. doi: 10.1016/j.pmrj.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999;53:1641–7. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 50.Freeman R, Chase KP, Risk MR. Quantitative sensory testing cannot differentiate simulated sensory loss from sensory neuropathy. Neurology. 2003;60:465–70. doi: 10.1212/wnl.60.3.465. [DOI] [PubMed] [Google Scholar]
- 51.Selim MM, Wendelschafer-Crabb G, Hodges JS, et al. Variation in quantitative sensory testing and epidermal nerve fiber density in repeated measurements. Pain. 2010;151:575–81. doi: 10.1016/j.pain.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 52.Narayanaswamy H, Facer P, Misra VP, et al. A longitudinal study of sensory biomarkers of progression in patients with diabetic peripheral neuropathy using skin biopsies. J Clin Neurosci. 2012;19:1490–6. doi: 10.1016/j.jocn.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 53.Lee G, Xiang Z, Brannagan TH, III, et al. Differential gene expression in chronic inflammatory demyelinating polyneuropathy (CIDP) skin biopsies. J Neurol Sci. 2010;290:115–22. doi: 10.1016/j.jns.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Lombardi R, Erne B, Lauria G, et al. IgM deposits on skin nerves in anti-myelin-associated glycoprotein neuropathy. Ann Neurol. 2005;57:180–7. doi: 10.1002/ana.20364. [DOI] [PubMed] [Google Scholar]
- 55.Pan CL, Tseng TJ, Lin YH, et al. Cutaneous innervation in Guillain-Barré syndrome: pathology and clinical correlations. Brain. 2003;126:386–97. doi: 10.1093/brain/awg039. [DOI] [PubMed] [Google Scholar]
- 56.Ceuterick-deGroote GC, De JP, Timmerman V, et al. Infantile demyelinating neuropathy associated with a de novo point mutation on Ser72 in PMP22 and basal lamina onion bulbs in skin biopsy. Pathol Res Pract. 2001;197:193–8. doi: 10.1078/0344-0338-00033. [DOI] [PubMed] [Google Scholar]
- 57.Lee JE, Shun CT, Hsieh SC, Hsieh ST. Skin denervation in vasculitic neuropathy. Arch Neurol. 2005;62:1570–3. doi: 10.1001/archneur.62.10.1570. [DOI] [PubMed] [Google Scholar]
- 58.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–47. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- 59.Dib-Hajj SD, Rush AM, Cummins TR, et al. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain. 2005;128:1847–54. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- 60.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Nav1.7 and human pain disorders. Trends Neurosci. 2007;30:555–63. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Cox JJ, Reimann F, Nicholas AK, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–8. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 63.Han C, Hoeijmakers JG, Liu S, et al. Functional profiles of SCN9A variants in dorsal root ganglion neurons and superior cervical ganglion neurons correlate with autonomic symptoms in small fibre neuropathy. Brain. 2012;135:2613–28. doi: 10.1093/brain/aws187. [DOI] [PubMed] [Google Scholar]
- 64.Han C, Hoeijmakers JG, Ahn HS, et al. Nav1.7-related small fiber neuropathy: impaired slow-inactivation and DRG neuron hyperexcitability. Neurology. 2012;78:1635–43. doi: 10.1212/WNL.0b013e3182574f12. [DOI] [PubMed] [Google Scholar]
- 65.Thakor DK, Lin A, Matsuka Y, et al. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5:14. doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Craner MJ, Klein JP, Renganathan M, et al. Changes of sodium channel expression in experimental painful diabetic neuropathy. Ann Neurol. 2002;52:786–92. doi: 10.1002/ana.10364. [DOI] [PubMed] [Google Scholar]
- 67.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–9. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 68.Shikuma C, Gerschenson M, Ananworanich J, et al. Determinants of epidermal nerve fibre density in antiretroviral-naive HIV-infected individuals. HIV Med. 2012;13:602–8. doi: 10.1111/j.1468-1293.2012.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valcour V, Yeh TM, Bartt R, et al. Acetyl-l-carnitine and nucleoside reverse transcriptase inhibitor-associated neuropathy in HIV infection. HIV Med. 2009;10:103–10. doi: 10.1111/j.1468-1293.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiffmann R, Hauer P, Freeman B, et al. Enzyme replacement therapy and intraepidermal innervation density in Fabry disease. Muscle Nerve. 2006;34:53–6. doi: 10.1002/mus.20550. [DOI] [PubMed] [Google Scholar]
- 71.Polydefkis M, Hauer P, Sheth S, et al. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–15. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 72•.Ebenezer GJ, O’Donnell R, Hauer P, et al. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain. 2011;134:1853–63. doi: 10.1093/brain/awr086. Mechanical axotomy and capsaicin were both used in this study to extensively characterize neurovascular repair. The results showed regenerative deficits in patients with diabetes (e.g., slowed Schwann cell migration and axonal regrowth). Blood vessel growth preceded other measures, suggesting a supportive role of blood vessels in axonal repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73••.Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann Neurol. 2010;68:888–98. doi: 10.1002/ana.22126. This study reported both sensory and autonomic fiber degeneration following topical application of capsaicin. Sensory fibers were found to degenerate more quickly and regeneration proceeded much more slowly than for autonomic sudomotor, vasomotor, and pilomotor fibers. This is the first study focusing on autonomic fibers following denervation by capsaicin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo KR, Chao CC, Hsieh PC, et al. Effect of glycemic control on sudomotor denervation in type 2 diabetes. Diabetes Care. 2012;35:612–6. doi: 10.2337/dc11-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giannoccaro MP, Donadio V, Gomis PC, et al. Somatic and autonomic small fiber neuropathy induced by bortezomib therapy: an immunofluorescence study. Neurol Sci. 2011;32:361–3. doi: 10.1007/s10072-010-0475-2. [DOI] [PubMed] [Google Scholar]
- 76.Wakabayashi K, Takahashi H, Ohama E, et al. Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol. 1993;60:609–12. [PubMed] [Google Scholar]
- 77.Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm. 2006;113:1169–76. doi: 10.1007/s00702-005-0431-0. [DOI] [PubMed] [Google Scholar]
- 78.Miki Y, Tomiyama M, Ueno T, et al. Clinical availability of skin biopsy in the diagnosis of Parkinson’s disease. Neurosci Lett. 2010;469:357–9. doi: 10.1016/j.neulet.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 79.Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson’s disease: evidence of a cutaneous denervation. Brain. 2008;131:1903–11. doi: 10.1093/brain/awn102. [DOI] [PubMed] [Google Scholar]
- 80.Robertson D, Gilman S. In: Multiple system atrophy. Robertson D, Biaggioni I, Burnstock G, Low PA, Paton J, editors. New York: Elsevier; 2012. pp. 453–7. [Google Scholar]
- 81.Donadio V, Cortelli P, Elam M, et al. Autonomic innervation in multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2010;81:1327–35. doi: 10.1136/jnnp.2009.198135. [DOI] [PubMed] [Google Scholar]
- 82.Kaufmann H, Goldstein DS. Pure autonomic failure: a restricted Lewy body synucleinopathy or early Parkinson disease? Neurology. 2010;74:536–7. doi: 10.1212/WNL.0b013e3181d26982. [DOI] [PubMed] [Google Scholar]
- 83.Singer W, Spies JM, McArthur J, et al. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. 2004;62:612–8. doi: 10.1212/01.wnl.0000110313.39239.82. [DOI] [PubMed] [Google Scholar]
- 84.Loseth S, Lindal S, Stalberg E, Mellgren SI. Intraepidermal nerve fibre density, quantitative sensory testing and nerve conduction studies in a patient material with symptoms and signs of sensory polyneuropathy. Eur J Neurol. 2006;13:105–11. doi: 10.1111/j.1468-1331.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 85.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–8. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 86.Li J, Bai Y, Ghandour K, et al. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain. 2005;128:1168–77. doi: 10.1093/brain/awh483. [DOI] [PubMed] [Google Scholar]
- 87.Provitera V, Nolano M, Pagano A, et al. Myelinated nerve endings in human skin. Muscle Nerve. 2007;35:767–75. doi: 10.1002/mus.20771. [DOI] [PubMed] [Google Scholar]
- 88.Katona I, Wu X, Feely SM, et al. PMP22 expression in dermal nerve myelin from patients with CMT1A. Brain. 2009;132:1734–40. doi: 10.1093/brain/awp113. [DOI] [PMC free article] [PubMed] [Google Scholar]


