Abstract
The horn-eyed ghost crab Ocypode ceratophthalma is a terrestrial brachyuran native to the Indo-Pacific region, including the islands of Hawaii. Here, multiple mass spectrometric platforms, including matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry (MALDI-TOF/TOF MS/MS) and nanoflow liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (nanoLC-ESI-Q-TOF MS/MS), were used to characterize the neuropeptidome of this species. In total, 156 peptide paracrines/hormones, representing 15 peptide families, were identified from the O. ceratophthalma supraesophageal ganglion (brain), eyestalk ganglia, pericardial organ and/or sinus gland, including 59 neuropeptides de novo sequenced here for the first time. Among the de novo sequenced peptides were isoforms of A-type allatostatin, B-type allatostatin, FMRFamide-like peptide (FLP), orcokinin, orcomyotropin and RYamide. Of particular note, were several novel FLPs including DVRAPALRLRFamide, an isoform of short neuropeptide F, and NRSNLRFamide, the orcokinins NFDEIDRSGYGFV and DFDEIDRSSFGFH, which exhibit novel Y for F and D for N substitutions at positions 10 and 1, respectively, and FDAYTTGFGHS, a member of the orcomyotropin family exhibiting a novel Y for F substitution at position 4. Taken collectively, the set of peptides described here represents the largest number of neuropeptides thus far characterized via mass spectrometry from any single crustacean, and provides a framework for future investigations of the physiological roles played by these molecules in this species.
Keywords: MALDI-TOF/TOF tandem mass spectrometry, nanoLC-ESI-Q-TOF tandem mass spectrometry, neuropeptide, neurohormone, Crustacea, de novo sequencing
1. Introduction
A large number of molecules are used by nervous systems for chemical communication. These substances can be divided into several broad classes, the largest and most diverse of which is the peptides. While many organisms have contributed to our knowledge of peptidergic signaling, work done on crustaceans has provided many important insights for over half a century [6,8,9]. For example, peptidergic neurosecretion was first formally demonstrated using a crustacean model [1,45], and the first invertebrate neuropeptide to be isolated and fully characterized was also from a member of this arthropod subphylum [17]. More recently, the crustacean stomatogastric and cardiac neuromuscular systems have been invaluable contributors to our understanding of the generation, maintenance, and modulation of rhythmically active behaviors. Specifically, these systems have been instrumental in discerning how locally-released and circulating neuropeptides allow for the production of an essentially infinite number of distinct behavioral outputs from a single, numerically simple, “hard-wired”, neural network [6,9,11,16,21,25,41,42,43,44,48,50,53]. In addition to their use as biomedical models, the diversity of species which comprise the Crustacea makes this group of animals a convenient model in which to assess the structural and functional evolution of peptides/peptide families [9]; there are approximately 67,000 extant species in this arthropod subphylum and its members inhabit many highly diverse aquatic and terrestrial environments [2].
Prior to the early-2000’s, the common strategy for crustacean peptide identification was to isolate a single neuropeptide biochemically/chromatographically from a large pool of starting tissue, and then to determine its structure using a combination of proteolytic cleavage and Edman analysis [6,9]. Recently, there has been a shift in the focus of crustacean neuropeptide discovery from the identification and structural determination of single peptides to the characterization of entire peptidomes, the full complement of neuropeptides used by an animal [6,9]. Mass spectrometry, with its highly sensitive, accurate mass matching and de novo sequencing capabilities, has been a major contributor to this shift in focus for the field [6,9,34].
At present, members of the Decapoda are by far the most thoroughly investigated crustaceans in terms of their neuropeptides [6,9]. This said, only a handful of species have been the subjects of large-scale mass spectrometric analyses, i.e. the penaeid shrimp Litopenaeus vannamei [38], the astacidean lobster Homarus americanus [4,5,37], and the brachyuran crabs Cancer borealis [29,33,40], Cancer productus [18,19], Carcinus maenas [36] and Callinectes sapidus [26,27,28], all of which are marine. The horn-eyed ghost crab, Ocypode ceratophthalma, is distinct from the other decapods whose peptidomes have been deduced in that it is primarily a terrestrial species, inhabiting the supralittoral zone of sandy beaches throughout the Indo-Pacific region [22]. Here, we present a multi-platform mass spectral characterization of the neuropeptidome of O. ceratophthalma (derived from selected portions of the central nervous system [supraesophageal and eyestalk ganglia], as well as its primary neuroendocrine organs [sinus gland and pericardial organ]), comparing it to those of the other decapods thus far surveyed.
2. Materials and methods
2.1. Animals
Horn-eyed ghost crabs, O. ceratophthalma, were collected by hand from the supralittoral zones of Malaekahana Beach (Laie, Oahu, HI) and the beach at Maunalua Bay adjacent to Paiko Lagoon/Kuli’ou’ou Beach Park (Honolulu, Oahu, HI); all animals were maintained in moist sand oC) until used for tissue collection (generally less than 24 hours after at room temperature (~22 collection).
2.2. Tissue collection
Prior to dissection, crabs were anesthetized by packing in ice for approximately 15 minutes, after which the eyestalk ganglia (including the sinus gland [SG]), supraesophageal ganglia (brain), pericardial organs (POs) and/or individual SGs were isolated by manual microdissection and immediately placed in ice-cold acidified methanol, i.e. 90% methanol (Fisher Scientific, Pittsburgh, PA; catalog No.: AC61009-0040 HPLC Grade)/9% glacial acetic acid (Fisher; catalog No.: A38S-212)/1% water (prepared using a PURELAB Plus Ultra-pure water filtration system [ELGA LabWater LLC, Woodridge, IL]). For all experiments, pooled tissues were collected: eyestalk ganglia, 30 in 500 μL of acidified methanol; brains, 30 in 500 μL of acidified methanol; SGs, 30 in 100 μL of acidified methanol; POs, 30 in 100 μL of acidified methanol. All pooled samples were stored at -80°C until used for tissue extraction.
2.3. Tissue extraction
Pooled tissues were homogenized in cold acidified methanol (see 2.2) using a handheld ground glass homogenizer (Wheaten Science; Millville, NJ), after which the tissue homogenate was centrifuged at 16,000xg using an Eppendorf 5415D tabletop centrifuge (Eppendorf AG, Hauppauge, NY). The resulting supernatant was collected and dried using a Savant SC 110 SpeedVac concentrator (Thermo Scientific, Asheville, NC) and then re-suspended in 50 μL of 0.1% formic acid (Fisher; catalog No.: AC14793-2500) in water for further separation and analysis.
2.4. Reversed phase-HPLC fractionation of tissue extracts
The extracts produced for each tissue were fractionated via high performance liquid chromatography (HPLC). Specifically, the re-suspended tissue supernatants described earlier (see 2.3) were vortexed and briefly centrifuged, after which they were subjected to separation using a Rainin Dynamax HPLC system equipped with a UV-D II absorbance detector (Rainin Instrument Inc., Woburn, MA). The mobile phases used for HPLC fractionation were de-ionized water containing 0.1% formic acid (Solution A) and acetonitrile (ACN; Fisher; catalog No.: AC610010040) containing 0.1% formic acid (Solution B). Approximately 50 μL of extract was injected onto a Macrosphere C18 column (2.1 mm i.d. x 250 mm length, 5 μm particle size; Alltech Assoc. Inc., Deerfield, IL). HPLC separation consisted of a 120-minute gradient of 5- 95% Solution B, with fractions automatically collected every two minutes using a Rainin Dynamax FC-4 fraction collector. Following separation, each fraction was dried using a Savant SC 110 SpeedVac concentrator and then resuspended in 10 μL of 0.1% formic acid.
2.5. MALDI-TOF/TOF mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry (MALDI-TOF/TOF MS/MS) was performed using a model 4800 MALDI-TOF/TOF analyzer (Applied Biosystems, Framingham, MA) equipped with a 200 Hz, 355 nm Nd:YAG laser; this instrument was used for off-line HPLC fraction screening. Off-line analysis of HPLC fractions was performed by spotting 0.4 μL of an HPLC fraction (see 2.4) on the MALDI sample plate and adding 0.4 μL of 5mg/mL α-cyano-4-hydroxy-cinnamic acid (CHCA); the resulting mixture was allowed to crystallize at room temperature (approximately 20°C). All acquisitions were performed in positive ion reflectron mode, and instrument parameters were set using 4000 Series Explorer software (Applied Biosystems). Mass spectra were obtained by averaging 1000 laser shots covering the mass range m/z 500-4000. Tandem mass spectrometric sequencing (MS/MS) was achieved by 1 kV collision induced dissociation (CID) using air.
2.6. NanoLC-ESI-Q-TOF mass spectrometry
2.6.1. Q-TOF Micromass spectrometer system
For some experiments, nanoflow liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (nanoLC-ESI-Q-TOF MS/MS) was performed using a Waters nanoAcquity UPLC system coupled to a Q-TOF Micromass spectrometer (Waters Corporation, Milford, MA). Here, an in-house prepared, C18 reversed phase capillary column (75 μm internal diameter x100 mm length, 3 μm particle size, 100Å pore size) was used for the second dimension separation of the HPLC described above. The mobile phases used for separation were: 0.1% formic acid in deionized water (mobile phase A) and 0.1% formic acid in ACN (mobile phase B). An aliquot of 5.0 μL of a tissue extract or HPLC fraction was injected and loaded onto the trap column (Zorbax 300SB-C18 Nano trapping column; Agilent Technologies, Santa Clara, CA) using mobile phase A at a flow rate of 10 μL/min for 10 minutes, after which the stream select module was switched to a position at which the trap column came in line with the analytical capillary column, and a linear gradient of 5% to 45% mobile phase B over 90 min was initiated. The nanoflow ESI source conditions were set as follows: capillary voltage 3200 V, sample cone voltage 35 V, extraction cone voltage 1 V, source temperature 100°C; data dependent acquisition was employed for the MS survey scan, selection of three precursor ions, and subsequent MS/MS of the selected parent ions. The MS scan range was from m/z 400-1800, and the MS/MS scan was from m/z 50-1800.
2.6.2. Synapt G2 HDMS mass spectrometer system
In addition to the system described in 2.6.1, nanoLC-ESI-Q-TOF MS/MS was also performed using a Waters nanoAcquity UPLC system coupled to a Synapt G2 HDMS mass spectrometer (Waters). Here, chromatographic separations were performed on a Waters BEH 130Å C18 reversed phase capillary column (150 mm X 75 μm, 1.7 μm). The mobile phases used were the same as those described in 2.6.1. An aliquot of 2.0 μL of an HPLC fraction was injected and loaded onto the Waters NanoEase trap column using 95% mobile phase A and 5% mobile phase B at a flow rate of 10 μL/min for 3 min. Following this loading, a linear gradient from 5 to 45% mobile phase B over 75 min was employed for separation. Data dependent acquisition was employed for the MS survey scan, the selection of three precursor ions, and subsequent MS/MS of the selected parent ions. The MS scan range was from m/z 400–2000, and the MS/MS scan was from m/z 50–2000.
3. Results
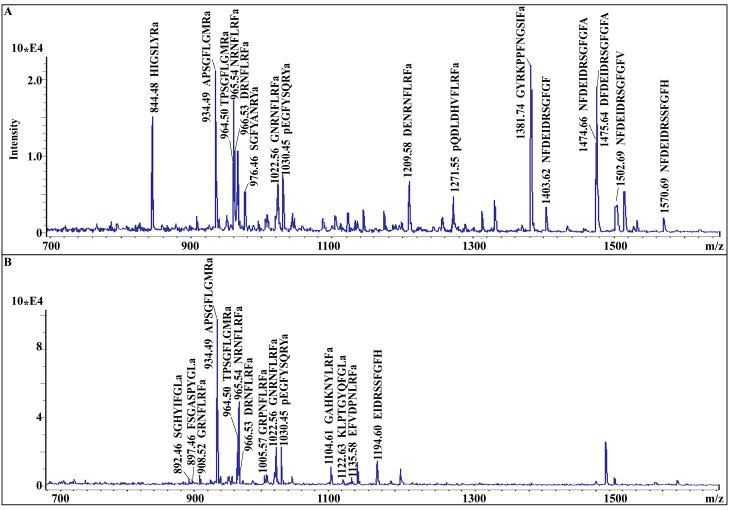
To determine the neuropeptidome of O. ceratophthalma, a strategy combining multiple mass spectral platforms was used. In the sections that follow, the identified peptides have been grouped into families of related isoforms and these are presented below in alphabetical order based on family name. Table 1 shows the sequences of the identified peptides, as well as the mass spectral platform(s) through which they were identified; Table 1 also provides information on the tissues in which the peptides were detected. Figure 1 shows examples of the MALDI-TOF/TOF MS/MS spectra used for the identification of known peptides based on accurate mass matching of predicted vs. detected mass-to-charge ratios (m/z). This figure also highlights the power of HPLC for reducing the chemical complexity of crude tissue extracts, thus allowing for increased peptide identification in fractionated samples. The remaining mass spectra presented here show examples of de novo peptide sequencing using the nanoLC-ESI-Q-TOF platform. Included in several figures (e.g. Fig. 2) are MS/MS spectra generated for the same peptide using each of the two instruments used in our study: Q-TOF Micromass and Synapt G2 HDMS; these spectra highlight the distinct, and complementary, information that each of the nanoLC-ESI-Q-TOF systems provides for de novo sequence assignment.
Table 1.
Ocypode ceratophthalma neuropeptides identified via mass spectrometry.
| Family | M+H | Sequence | Tissue | |||
|---|---|---|---|---|---|---|
| Br | PO | SG | ES | |||
| A-AST | 769.39 | EAYAGFLa | + | |||
| A-AST | 794.42 | AGPYAFGLa | + | |||
| A-AST | 795.40 | EPYAFGLa | + | |||
| A-AST | 854.40 | DGPYSFGLa | + | |||
| A-AST | 879.48 | RGPYAFGLa | + | |||
| A-AST | 883.43 | SNPYSFGLa | + | |||
| A-AST | 892.47 | SGHYIFGLa | + | |||
| A-AST | 897.46 | FSGASPYGLa | + | |||
| A-AST | 909.49 | ARPYSFGLa | + | |||
| A-AST | 918.40 | SDMYSFGLa | + | |||
| A-AST | 925.49 | SRPYSFGLa | + | |||
| A-AST | 937.49 | PRDYAFGLa | + | + | + | |
| A-AST | 939.46 | EPQYSFGLa | + | + | + | |
| A-AST | 939.50 | TRPYSFGLa | + | |||
| A-AST | 1122.63 | KLPTGYQFGLa | + | |||
| A-AST | 1384.61 | YDFEASSYSFGLa | + | + | + | |
| B-AST | 1031.51 | AWSNLGQAWa | + | + | + | |
| B-AST | 1061.51 | DGSSNLRGAWa | + | + | ||
| B-AST | 1137.54 | TGWSSTSRAWa | + | + | ||
| B-AST | 1153.54 | TGWSVFQGSWa | + | |||
| B-AST | 1182.57 | TSWGKFQGSWa | + | + | ||
| B-AST | 1198.56 | TSWGKYQGSWa | + | |||
| B-AST | 1220.58 | SGDWSSLRGAWa | + | + | + | |
| B-AST | 1222.58 | GNWNKFQGSWa | + | + | ||
| B-AST | 1236.49 | SDGWSPSGDGSWa | + | |||
| B-AST | 1252.59 | NNWSKFQGSWa | + | |||
| B-AST | 1253.61 | TQWSKFQGSWa | + | |||
| B-AST | 1254.51 | DDWSQFQGSWa | + | |||
| B-AST | 1293.63 | STNWSSLRSAWa | + | + | + | |
| B-AST | 1325.56 | STNWSSCPTSAWa | + | |||
| B-AST | 1458.67 | SPNDWAHFRGSWa | + | + | ||
| CCAP | 956.37 | PFCNAFTGCa | + | + | ||
| CPRP | 863.46 | LPSAHPLE | + | |||
| CPRP | 1367.61 | GPAESSGESAHPLE | + | |||
| CPRP | 1523.71 | RGPAESSGESAHPLE | + | |||
| CPRP | 1576.74 | SLRGPAESSGESALSE | + | |||
| CPRP | 1636.79 | LRGPAESSGESAHPLE | + | |||
| CPRP | 1723.82 | SLRGPAESSGESAHPLE | + | + | ||
| CPRP | 1751.82 | SLRGPAESDGESAHPLE | + | + | ||
| CPRP | 1824.87 | TSLRGPAESSGESAHPLE | + | + | ||
| CPRP | 1937.96 | LTSLRGPAESSGESAHPLE | + | |||
| CPRP | 2051.04 | LLTSLRGPAESSGESAHPLE | + | |||
| FLP - myosuppressin | 1271.64 | pQDLDHVFLRFa | + | + | + | |
| FLP - myosuppressin | 1288.68 | QDLDHVFLRFa | + | + | + | |
| FLP - sNPF | 887.56 | PSLRLRFa | + | + | + | |
| FLP - sNPF | 905.52 | PSMRLRFa | + | + | ||
| FLP - sNPF | 1105.63 | SMPSLRLRFa | + | |||
| FLP - sNPF | 1312.80 | DVRAPALRLRFa | + | |||
| FLP - extended -FLRFa | 695.40 | NFLRFa | + | |||
| FLP - extended -FLRFa | 908.52 | GRNFLRFa | + | |||
| FLP - extended -FLRFa | 965.54 | NRNFLRFa | + | + | + | + |
| FLP - extended -FLRFa | 966.53 | DRNFLRFa | + | + | + | |
| FLP - extended -FLRFa | 994.51 | NPSDFLRFa | + | + | ||
| FLP - extended -FLRFa | 1005.57 | GRPNFLRFa | + | + | ||
| FLP - extended -FLRFa | 1007.58 | PKSNFLRFa | + | |||
| FLP - extended -FLRFa | 1019.59 | APRNFLRFa | + | |||
| FLP - extended -FLRFa | 1022.56 | GNRNFLRFa | + | + | + | |
| FLP - extended -FLRFa | 1023.55 | GDRNFLRFa | + | + | + | |
| FLP - extended -FLRFa | 1031.59 | AHKNFLRFa | + | |||
| FLP - extended -FLRFa | 1059.60 | AHRNFLRFa | + | |||
| FLP - extended -FLRFa | 1073.55 | TNYGGFLRFa | + | |||
| FLP - extended -FLRFa | 1122.63 | RDRNFLRFa | + | |||
| FLP - extended -FLRFa | 1137.59 | DGNRNFLRFa | + | + | + | |
| FLP - extended -FLRFa | 1144.59 | AYNQSFLRFa | + | + | + | + |
| FLP - extended -FLRFa | 1190.64 | NQPGVNFLRFa | + | |||
| FLP - extended -FLRFa | 1208.66 | SVGNRNFLRFa | + | + | ||
| FLP - extended -FLRFa | 1209.61 | DENRNFLRFa | + | + | + | + |
| FLP - extended -YLRFa | 926.52 | SKNYLRFa | + | |||
| FLP - extended -YLRFa | 1104.61 | GAHKNYLRFa | + | |||
| FLP - extended -YLRFa | 1173.62 | YGNKNYLRFa | + | |||
| FLP - extended -NLRFa | 905.51 | NRSNLRFa | + | + | ||
| FLP - extended -NLRFa | 1135.59 | EFVDPNLRFa | + | |||
| Orcokinin | 908.41 | NFDEIDR | + | + | ||
| Orcokinin | 909.39 | DFDEIDR | + | |||
| Orcokinin | 995.44 | NFDEIDRS | + | |||
| Orcokinin | 1052.46 | NFDEIDRSG | + | |||
| Orcokinin | 1053.45 | DFDEIDRSG | + | |||
| Orcokinin | 1066.48 | NFDEIDRSA | + | |||
| Orcokinin | 1082.47 | NFDEIDRSS | + | |||
| Orcokinin | 1098.52 | EIDRSGFGFA | + | |||
| Orcokinin | 1156.53 | FDEIDRSGFA | + | + | ||
| Orcokinin | 1186.54 | FDEIDRSSFA | + | + | ||
| Orcokinin | 1194.55 | EIDRSSFGFH | + | |||
| Orcokinin | 1198.55 | NFDEIDRSGFa | + | + | ||
| Orcokinin | 1213.55 | DEIDRSGFGFA | + | |||
| Orcokinin | 1228.56 | NFDEIDRSSFa | + | |||
| Orcokinin | 1229.54 | NFDEIDRSSF | + | |||
| Orcokinin | 1256.55 | NFDEIDRSGFG | + | + | + | + |
| Orcokinin | 1257.54 | DFDEIDRSGFG | + | + | ||
| Orcokinin | 1270.57 | NFDEIDRSGFA | + | + | + | + |
| Orcokinin | 1271.55 | DFDEIDRSGFA | + | + | + | |
| Orcokinin | 1286.56 | NFDEIDRSSFG | + | + | + | + |
| Orcokinin | 1287.55 | DFDEIDRSSFG | + | + | ||
| Orcokinin | 1298.60 | NFDEIDRSGFV | + | |||
| Orcokinin | 1300.58 | NFDEIDRSSFA | + | + | + | |
| Orcokinin | 1301.56 | DFDEIDRSSFA | + | + | ||
| Orcokinin | 1319.59 | FDEIDRSSFGF | + | |||
| Orcokinin | 1328.58 | NFDEIDRSDFA | + | |||
| Orcokinin | 1360.62 | FDEIDRSGFGFA | + | |||
| Orcokinin | 1388.65 | FDEIDRSGFGFV | + | |||
| Orcokinin | 1390.63 | FDEIDRSSFGFA | + | |||
| Orcokinin | 1403.62 | NFDEIDRSGFGF | + | + | + | + |
| Orcokinin | 1404.61 | DFDEIDRSGFGF | + | + | + | |
| Orcokinin | 1433.63 | NFDEIDRSSFGF | + | + | + | + |
| Orcokinin | 1434.62 | DFDEIDRSSFGF | + | + | + | |
| Orcokinin | 1474.66 | NFDEIDRSGFGFA | + | + | + | |
| Orcokinin | 1475.64 | DFDEIDRSGFGFA | + | + | + | + |
| Orcokinin | 1501.71 | NFDEIDRSGFGFVa | + | |||
| Orcokinin | 1502.69 | NFDEIDRSGFGFV | + | + | + | + |
| Orcokinin | 1503.68 | DFDEIDRSGFGFV | + | + | ||
| Orcokinin | 1504.67 | NFDEIDRSSFGFA | + | + | + | |
| Orcokinin | 1505.65 | DFDEIDRSSFGFA | + | + | ||
| Orcokinin | 1518.68 | NFDEIDRSGYGFV | + | |||
| Orcokinin | 1532.70 | NFDEIDRSSFGFV | + | + | + | |
| Orcokinin | 1533.69 | DFDEIDRSGFGFV | + | |||
| Orcokinin | 1548.66 | DFDEIDRSSFGFN | + | |||
| Orcokinin | 1554.70 | NFDEIDRTGFGFH | + | |||
| Orcokinin | 1570.69 | NFDEIDRSSFGFH | + | + | + | |
| Orcokinin | 1571.68 | DFDEIDRSSFGFH | + | |||
| Orcomyotropin | 1099.30 | FDAFTTGFGH | + | |||
| Orcomyotropin | 1168.54 | FPAFTTGFGHS | + | + | + | |
| Orcomyotropin | 1186.52 | FDAFTTGFGHS | + | + | + | + |
| Orcomyotropin | 1202.51 | FDAYTTGFGHS | + | |||
| OPRP | 1062.56 | TPRDIANLY | + | |||
| PDH | 1397.80 | NSELINSILGLPK | + | |||
| PDH | 1627.91 | NSELINSILGLPKVM | + | |||
| PDH | 1812.99 | SELINSILGLPKVMNDAa | + | |||
| PDH | 1927.03 | NSELINSILGLPKVMNDAa | + | + | + | |
| PDH | 1943.03 | NSELINSILGLPKVM(O)NDAa | + | + | ||
| Proctolin | 649.37 | RYLPT | + | + | + | + |
| Pyrokinin | 878.52 | LYFAPRLa | + | |||
| Pyrokinin | 1109.58 | TDGFAFSPRLa | + | |||
| RYamide | 784.41 | FVGGSRYa | + | + | + | |
| RYamide | 832.41 | FYANRYa | + | |||
| RYamide | 862.42 | FYSQRYa | + | |||
| RYamide | 959.47 | SGFYAPRYa | + | |||
| RYamide | 975.50 | SGFYALRYa | + | |||
| RYamide | 976.46 | SGFYANRYa | + | + | ||
| RYamide | 977.45 | SGFYADRYa | + | |||
| RYamide | 992.46 | SGFYSNRYa | + | |||
| RYamide | 993.44 | SGFYSDRYa | + | |||
| RYamide | 1008.44 | SGFYCNRYa | + | |||
| RYamide | 1030.45 | pEGFYSQRYa | + | + | + | |
| RYamide | 1110.53 | SGFNSPSPRYa | + | |||
| RYamide | 1114.58 | SSRFVGGSRYa | + | |||
| SIFamide | 1161.65 | RKPPFNGSIFa | + | |||
| SIFamide | 1381.74 | GYRKPPFNGSIFa | + | + | + | |
| TRP | 766.18 | SGFLGMRa | + | + | ||
| TRP | 863.46 | PSGFLGMRa | + | + | ||
| TRP | 934.49 | APSGFLGMRa | + | + | + | |
| TRP | 950.49 | APSGFLGM(O)Ra | + | + | + | |
| TRP | 964.50 | TPSGFLGMRa | + | + | + | |
| TRP | 980.50 | TPSGFLGM(O)Ra | + | + | + | |
| TRP | 992.50 | APSGFLGMRG | + | + | ||
| Other | 794.50 | HIGSLLRa | + | |||
| Other | 844.48 | HIGSLYRa | + | + | + | |
Peptides shown in blue are novel peptides de novo sequenced here for the first time using a Micromass nanoLC-ESI-Q-TOF MS/MS system. Peptides indicated in red are novel peptides de novo sequenced for the first time using a SYNAPT G2 nanoLC-ESI-Q-TOF MS/MS system. Peptides indicated in green are novel peptides de novo sequenced here by both the Micromass and the SYNAPT G2 systems. Peptides shown in black are ones known from other crustaceans and identified here via accurate mass matching via
MALDI-TOF/TOF MS/MS. It should be noted that some of the peptides shown in blue, red or green were also identified by MALDI-TOF/TOF.
Tissue abbreviations: Br, brain; ES, eyestalk ganglia; PO, pericardial organ; SG, sinus gland
Peptide family abbreviations: A-AST, A-type allatostatin; B-AST, B-type allatostatin; CCAP, crustacean cardioactive peptide; CPRP, crustacean hyperglycemic hormone precursor-related peptide; FLP, FMRFamide-like peptide; OPRP, orcokinin/orcomyotropin precursor-related peptide; PDH, pigment dispersing hormone; sNPF, short neuropeptide F; TRP, tachykinin-related peptide.
Other abbreviations: a, amide group; M(O), oxidized methionine
Figure 1.
Identification of neuropeptides in the brain of Ocypode ceratophthalma via MALDI-TOF/TOF accurate mass matching. (A) Detection of peptides in crude brain extract. (B) Detection of peptides in an HPLC separated fraction (Fraction 6) of brain extract.
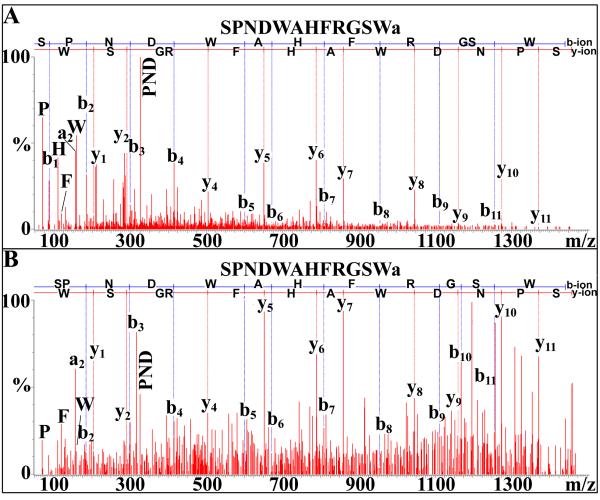
Figure 2.
NanoLC-ESI-Q-TOF de novo sequencing of SPNDWAHFRGSWamide, a novel B-type allatostatin, from the pericardial organ using (A) Q-TOF Micromass or (B) SYNAPT G2 HDMS systems. In this and all other de novo sequencing figures, b-ions represent m/z peaks where charge is maintained on amino (N)-terminal peptide fragments, while y-ions represent peaks where charge is maintained on carboxy (C)-terminal fragments.
3.1. A-type allatostatin
Sixteen peptides possessing –YXFGLamide carboxyl (C)-termini (where X represents a variable residue), the hallmark of the A-type allatostatin (A-AST) family [9], were identified from O. ceratophthalma neural extracts (Table 1). Of these peptides, 12 were isoforms known from other species, for example RGPYAFGLamide, FSGASPYGLamide (Fig. 1B) and TRPYSFGLamide, all of which are known Carcinus maenas isoforms [36]. The remaining four A-ASTs, SGHYIFGLamide (Fig. 1B), EPQYSFGLamide, KLPTGYQFGLamide (Fig. 1B), and YDFEASSYSFGLamide, were identified here for the first time.
A-ASTs were identified in each of the tissues analyzed (Table 1). However, the PO was by far the richest source of members of this peptide family, with nine of the 16 isoforms detected in this tissue (Table 1). In contrast, the SG had the fewest A-ASTs, with just two peptides identified in this neuroendocrine organ (Table 1). Interestingly, only three of the 16 A-AST isoforms were identified from multiple tissues (Table 1).
3.2. B-type allatostatin
Fifteen peptides possessing the C-terminal motif –WX6Wamide (where X6 represents six variable amino acids), the hallmark of the B-type allatostatin (B-AST) family [9], were identified in O. ceratophthalma (Table 1). Of these peptides, nine, DGSSNLRGAWamide, TGWSSTSRAWamide, TGWSVFQGSWamide, TSWGKYQGSWamide, SDGWSSLRGSWamide, TQWSKFQGSWamide, DDWSQFQGSWamide, STNWSSCPTSAWamide and SPNDWAHFRGSWamide (Fig. 2) are novel B-AST isoforms, sequenced here for the first time. The remaining six B-ASTs are known crustacean isoforms, e.g. AWSNLGQAWamide, which was originally identified from C. maenas [36].
The PO was by far the richest source of B-ASTs, with all 14 isoforms present in this tissue (Table 1). Seven B-ASTs were present in the brain, with four sequenced from the eyestalk ganglia (Table 1). Interestingly, no B-type peptides were detected in the SG (Table 1). Eight of the 14 B-ASTs were present in multiple tissues (Table 1).
3.3. Crustacean cardioactive peptide
The peptide PFCNAFTGCamide, commonly referred to as crustacean cardioactive peptide or CCAP [9], was identified in both the brain and PO of O. ceratophthalma (Table 1); this peptide has previously been identified in a large number of decapod species (e.g. [10,19,26,27,33,37,38,40,52]).
3.4. Crustacean hyperglycemic hormone precursor-related peptide
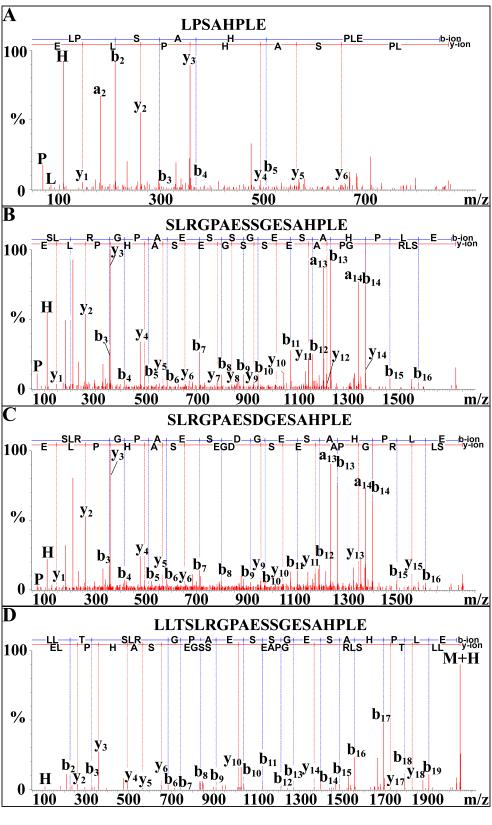
Ten novel, apparently truncated, crustacean hyperglycemic hormone precursor-related peptides (CPRPs), LPSAHPLE (Fig. 3A), GPAESSGESAHPLE, RGPAESSGESAHPLE, SLRGPAESSGESALSE (Fig. 3B), LRGPAESSGESAHPLE, SLRGPAESSGESAHPLE, SLRGPAESDGESAHPLE (Fig. 3C), TSLRGPAESSGESAHPLE, LTSLRGPAESSGESAHPLE and LLTSLRGPAESSGESAHPLE (Fig. 3D), were sequenced from the eyestalk ganglia (5 of the 10 peptides) and/or SG (8 of the 10 truncated CPRPs) of O. ceratophthalma (Table 1). Three of the 10 peptides were sequenced from both tissues (Table 1).
Figure 3.
NanoLC-ESI-Q-TOF de novo sequencing of putative truncated crustacean hyperglycemic hormone precursor-related peptides (CPRPs) from the sinus gland via Q-TOF Micromass. (A) De novo sequencing of LPSAHPLE. (B) De novo sequencing of SLRGPAESSGESALSE. (C) De novo sequencing of SLRGPAESDGESAHPLE. (D) De novo sequencing of LLTSLRGPAESSGESAHPLE.
Alignment of the 10 truncated CPRPs (Fig. 4) suggests that at least four full-length peptides are produced in the O. ceratophthalma eyestalk, with seven of the 10 peptides likely derived from one full-length CPRP, and the remaining three truncations representing portions of three other distinct CPRPs. This complement of four full-length CPRPs, if complete, would be identical to that seen in the XO-SG systems of several other brachyurans, for example Cancer productus [18,24,57].
Figure 4.
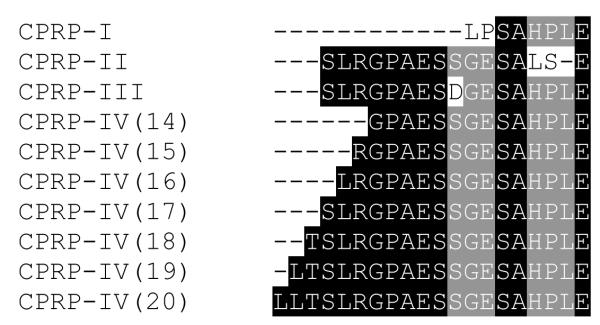
Sequence alignment of truncated crustacean hyperglycemic hormone precursor-related peptides (CPRPs) using the online software program MAFFT version 6 (http://mafft.cbrc.jp/alignment/software/; [30,31]). Three of the ten truncations (CPRP-I, CPRP-II and CPRP-III) possess sequences that suggest they are from CPRPs distinct from the remaining seven peptides (which appear to be derived from a common CPRP; CPRP-IV). In this figure, amino acids that are common to all truncations in their regions of overlap are highlighted in black, while residues that are shared by all but one peptide are highlighted in gray; variable residues (as well as a single apparent deletion) are unhighlighted in this figure. The numbers in parentheses to the right of the CPRP-IVs show the number of amino acid residues contained in the truncation.
3.5. FMRFamide-like peptide
In crustaceans, a number of distinct subgroups of peptides form what is commonly referred to as the FMRFamide-like peptide (FLP) or RFamide superfamily [9]. The FLP subgroups include the myosuppressin (–HVFLRFamide C-terminal consensus motif), neuropeptide F (typically 36 amino acids in overall length and possessing the C-terminal motif – GRPRFamide), short neuropeptide F (sNPF; typically ~10 amino acids long and possessing – RXRFamide C-termini, where X is a variable residue) and sulfakinin (possessing the C-terminal consensus motif –Y(SO3H)GHM/LRFamide) subfamilies, as well as a number of other N-terminally extended –(F/Y)(L/V/I)RFamides [8,9]. As described in detail below, a total of 30 FLPs were sequenced from the neural extracts of O. ceratophthalma (Table 1), including eight novel members of this peptide superfamily.
3.5.1. Myosuppressin
Two peptides possessing –HVFLRFamide C-termini, pQDLDHVFLRFamide (Fig. 1A) and QDLDHVFLRFamide, were identified from the brain, eyestalk ganglia and SG of O. ceratophthalma (Table 1). Both myosuppressins are known, broadly conserved crustacean isoforms (e.g. [37,56]).
3.5.2. Short neuropeptide F
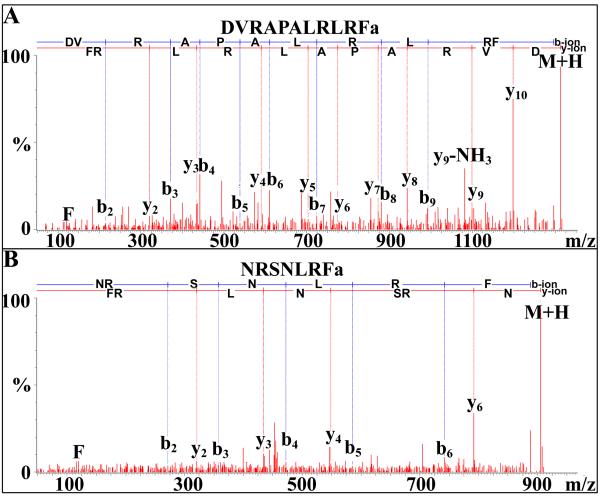
Four peptides exhibiting –RLRFamide C-termini were identified from the eyestalk ganglia (all four isoforms), brain (two of the four peptides) and/or SG (one peptide) of O. ceratophthalma (Table 1). Three of the four sNPFs are known crustacean isoforms, e.g. SMPSLRLRFamide, a broadly conserved crustacean sNPF (e.g. [29,36,37,38,40,49]). The remaining sNPF, DVRAPALRLRFamide (Fig. 5A), is novel, being sequenced from the eyestalk ganglia for the first time here (Table 1). Of the four sNPFs, two were detected in multiple tissues (Table 1).
Figure 5.
NanoLC-ESI-Q-TOF de novo sequencing of novel FMRFamide-like peptides from the eyestalk ganglia via SYNAPT G2 HDMS. (A). De novo sequencing of the short neuropeptide F isoform DVRAPALRLRFamide. (B) De novo sequencing of NRSNLRFamide.
3.5.3. Other RFamides
Nineteen amino (N)-terminally extended –FLRFamides were sequenced from the neural extracts of O. ceratophthalma (Table 1), 15 from the brain, 10 each from the PO and eyestalk ganglia, and four from the SG. Ten of the 19 identified peptides were found in multiple tissues (Table 1). While the majority of the N-terminally extended –FLRFamides (16 of the 19 peptides) are previously known isoforms, e.g. RDRNFLRFamide and APRNFLRFamide from C. productus [19], three, AYNQSFLRFamide, NQPGVNFLRFamide and SVGNRNFLRFamide, were novel identifications, described here for the first time.
Three FLPs possessing –YLRFamide C-termini were identified from O. ceratophthalma brain (2 peptides) or eyestalk ganglia (1 peptide), including the novel isoform YGNKNYLRFamide sequenced from eyestalk extracts (Table 1).
In addition to the –F/YLRFamides, two peptides possessing –NLRFamide C-termini, including the novel isoform NRSNLRFamide (Figs. 1A-B and 5B), were sequenced from the eyestalk and/or brain of O. ceratophthalma (Table 1).
3.6. Orcokinin
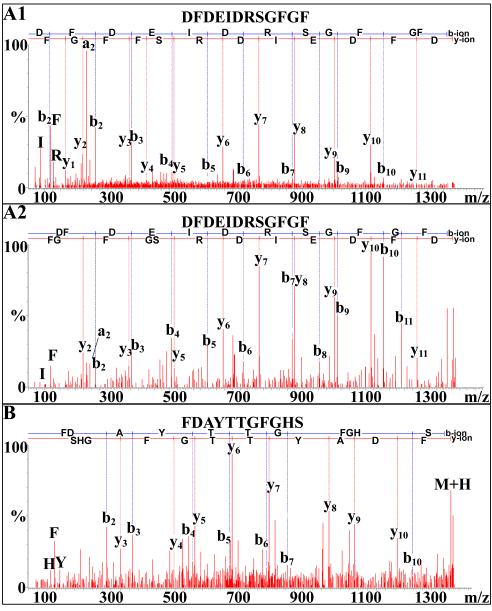
Forty-seven peptides showing sequence homology to members of the orcokinin family (full-length, mature peptides typically being 13 amino acids long and possessing the N-terminal consensus motif NFDEIDR-; [9]), were identified from the neural extracts of O. ceratophthalma (Table 1). Of these peptides, which include putative full-length peptides, truncations and amidated isoforms, 19 are novel, being identified for the first time here: NFDEIDR, DFDEIDR, NFDEIDRS, NFDEIDRSG, DFDEIDRSG, NFDEIDRSS, EIDRSSFGFH (Fig. 1B), NFDEIDRSSF, NFDEIDRSGFV, FDEIDRSSFGF, NFDEIDRSDFA, FDEIDRSGFGFV, FDEIDRSSFGFA, DFDEIDRSGFGF (Fig. 6A), DFDEIDRSSFGF, NFDEIDRSGFGFVamide, NFDEIDRSGYGFV, NFDEIDRSSFGFH (Fig. 1A) and DFDEIDRSSFGFH. The remaining peptides were previously described from other crustaceans, e.g. the amidated orcokinin variants NFDEIDRSGFamide and NFDEIDRSSFamide from H. americanus and C. maenas, respectively [36,37].
Figure 6.
NanoLC-ESI-Q-TOF de novo sequencing of novel orcokinin and orcomyotropin peptides. (A) De novo sequencing of the putative truncated orcokinin DFDEIDRSGFGF from the pericardial organ via (A1) Q-TOF Micromass and (A2) SYNAPT G2 HDMS. (B) De novo sequencing of the orcomyotropin FDAYTTGFGHS from the eyestalk ganglia via SYNAPT G2 HDMS.
While orcokinins were detected in all the O. ceratophthalma tissues examined, the PO was a particularly rich source of members of this family, with 37 of the 47 peptides identified in this tissue (Table 1). Of the 47 orcokinins identified here, 24 were present in multiple tissues (Table 1).
3.7. Orcomyotropin
Four peptides showing sequence homology to FDAFTTGFamide [15], commonly referred to as orcomyotropin [9], were identified from the neural extracts of O. ceratophthalma (Table 1). All four peptides were unamidated, C-terminally extended isoforms (Table 1), three being previously known variants, e.g. the broadly conserved FDAFTTGFGHS (e.g. [26,27,36,37,38,40,51]), and the fourth, FDAYTTGFGHS (Fig. 6B), a novel isoform sequenced from the eyestalk ganglia for the first time here. All tissues contained at least one isoform of orcomyotropin, and two of the isoforms were present in multiple tissues (Table 1).
3.8. Orcokinin/orcomyotropin precursor-related peptide
In decapods, multiple orcokinin and one copy of orcomyotropin are encoded on a common precursor protein [12,60]. In addition, a number of precursor-related peptides (PRP) are encoded with these peptides on the prepro-orcokinin/orcomyotropin [12,60]. Here, we have sequenced TPRDIANLY from the SG of O. ceratophthalma (Table 1); this peptide is identical to an orcokinin/orcomyotropin precursor-related peptide recently described from C. sapidus [26].
3.9. Pigment dispersing hormone
The peptide NSELINSILGLPKVMNDAamide, commonly referred to as β-pigment dispersing hormone or β-PDH [9], was identified from the brain, eyestalk ganglia and SG of O. ceratophthalma (Table 1); this peptide has previously been identified from a wide variety of decapod species, including a number of brachyurans (e.g. [19,23,26,32,36,40,46]). In addition, three novel, putative truncations of β-PDH (one from the SG and two from the eyestalk ganglia; Table 1), and a novel oxidized version of this peptide (from the brain and SG; Table 1), were sequenced here for the first time.
3.10. Proctolin
The peptide RYLPT, commonly referred to as proctolin [9], was detected in all of the O. ceratophthalma tissues examined in this study (Table 1); proctolin has previously been identified from a wide variety of crustaceans (e.g. [19,26,27,33,36,37,38,40,47,59]).
3.11. Pyrokinin
Two peptides exhibiting the C-terminal motif –FXPRLamide (where X is a variable residue), the hallmark of the pyrokinin family [9], were identified in O. ceratophthalma (Table 1). These peptides, LYFAPRLamide (detected in the eyestalk ganglia) and DTGFAFSPRLamide (identified in the PO), are known crustacean pyrokinins, with the former previously sequenced from C. borealis [40] and the latter a known C. maenas isoform [36].
3.12. RYamide
Thirteen peptides possessing the C-terminal motif –RYamide, the hallmark of the RYamide family [9], were identified in O. ceratophthalma (Table 1). Of these peptides, five, SGFYALRYamide, SGFYSNRYamide (Fig. 7A), SGFYSDRYamide (Fig. 7B), SGFYCNRYamide (Fig. 7C) and SGFNSPSPRYamide, are novel isoforms, sequenced here for the first time. The remaining eight RYamides are known crustacean isoforms, e.g. the broadly conserved pEGFYSQRYamide (Fig. 1A-B) [33,55].
Figure 7.
NanoLC-ESI-Q-TOF de novo sequencing of novel RYamide isoforms from the pericardial organ via SYNAPT G2 HDMS. (A) De novo sequencing of SGFYSNRYamide. (B) De novo sequencing of SGFYSDRYamide. (C) De novo sequencing of SGFYCNRYamide.
The PO was by far the richest source of RYamides, with all 13 isoforms present in this tissue (Table 1). In contrast, only three RYamides were identified in the brain, with but a single isoform each present in the eyestalk ganglia and SG (Table 1). Only three of the 13 RYamides were present in multiple tissues (Table 1).
3.13. SIFamide
Two peptides exhibiting the C-terminal motif –SIFamide, the hallmark of the SIFamide family [9], were identified in O. ceratophthalma: GYRKPPFNGSIFamide (Fig. 1A) and RKPPFNGSIFamide (Table 1). Both peptides are known, broadly conserved crustacean isoforms (e.g. [49,56]), with the former detected in brain, eyestalk ganglia and SG extracts and the latter identified from brain only (Table 1).
3.14. Tachykinin-related peptide
Seven peptides possessing the C-terminal motif –FXGXRamide (where the two Xs represent variable amino acids), the hallmark of the tachykinin-related peptide (TRP) family [9], were identified from O. ceratophthalma tissue extracts (Table 1). All of the TRPs appear to be related to the mature, full-length isoforms APSGFLGMRamide or TPSGFLGMRamide, with one partially processed, two oxidized, and two truncated versions of them included in the collection of peptides identified from O. ceratophthalma (Table 1). APSGFLGMRamide (Fig. 1A-B) or TPSGFLGMRamide (Fig. 1A-B) are previously known crustacean TRPs (e.g. [7,56,58]), and the partially processed, oxidized and truncated versions of them have also been previously described from other decapods (e.g. [7,37,58]).
All of the sequenced TRPs were present in extracts of the eyestalk ganglia, with six of the seven also sequenced from the brain (Table 1). Three of the seven TRPs were detected in the SG, with two sequenced from the PO (Table 1). All of the TRPs were detected in at least two of the four tissues sampled in our study (Table 1).
3.15. Other peptides
In addition to members of the families described above, two other peptides were identified here from the neural extracts of O. ceratophthalma: HIGSLYRamide (from the brain [Fig. 1A and Table 1], eyestalk ganglia and SG) and HIGSLLRamide (from the eyestalk ganglia only [Table 1]). HIGSLYRamide is a known crustacean neuropeptide, having originally been identified from C. productus [19]; HIGSLLRamide is described here for the first time.
4. Discussion
4.1. The neuropeptidome of Ocypode ceratophthalma
In the study presented here, MALDI-TOF/TOF MS/MS and nanoLC-ESI-Q-TOF MS/MS were used to elucidate the neuropeptidome of the crab O. ceratophthalma. In total 156 peptides were identified using this combined approach, of which 59 were novel, being described here for the first time. The peptides identified included members of 15 families, and the collection, in its totality, represents the largest number of peptides thus far characterized via mass spectrometry from any crustacean in a single study.
Members of the orcokinin family were by far the largest single group of peptides identified from O. ceratophthalma, with 47 peptides characterized. In addition, a large number of FLPs, 30 peptides, were identified from the neural tissues of this species. Interestingly, several highly conserved peptides/peptide families that are present in the peptidomes of other decapods went undetected in our study, including members of the crustacean hyperglycemic hormone (CHH) superfamily (i.e. CHH, molt-inhibiting hormone [MIH] and mandibular organ-inhibiting hormone [MOIH]), the C-type allatostatins (C-ASTs), corazonin, and red pigment concentrating hormone (RPCH). It is not surprising that we did not characterize isoforms of CHH, MIH or MOIH, as these peptides are typically over 70 amino acids in length [9], too long to be effectively sequenced using the methodology/MS platforms employed here. In contrast, the known, and highly conserved, decapod C-type ASTs (pQIRYHQCYFNPISCF and SYWKQCAFNAVSCFamide), corazonin (pQTFQYSRGWTNamide) and RPCH (pELNFSPGWamide), see [9] for a recent review of these peptides in decapod species, should have been detectable via the methods we used.
Several possibilities exist for our failure to identify the C-ASTs, corazonin and RPCH in O. ceratophthalma. First, it is possible that they are simply absent in the tissues surveyed. This seems unlikely as previous mass spectral work has identified both C-type AST peptides in the brain and PO of a number of crustacean species, including brachyurans [14,39,54]. Likewise, corazonin has previously been detected via MS in crustacean brains and/or POs [33,36,38,40], with RPCH commonly found via MS in SGs [13,19,36,37,40]. Moreover, immunohistochemistry suggests the presence of at least corazonin-like and RPCH-like immunoreactive neurons in the brain of O. ceratophthalma (A.E. Christie, unpublished). More likely is that these peptides are present in O. ceratophthalma, but in amounts that are below our levels of detection, or that signals derived from their ionized forms have been suppressed by other peptides/compounds present in the analyzed extracts. Alternatively, it is possible that the native O. ceratophthalma isoforms of C-AST, corazonin and/or RPCH differ in amino acid composition and/or post-translational modification from those of other decapods. Clearly, additional study will be needed to clarify this issue.
4.2. Relative contributions of different mass spectral platforms to the elucidation of a neuropeptidome
In this study, a mass spectrometric strategy combining MALDI-TOF/TOF and nanoLC-ESI-Q-TOF was used to elucidate the neuropeptidome of O. ceratophthalma. Specifically, the highly sensitive and accurate mass measurements provided by MALDI-TOF/TOF were used to identify known peptides based on their mass-to-charge ratios (m/z), while nanoLC-ESI-Q-TOF MS/MS was employed to de novo sequence peptides from the O. ceratophthalma nervous system. These platforms, while using different ionization methods, are complementary to one another and, in combination with advanced separation technology, enable the discovery of a maximum number of neuropeptides. For example, 35 peptides were detected in the brain using MALDI-TOF/TOF, 53 were identified from this tissue by combining the results from Micromass and SYNAPT G2 ESI-Q-TOF; 16 brain peptides were detected by both platforms.
Off-line HPLC separation greatly simplifies the chemical complexity of biological matrices, such as crude neural tissue extracts, by removing salts, lipids and proteins that can interfere with the ionization efficiency of neuropeptides, the targets of the study presented here. For example, we were able to detect only 17 neuropeptides via MALDI-TOF/TOF in crude extract of the O. ceratophthalma brain, whereas 35 were revealed after the extract was subjected to HPLC separation. This finding highlights the advantage of incorporating separation techniques to reduce sample complexity and increase dynamic range. In addition, the off-line HPLC can be coupled to a nano UPLC, which is connected directly to the ESI-Q-TOF, and serves as an additional dimension in 2D-LC for further separation of neuropeptides. Again, the combination of microscale separation methods and complementary mass spectral techniques provides enhanced neuropeptidome coverage.
Two ESI-Q-TOF mass spectrometers were used in this study, a Q-TOF Micromass and a SYNAPT G2 HDMS. While the principles governing the function of these two instruments is the same, different setup and detection parameters make the peptide fragmentation patterns generated from these two instruments distinct. For example, the Q-TOF Micromass instrument produced more intense fragment ions in the low mass range than did the SYNAPT G2 HDMS system, and therefore provided more complete information on immonium ions, which are indicative of a peptide’s amino acid composition. In contrast, the SYNAPT G2 HDMS provided higher sensitivity and better coverage than did the Q-TOF Micromass mass spectrometer, as the ion optics and resolution have been improved in this new generation instrument. Moreover, the SYNAPT G2 provided more information on large fragment ions than did the Q-TOF Micromass system, which helped in the elucidation of peptide sequence structure. Additionally, for some peptide families, e.g. the orcokinins, more b-type ions are detected using the SYNAPT G2 instrument; typically only a series of y-type fragment ions at high mass range were detected for these peptides using the Q-TOF Micromass mass spectrometer, which makes deduction of peptide sequences more difficult. Thus, the complementary information provided by the Q-TOF Micromass and SYNAPT G2 HDMS instruments greatly improved our ability for ESI-Q-TOF de novo sequencing.
4.3. Potential uses of Ocypode ceratophthalma peptidomic data
As stated earlier, the horn-eyed ghost crab O. ceratophthalma is primarily a terrestrial species [22]. Thus, its modulation of physiological processes like water and ion transport, respiration, etc., may be under quite different hormonal control from freshwater and marine species. The catalog of neuropeptides identified here provides a foundation for future investigation of the hormonal control of these and other processes in this terrestrial decapod species. Interestingly, ghost crabs are often billed as among the fastest land crustaceans [20]; they also construct elaborate burrows using their chelipeds and walking legs [22]. These behaviors are undoubtedly controlled by the thoracic nervous system, which provides innervation to the leg musculature. The catalog of peptides identified here now allows for investigation of how these molecules are able to modulate/integrate the distinct and complex movements necessary for enabling these behaviors. Finally, ghost crabs have long been known to exhibit robust circadian rhythms in locomotion [3] and color change [35]. The catalog of peptides presented here now positions us to begin to assess which of, and how, these molecules may function as output signals from the circadian system to affect these and other daily rhythms in physiology and behavior.
The first description of a peptidome from a terrestrial crustacean
156 peptides, representing 15 distinct families, characterized
~40% of the identified peptides are novel
The peptidome is the largest thus far identified via MS from any crustacean
Data provide a framework for future physiological investigations
Acknowledgements
We thank Dr. Ian Cooke for providing us access to lodging and a base for field collection and dissection at Malaekahana Beach. We also thank Dr. Daniel Hartline, Dr. Petra Lenz, Nico Hartline, Kara Chin, and Christina Linkem for their help in collecting some of the animals used in this study, and Tiana Fontanilla for reading and commenting on early drafts of this manuscript. Support for this study was provided by the National Science Foundation (CHE-0957784 to L.L.), the National Institutes of Health (1R01DK071801 to L.L.) and the Cades Foundation of Honolulu, Hawaii (to A.E.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bliss DE. Metabolic effects of sinus gland or eyestalk removal in the land crab, Gecarcinus lateralis. Anat. Rec. 1951;111:502–503. [Google Scholar]
- [2].Brusca RC, Brusca GJ. Invertebrates. Second Edition Sinauer Associates; Sunderland, MA: 2003. [Google Scholar]
- [3].Burrows M, Hoyle G. The mechanism of rapid running in the ghost crab, Ocypode ceratophthalma. J. Exp. Biol. 1973;58:327–349. [Google Scholar]
- [4].Cape SS, Rehn KJ, Ma M, Marder E, Li L. Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic lobster, Homarus americanus. J. Neurochem. 2008;105:609–702. doi: 10.1111/j.1471-4159.2007.05154.x. [DOI] [PubMed] [Google Scholar]
- [5].Chen R, Jiang X, Conaway MC, Mohtashemi I, Hui L, Viner R, Li L. Mass spectral analysis of neuropeptide expression and distribution in the nervous system of the lobster Homarus americanus. J. Proteome Res. 2010;9:818–832. doi: 10.1021/pr900736t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christie AE. Crustacean neuroendocrine systems and their signaling agents. Cell Tissue Res. 2011;345:41–67. doi: 10.1007/s00441-011-1183-9. [DOI] [PubMed] [Google Scholar]
- [7].Christie AE, Lundquist CT, Nässel DR, Nusbaum MP. Two novel tachykinin-related peptides from the nervous system of the crab Cancer borealis. J. Exp. Biol. 1997;200:2279–2294. doi: 10.1242/jeb.200.17.2279. [DOI] [PubMed] [Google Scholar]
- [8].Christie AE, McCoole MD. From genes to behavior: investigations of neurochemical signaling come of age for the model crustacean Daphnia pulex. J. Exp. Biol. 2012;215:2535–2544. doi: 10.1242/jeb.070565. [DOI] [PubMed] [Google Scholar]
- [9].Christie AE, Stemmler EA, Dickinson PS. Crustacean neuropeptides. Cell. Mol. Life Sci. 2010;67:4135–4169. doi: 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chung JS, Wilcockson DC, Zmora N, Zohar Y, Dircksen H, Webster SG. Identification and developmental expression of mRNAs encoding crustacean cardioactive peptide (CCAP) in decapod crustaceans. J. Exp. Biol. 2006;209:3862–3872. doi: 10.1242/jeb.02425. [DOI] [PubMed] [Google Scholar]
- [11].Cook IM. Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol. Bull. 2002;202:108–136. doi: 10.2307/1543649. [DOI] [PubMed] [Google Scholar]
- [12].Dickinson PS, Stemmler EA, Barton EE, Cashman CR, Gardner NP, Rus S, Brennan HR, McClintock TS, Christie AE. Molecular, mass spectral, and physiological analyses of orcokinins and orcokinin precursor-related peptides in the lobster Homarus americanus and the crayfish Procambarus clarkii. Peptides. 2009;30:297–317. doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dickinson PS, Stemmler EA, Christie AE. The pyloric neural circuit of the herbivorous crab Pugettia producta shows limited sensitivity to several neuromodulators that elicit robust effects in more opportunistically feeding decapods. J. Exp. Biol. 2008;211:1434–1447. doi: 10.1242/jeb.016998. [DOI] [PubMed] [Google Scholar]
- [14].Dickinson PS, Wiwatpanit T, Gabranski ER, Ackerman RJ, Stevens JS, Cashman CR, Stemmler EA, Christie AE. Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J. Exp. Biol. 2009;212:1140–1152. doi: 10.1242/jeb.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dircksen H, Burdzik S, Sauter A, Keller R. Two orcokinins and the novel octapeptide orcomyotropin in the hindgut of the crayfish Orconectes limosus: identified myostimulatory neuropeptides originating together in neurones of the terminal abdominal ganglion. J. Exp. Biol. 2000;203:2807–2818. doi: 10.1242/jeb.203.18.2807. [DOI] [PubMed] [Google Scholar]
- [16].Fénelon V, Le Feuvre Y, Bem T, Meyrand P. Maturation of rhythmic neural networks: role of central modulatory inputs. J. Physiol. Paris. 2003;97:59–68. doi: 10.1016/j.jphysparis.2003.10.007. [DOI] [PubMed] [Google Scholar]
- [17].Fernlund P, Josefsson L. Crustacean color-change hormone: Amino acid sequence and chemical synthesis. Science. 1972;177:173–175. doi: 10.1126/science.177.4044.173. [DOI] [PubMed] [Google Scholar]
- [18].Fu Q, Christie AE, Li L. Mass spectrometric characterization of crustacean hyperglycemic hormone precursor-related peptides (CPRPs) from the sinus gland of the crab, Cancer productus. Peptides. 2005;26:2137–2150. doi: 10.1016/j.peptides.2005.03.040. [DOI] [PubMed] [Google Scholar]
- [19].Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: An anatomical and mass spectrometric investigation. J. Comp. Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- [20].Hafemann DR, Hubbard JI. On the rapid running of ghost crabs (Ocypode Ceratophthalma) J. Exp. Zool. 1969;170:25–31. [Google Scholar]
- [21].Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- [22].Hoover JP. Hawaii’s Sea Creatures. Revised Edition Mutual Publishing LLC; Honolulu, HI: 2010. [Google Scholar]
- [23].Hsu YW, Stemmler EA, Messinger DI, Dickinson PS, Christie AE, de la Iglesia HO. Cloning and differential expression of two β-pigment-dispersing hormone (β-PDH) isoforms in the crab Cancer productus: evidence for authentic β-PDH as a local neurotransmitter and β-PDH II as a humoral factor. J. Comp. Neurol. 2008;508:197–211. doi: 10.1002/cne.21659. [DOI] [PubMed] [Google Scholar]
- [24].Hsu YW, Weller JR, Christie AE, de la Iglesia HO. Molecular cloning of four cDNAs encoding prepro-crustacean hyperglycemic hormone (CHH) from the eyestalk of the red rock crab Cancer productus: identification of two genetically encoded CHH isoforms and two putative post-translationally derived CHH variants. Gen. Comp. Endocrinol. 2008;155:517–525. doi: 10.1016/j.ygcen.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [25].Hudson AE, Archila S, Prinz AA. Identifiable cells in the crustacean stomatogastric ganglion. Physiology (Bethesda) 2010;25:311–318. doi: 10.1152/physiol.00019.2010. [DOI] [PubMed] [Google Scholar]
- [26].Hui L, Cunningham R, Zhang Z, Cao W, Jia C, Li L. Discovery and characterization of the Crustacean hyperglycemic hormone precursor related peptides (CPRP) and orcokinin neuropeptides in the sinus glands of the blue crab Callinectes sapidus using multiple tandem mass spectrometry techniques. J. Proteome Res. 2011;10:4219–4229. doi: 10.1021/pr200391g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hui L, Xiang F, Zhang Y, Li L. Mass spectrometric elucidation of the neuropeptidome of a crustacean neuroendocrine organ. Peptides. 2012;36:230–239. doi: 10.1016/j.peptides.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hui L, Zhang Y, Wang J, Cook A, Ye H, Nusbaum MP, Li L. Discovery and functional study of a novel crustacean tachykinin neuropeptide. ACS Chem. Neurosci. 2011;2:711–722. doi: 10.1021/cn200042p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huybrechts J, Nusbaum MP, Bosch LV, Baggerman G, De Loof A, Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab, Cancer borealis. Biochem. Biophys. Res. Commun. 2003;308:535–544. doi: 10.1016/s0006-291x(03)01426-8. [DOI] [PubMed] [Google Scholar]
- [30].Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- [32].Klein JM, Mohrherr CJ, Sleutels F, Riehm JP, Rao KR. Molecular cloning of two pigment-dispersing hormone (PDH) precursors in the blue crab Callinectes sapidus reveals a novel member of the PDH neuropeptide family. Biochem. Biophys. Res. Commun. 1994;205:410–416. doi: 10.1006/bbrc.1994.2680. [DOI] [PubMed] [Google Scholar]
- [33].Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J. Neurochem. 2003;87:642–656. doi: 10.1046/j.1471-4159.2003.02031.x. [DOI] [PubMed] [Google Scholar]
- [34].Li L, Sweedler JV. Peptides in the brain: measurement approaches and challenges. Annu. Rev. Anal. Chem. 2008;1:451–483. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- [35].Little G. Chromatophore responses in relation to the photoperiod and background color in the Hawaiian ghost crab, Ocypode ceratophthalma (Pallas) Pacific Sci. 1967;21:77–84. [Google Scholar]
- [36].Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen. Comp. Endocrinol. 2009;161:320–334. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma M, Chen R, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC, Goy MF, Christie AE, Li L. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen. Comp. Endocrinol. 2008;156:395–409. doi: 10.1016/j.ygcen.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma M, Gard AL, Xiang F, Wang J, Davoodian N, Lenz PH, Malecha SR, Christie AE, Li L. Combining in silico transcriptome mining and biological mass spectrometry for neuropeptide discovery in the Pacific white shrimp Litopenaeus vannamei. Peptides. 2010;31:27–43. doi: 10.1016/j.peptides.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ma M, Szabo TM, Jia C, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma M, Wang J, Chen R, Li L. Expanding the crustacean neuropeptidome using a multifaceted mass spectrometric approach. J. Proteome Res. 2009;8:2426–2437. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Ann. Rev. Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- [42].Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nusbaum MP, Blitz DM. Neuropeptide modulation of microcircuits. Curr. Opin. Neurobiol. 2012;22:592–601. doi: 10.1016/j.conb.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The role of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- [45].Passano LM. The X-organ-sinus gland system neurosecretory system of crabs. Anat. Rec. 1951;111:502. [Google Scholar]
- [46].Rao KR, Riehm JP, Zahnow CA, Kleinholz LH, Tarr GE, Johnson L, Norton S, Landau M, Semmes OJ, Sattelberg RM, Jorenby WH, Hintz MF. Characterization of a pigment-dispersing hormone in eyestalks of the fiddler crab Uca pugilator. Proc. Natl. Acad. Sci. USA. 1985;82:5319–5322. doi: 10.1073/pnas.82.16.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schwarz TL, Lee GM, Siwicki KK, Standaert DG, Kravitz EA. Proctolin in the lobster: the distribution, release, and chemical characterization of a likely neurohormone. J. Neurosci. 1984;4:1300–1311. doi: 10.1523/JNEUROSCI.04-05-01300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Selverston AI, Ayers J. Oscillations and oscillatory behavior in small neural circuits. Biol. Cybern. 2006;95:537–554. doi: 10.1007/s00422-006-0125-1. [DOI] [PubMed] [Google Scholar]
- [49].Sithigorngul P, Pupuem J, Krungkasem C, Longyant S, Chaivisuthangkura P, Sithigorngul W, Petsom A. Seven novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant tiger prawn Penaeus monodon. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002;131:325–337. doi: 10.1016/s1096-4959(01)00499-7. [DOI] [PubMed] [Google Scholar]
- [50].Skiebe P. Neuropeptides are ubiquitous chemical mediators: using the stomatogastric nervous system as a model system. J. Exp. Biol. 2001;204:2035–2048. doi: 10.1242/jeb.204.12.2035. [DOI] [PubMed] [Google Scholar]
- [51].Skiebe P, Dreger M, Börner J, Meseke M, Weckwerth W. Immunocytochemical and molecular data guide peptide identification by mass spectrometry: orcokinin and orcomyotropin-related peptides in the stomatogastric nervous system of several crustacean species. Cell. Mol. Biol. (Noisy-le-grand) 2003;49:851–871. [PubMed] [Google Scholar]
- [52].Stangier J, Hilbich C, Beyreuther K, Keller R. Unusual cardioactive peptide (CCAP) from pericardial organs of the shore crab Carcinus maenas. Proc. Natl. Acad. Sci. USA. 1987;84:575–579. doi: 10.1073/pnas.84.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stein W. Modulation of stomatogastric rhythms. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- [54].Stemmler EA, Bruns EA, Cashman CR, Dickinson PS, Christie AE. Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. Gen. Comp. Endocrinol. 2010;165:1–10. doi: 10.1016/j.ygcen.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stemmler EA, Bruns EA, Gardner NP, Dickinson PS, Christie AE. Mass spectrometric identification of pEGFYSQRYamide: a crustacean peptide hormone possessing a vertebrate neuropeptide Y (NPY)-like carboxy-terminus. Gen. Comp. Endocrinol. 2007;152:1–7. doi: 10.1016/j.ygcen.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stemmler EA, Cashman CR, Messinger DI, Gardner NP, Dickinson PS, Christie AE. High-mass-resolution direct-tissue MALDI-FTMS reveals broad conservation of three neuropeptides (APSGFLGMRamide, GYRKPPFNGSIFamide and pQDLDHVFLRFamide) across members of seven decapod crustaean infraorders. Peptides. 2007;28:2104–2115. doi: 10.1016/j.peptides.2007.08.019. [DOI] [PubMed] [Google Scholar]
- [57].Stemmler EA, Hsu YW, Cashman CR, Messinger DI, de la Iglesia HO, Dickinson PS, Christie AE. Direct tissue MALDI-FTMS profiling of individual Cancer productus sinus glands reveals that one of three distinct combinations of crustacean hyperglycemic hormone precursor-related peptide (CPRP) isoforms are present in individual crabs. Gen. Comp. Endocrinol. 2007;154:184–192. doi: 10.1016/j.ygcen.2007.06.025. [DOI] [PubMed] [Google Scholar]
- [58].Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J. Neurochem. 2007;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- [59].Sullivan RE. A proctolin-like peptide in crab pericardial organs. J. Exp. Zool. 1979;210:543–552. [Google Scholar]
- [60].Yasuda-Kamatani Y, Yasuda A. Identification of orcokinin gene-related peptides in the brain of the crayfish Procambarus clarkii by the combination of MALDI-TOF and on-line capillary HPLC/Q-TOF mass spectrometries and molecular cloning. Gen. Comp. Endocrinol. 2000;118:161–172. doi: 10.1006/gcen.1999.7453. [DOI] [PubMed] [Google Scholar]