Abstract
Gangliosides are sialic acid-containing glycosphingolipids that are most abundant in the nervous system. Heterogeneity and diversity of the structures in their carbohydrate chains are characteristic hallmarks of these lipids; so far, 188 gangliosides with different carbohydrate structures have been identified in vertebrates. The molecular structural complexity increases manifold if one considers heterogeneity in the lipophilic components. The expression levels and patterns of brain gangliosides are known to change drastically during development. In cells, gangliosides are primarily, but not exclusively, localized in the outer leaflets of plasma membranes and are integral components of cell surface microdomains with sphingomyelin and cholesterol from which they participate in cell-cell recognition, adhesion, and signal transduction. In this brief review, we discuss the structures, metabolism and functions of gangliosides.
Keywords: Glycosyltransferase, glycosphingolipid, knockout mouse, stem cell, neural stem cell
1. Structures and metabolism of gangliosides
Glycolipids are biomolecules containing one or more carbohydrate residues linked to a hydrophobic lipid moiety through a glycosidic linkage. Glycolipids containing either a sphingoid or a ceramide as the hydrophobic lipid moiety are referred to as glycosphingolipids. Glycosphingolipids possess highly heterogeneous and diverse molecular structures in their carbohydrate chains and the lipid moieties. Based on their basic carbohydrate structures, glycosphingolipids are classified into the following series, namely, ganglio-, isoganglio-, lacto-, neolacto-, lactoganglio-, globo-, isoglobo-, muco-, gala-, neogala-, mollu-, arthro-, schisto- and spirometo-series (Table 1). Acidic glycosphingolipids containing one or more sialic acid (N-acetylneuraminic acid or N-glycolylneuraminic acid) residue(s) in their carbohydrate moiety are especially referred to as gangliosides. Figure 1 depicts a common brain ganglioside, GM1. As of 2004, 188 gangliosides have been identified in vertebrate tissues1).
Table 1.
Carbohydrate structures of glycosphingolipids
| Series | Basic structure | Abbreviation |
|---|---|---|
| Globo | GalNAcβ1 −3 Galα1 −4Galβ1 −4Glcβ1 −1 'Cer | Gb |
| Isoglobo | GalNAcβ1 −3 Galα1 −3 Galβ1 −4Glcβ1 −1 'Cer | iGb |
| Ganglio | Galβ1 −3 GalNAcβ1 −4Galβ1 −4Glcβ1 −1 'Cer | Gg |
| Isoganglio | Galβ1–3 GalNAcβ1–3 Galβ1–4Glcβ1–1'Cer | iGg |
| Lacto | Galβ1 −3 GlcNAcβ1 −3 Galβ1 −4Glcβ1 −1 'Cer | Lc |
| Neolacto | Galβ1 −4GlcNAcβ1 −3 Galβ1 −4Glcβ1 −1 'Cer | nLc |
| Lactoganglio | GalNAcβ1–4(GlcNAcβ1–3)Galβ1–4Glcβ1–1'Cer | LcGg |
| Muco | Galβ1–4Galβ1–4Glcβ1–1'Cer | Mc |
| Gala | Galα1–4Galβ1–1'Cer | Ga |
| Neogala | Galβ1–6Galβ1–6Galβ1–1'Cer | |
| Mollu | GlcNAcβ1–2Manα1–3Manβ1–4Glcβ1 −1'Cer | Mu |
| Arthro | GalNAcβ1–4GlcNAcβ1–3Manβ1–4Glcβ1–1'Cer | At |
| Schisto | GalNAcβ1–4Glcβ1–1'Cer | |
| Spirometo | Galβ1–4Glcβ1–3Galβ1–1'Cer |
Figure 1.
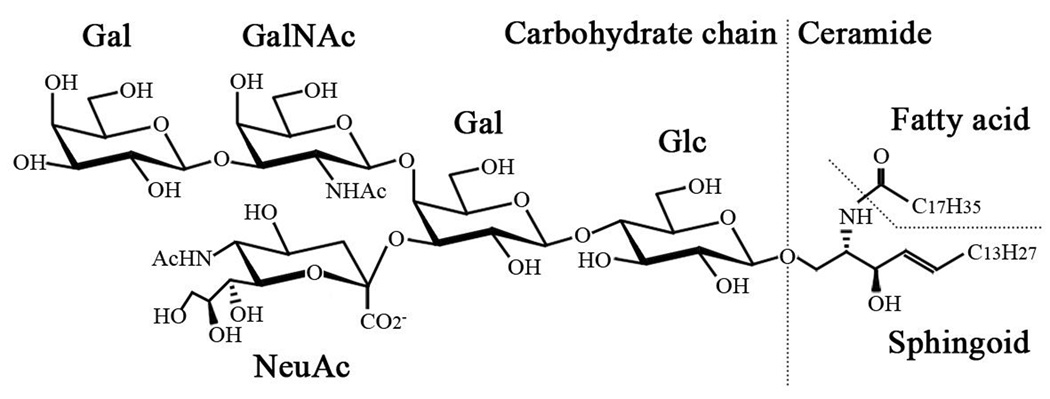
Structure of GM1 ganglioside.
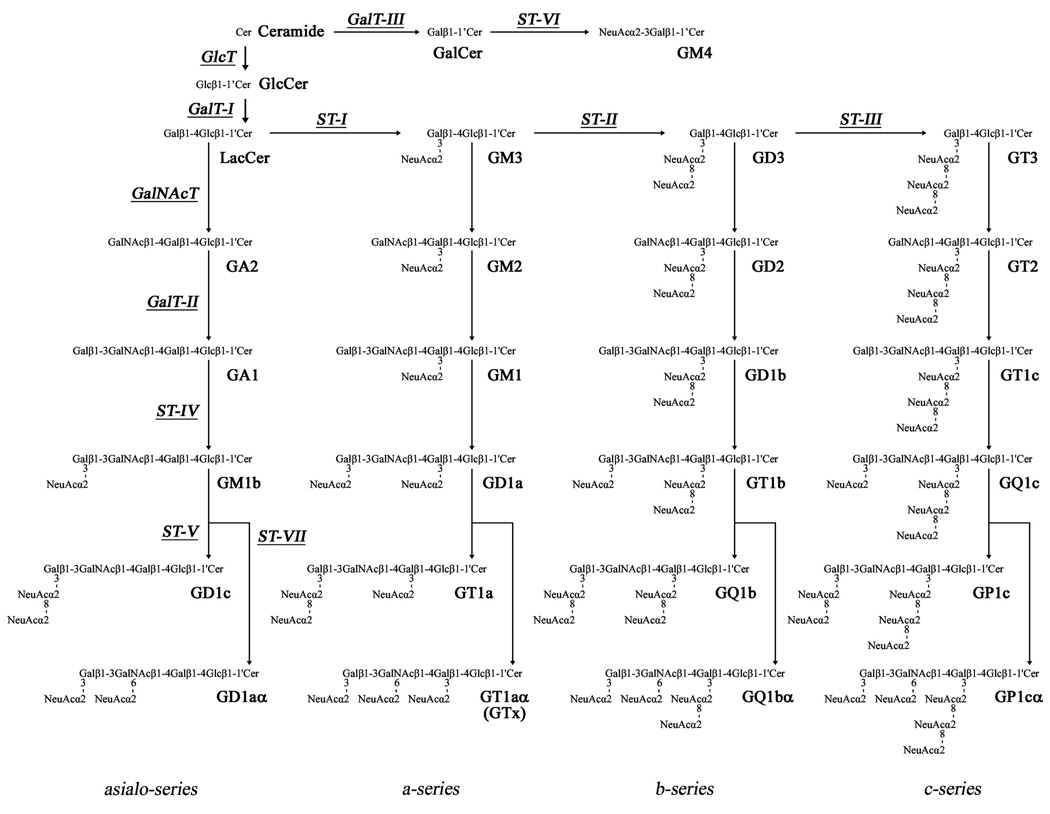
Figure 2 shows the structures and metabolic pathways of ganglio-series gangliosides. Ganglio-series glycosphingolipids having 0, 1, 2 and 3 sialic acid residue(s) linked to the inner galactose residue are classified into asialo-, a-, b- and c-series gangliosides, respectively. In addition, gangliosides having sialic acid residue(s) linked to the inner N-acetylgalactosamine residue, such as GT1aα originally reported as GTx2), are classified as α-series gangliosides.
Figure 2.
Structures and biosynthetic pathways of ganglio-series gangliosides. The nomenclature for gangliosides and the components are based on those of Svennerholm53) and IUPAC-IUBMB Joint Commission on Biochemical Nomenclature54), respectively. Glycosyltransferases catalyzing the synthesis of glycosphingolipids, including gangliosides, are underlined. GD1α, GT1aα, GQ1bα, and GP1cα are classified as belonging to the α-series gangliosides.
Glycosphingolipids including these gangliosides are primarily synthesized in the endoplasmic reticulum and further modified in the Golgi apparatus by sequential addition of additional carbohydrate moieties3) to an existing acceptor lipid molecule. The reaction is catalyzed by a series of specific glycosyltransferases. With the exception of GM4, which is derived from galactosylceramide (GalCer), most gangliosides are synthesized from lactosylceramide (LacCer). First, a simple ganglioside, GM3, is synthesized by addition of a sialic acid to LacCer by CMP-sialic acid: LacCer α2–3 sialyltransferase (ST-I or GM3 synthase). GD3 and GT3 are synthesized by sequential addition of sialic acids to GM3 and GD3 by CMP-sialic acid: GM3 α2–8 sialyltransferase (ST-II or GD3 synthase) and CMP-sialic acid: GD3 α2–8 sialyltransferase (ST-III or GT3 synthase), respectively. GM3, GD3 and GT3 further serve as precursors of more complex gangliosides belonging to the a-, b- and c-series, respectively. Elaboration of these simple gangliosides to complex gangliosides is catalyzed by UDP-GalNAc: LacCer/GM3/GD3/GT3 β1–4 N-acetylgalactosaminyltransferase (GalNAcT or GA2/GM2/GD2/GT2 synthase), UDP-Gal: GA2/GM2/GD2/GT2 β1–3 galactosyltransferase (GalT-II or GA1/GM1/GD1b/GT1c synthase), CMP-sialic acid: GA1/GM1/GD1b/GT1c α2–3 sialyltransferase (ST-IV or GM1b/GD1a/GT1b/GQ1c synthase), and CMP-sialic acid: GM1b/GD1a/GT1b/GQ1c α2–8 sialyltransferase (ST-V or GD1α/GT1aα/GQ1bα/GP1cα). Asialo-series gangliosides are also synthesized from LacCer by these glycosyltransferases along a different pathway.
Interestingly, the expression levels and patterns of ganglio-series gangliosides undergo dramatic changes during brain development4,5). For instance, in human and rodent embryonic brains, the predominant gangliosides are simple GM3 and GD3. As the brain develops, the expression of these simple gangliosides is down-regulated with concomitant up-regulation of complex gangliosides such as GM1, GD1a, GD1b and GT1b. This change in expression levels and patterns of gangliosides can be largely attributed to the developmental change of the expression levels and patterns of ganglioside synthases (glycosyltransferases)6,7) that are spatiotemporally regulated, both at the transcriptional and post-translational levels, by multiple systems, including transcription factors7) and probably epigenetic modifications8).
2. Functions of gangliosides
Gangliosides are ubiquitously found in tissues and body fluids, and are more abundantly expressed in the nervous system9). In cells, gangliosides are primarily, but not exclusively, localized in the outer leaflets of plasma membranes. On the cell surface, gangliosides are involved in cell-cell recognition and adhesion and signal transduction within specific cell surface microdomains, termed caveolae10), lipid rafts11), or glycosphingolipid-enriched microdomains12), with other membrane components such as sphingomyelin and cholesterol. Evidence is accumulating that gangliosides are colocalized in the microdomain structures with signaling molecules and adhesion molecules. In addition to cell plasma membranes, gangliosides have been shown to be present on nuclear membranes, and they have recently been proposed to play important roles in modulating intracellular and intranuclear calcium homeostasis and the ensuing cellular functions13).
The biological importance of gangliosides has been revealed by analyses of genetically engineered mice deficient in ganglioside synthases (Table 2). In histological studies of ST-I knockout mice, selective degeneration of the organ of Corti (sensory organ of hearing in the cochlea) occurs, coincidently with the onset of hearing loss. The loss of GM3 in this mutant may contribute to complete deafness14). This observation implicates a role for GM3 ganglioside and its derivatives in the functional maturation of the cochlea during early development. Recently, it has been reported that the ST-I knockout mice exhibit a phenotype resembling attention-deficit hyperactivity disorder15), thus indicating a novel role of glycosphingolipids for maintaining neuropsychological balance.
Table 2.
Phenotypes of ganglioside synthase-KO mice
| Disrupted gene | Gangliosides expressed in brain* | Phenotypes of the nervous system |
|---|---|---|
| ST-I | GM1b, GD1α14) | (Viable) |
| Complete hearing loss14) | ||
| Degeneration of the sensory organ of hearing in cochlea14) | ||
| Attention-deficit hyperactivity disorder-like behavior15) | ||
| ST-II | GM1, GD1a16,23,25) | (Viable) |
| GM3, GD1a in embryo16) | Impaired regeneration of the lesioned hypoglossal nerve16) | |
| GalNAcT | GM3, GD317,23,25) | (Viable) |
| Decreased myelination and axonal degeneration in CNS/PNS19) | ||
| Demyelination in PNS19) | ||
| Reduction in neural conduction velocity from the tibial nerve to the somatosensory cortex17) | ||
| Sensory nerve-dominant nerve degeneration and synaptic remodeling20) | ||
| ST-I/GalNAcT | (ganglioside deficient) 26) | (Viable; death soon after weaning) 26) |
| Axonal degeneration and perturbed axon-glia interaction in CNS26) | ||
| ST-II/GalNAcT | GM323–25) | (Viable; shortened life span) 25) |
| Sudden death in response to lethal sound-induced seizures25) | ||
| Neurodegeneration by dysfunction of complement systems and inflamation23,24) | ||
| GEM/raft transfiguration, complement activation, local inflammation23,24) | ||
| Progressive dysfunction of motor coordination, marked deterioration in memory and learning21,22) | ||
| Suppressed function of muscarinic type acetylcholine receptors22) |
In wildtype mice, the predominant gangliosides are GM3 and GD3 in embryonic brains and GM1, GD1a, GD1b and GT1b in adult brains.
ST-II knockout mice, deficient in b- and c-series gangliosides, exhibit intact nervous tissue morphology; however, the regenerative ability of injured hypoglossal nerve in these mice is found to be severely impaired16).
Mice lacking complex hexosamine-containing “brain-type” gangliosides, such as GM1, GD1a, GD1b and GT1b, caused by GalNAcT gene disruption show apparently normal histogenesis of brain and gross behavior17), but impaired motor coordination in older animals18). Interestingly, the nerve conduction velocity is significantly lower in GalNAcT knockout mice than in the wild type, as demonstrated by analyses of evoked potentials of contralateral S1 somatosensory cortex after stimulating the peripheral tibial nerve at the Achilles tendon17), suggesting the involvement of complex gangliosides in neural functions, such as neuronal transmission, or in structural maintenance of the nervous system. The latter hypothesis has been supported by detailed morphological analyses with electron microscopy of the GalNAcT knockout mice: those animals revealed decreased myelination and axonal degeneration in sciatic nerves and demyelination in optic nerves19,20), as well as neural degeneration, glial enlargement and synaptic remodeling in the dorsal horn of the spinal cord and dorsal root ganglia, especially in the sensory nerve system20).
When GalNAcT and ST-II genes are disrupted simultaneously, the double knockout mice express primarily GM3 with no “brain-type” gangliosides. These mice exhibit weight loss, progressive motor and sensory dysfunctions and deterioration in spatial learning and memory with aging21,22). Additionally, the responses to treatment with oxotremorine, an agonist of muscarinic acetylcholine receptors (mAChRs), [0]are markedly attenuated, indicating the impairment of mAChR functions in the GM3-only mice22), while there is no clear causal association with any aforementioned neurological abnormalities. Likewise, substantial degeneration of Purkinji neurons has also been reported in the cerebellar cortex of the double knockout mice, which may possibly result from regional complement activation and inflammatory reactions, as shown by deposits of C1q complement in the cerebella23) and a degeneration-rescuing effect by the crossbreed carrying the disrupted gene of C3 complement24).
A striking phenotype of high susceptibility to sound-induced seizures has been shown in another derived line of the double knockout mouse with a distinct genetic background, C57BL/625). These ST-II/GalNAcT double knockout animals also display a shortened life span, typically with the death of 50% of the mice by 30 weeks of age.
Mice lacking all ganglioside expression resulting from knockout of both GalNAcT and ST-I genes suffer severe lethality. The majority of the double knockout mice die soon after weaning at 3 weeks26). The ganglioside-deficient mice reveal prominent vacuolization pathology in the cerebellar and spinal white matters, along with enhanced cell apoptosis, axonal degeneration and perturbed axon-glia interactions in the cerebral cortex under histopathological examinations26).
Despite the neurological abnormalities that have been observed in ganglioside synthase knockout mice, it remains to be elucidated whether those phenotypes result from functional deficiency of the particular ganglioside product(s) and/or from an acquired consequence of the anomalous accumulation of substrate precursors.
3. Gangliosides in stem cells
Gangliosides are gaining increasing attention recently in the field of stem cell biology. Stem cells are undifferentiated cells endowed with a high potential for proliferation and the capacity for self-renewal with retention of pluripotency or multipotency to differentiate into their progenies. Stem cells have attracted considerable attention in recent years because of their biological and clinical potentials for regenerative medicine. A number of unique ganglioside markers have been identified in stem cells27,28). For instance, SSEA-4 (a globo-series ganglioside having an NeuAcα2–3Galβ1–3GalNAcβ1-R structure29)) is specifically expressed in human pluripotent embryonic stem cells30) and induced pluripotent stem cells31,32), GD3 is expressed in mouse and human mouse neural stem cells33,34), and GD234,35) and SSEA-436) are expressed in human mesenchymal stem cells. Gangliosides are primarily localized on the cell surface. Thus, gangliosides can be used as specific cell surface marker molecules for identifying or isolating these stem cells27,28).
Also, in brain cancer stem cells, a subpopulation of brain cancer cells has been reported. These cells exhibit stem cell-like characteristics, such as the ability for self-renewal and multipotency in addition to the capability to sustain brain tumor formation. These cells also express c-series gangliosides, also known as A2B5 antigens characteristic of embryonic cells37,38). These gangliosides can be utilized not only as biomarkers for cancer stem cells, but also as targets for the treatment of brain tumors. Studies of stem cell gangliosides should prove to be a fertile area of research in the future.
4. Gangliosides and diseases
Gangliosides are involved in the pathology of many diseases. For example, Guillain-Barré syndrome, an acute polyradiculoneuropathy that leads to acute quadriplegia, is caused by an autoimmune response to cell surface gangliosides39). In influenza, a well known viral infectious disease, influenza A viruses recognize sialic acid residues of gangliosides and glycoproteins on cell surfaces as receptor molecules for invasion of host cells40). Lysosomal storage diseases such as GM1 gangliosidosis and GM2 gangliosidosis (Tay-Sachs disease and Sandhoff disease) are caused by defects in the lysosomal glycosidases or specific co-activators, resulting in accumulation of the substrates, such as glycosphingolipids, including gangliosides. Human autosomal recessive infantile-onset symptomatic epilepsy syndrome, associated with developmental stagnation and blindness found in Old Order Amish pedigree, is caused by a nonsense mutation of ST-I (GM3 synthase)41). It has been recently suggested that GM3 in cell surface microdomains is involved in insulin resistance in type 2 diabetes, the most common metabolic disorder characterized by high blood glucose42).
In addition, the onset of Alzheimer’s disease, the most common form of dementia and neurodegenerative disease, has been proposed to be initiated by aggregation of amyloid-β peptide caused by gangliosides43,44). More recently, we found an increase of Chol-1α antigens, GQ1bα and GT1aα, which are specifically expressed in cholinergic neurons45,46) in the brain of Alzheimer’s disease model transgenic mice47). The increase of Chol-1α gangliosides may present evidence for generation of cholinergic neurons and neurogenesis in Alzheimer’s disease brains.
Mounting evidence suggests that gangliosides modulate aggressive angiogenesis commonly found to support tumor growth. Seyfried and co-investigators48) reported that GM3 and GD1a had an opposite effect on the responsiveness of human umbilical vein endothelial cells to vascular endothelial growth factor that promotes the endothelial cell survival, growth and migration. This is in concert with our earlier observation that GM3, a major endothelial cell ganglioside49), was a natural angiogenesis suppressor, but GD1a, shed from the surface of certain tumor cells, could induce angiogenesis. This observation suggests that exogenously administered GM3 may have therapeutic potential for reducing angiogenesis for tumor suppression.
These above reports suggest that gangliosides are important for prevention and treatment of certain diseases. In fact, there is a series of studies showing the neurotrophic effects of intracerebroventricularly administrated GM1, which is reported to improve the cognitive function in patients with Alzheimer’s disease50).
5. Conclusion
It has been about 7 decades since Ernst Klenk, a German chemist and lipidologist (1896–1971), first isolated gangliosides from the human brain51,52). Early research in the ganglioside field was focused on structural analysis of these molecules. With the advent of modern methodologies, many novel structures were identified that form the basis for further studies into gaining a better understanding of their cellular and subcellular localization in various tissue sources. This was followed by extensive investigations of their biosynthetic pathways and the regulatory mechanisms of their metabolism. Many biosynthetic and catalytic enzymes responsible for their metabolism have been characterized and glycogenes coding for these enzymes cloned and studied. These efforts have formed a firm foundation for elucidating the biological functions of these molecules. Future research should be focused on their roles not only as structural components of biomembranes, but also their functions in cell-cell recognition and adhesion, and mediators in signal transduction.
Acknowledgments
This study was supported by USPHS grants (NS11853–35 and NS26994–21) and a grant from the Childrens’ Medical Research Foundation, Chicago, IL, to RKY. RKY also grateful acknowledges the contributions from many of his past and present collaborators for the work done in his laboratory.
References
- 1.Yu RK, Yanagisawa M, Ariga T. Glycosphingolipid structures. In: Kamerling JP, editor. Comprehensive Glycoscience. Oxford, UK: Elsevier; 2007. pp. 73–122. [Google Scholar]
- 2.Nakamura K, Inagaki F, Tamai Y. A novel ganglioside in dogfish brain. Occurrence of a trisialoganglioside with a sialic acid linked to N-acetylgalactosamine. J. Biol. Chem. 1988;263:9896–9900. [PubMed] [Google Scholar]
- 3.Maccioni HJ. Glycosylation of glycolipids in the Golgi complex. J. Neurochem. 2007;103(Suppl 1):81–90. doi: 10.1111/j.1471-4159.2007.04717.x. [DOI] [PubMed] [Google Scholar]
- 4.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 2007;103:2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem. 1988;50:1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishii A, Ikeda T, Hitoshi S, Fujimoto I, Torii T, Sakuma K, Nakakita S, Hase S, Ikenaka K. Developmental changes in the expression of glycogenes and the content of N-glycans in the mouse cerebral cortex. Glycobiology. 2007;17:261–276. doi: 10.1093/glycob/cwl076. [DOI] [PubMed] [Google Scholar]
- 7.Yu RK, Ariga T, Yanagisawa M, Zeng G. Gangliosides in the nervous system: Biosynthesis and degradation. In: Fraser-Reid B, Tatsuka K, Thiem J, editors. Glycoscience. Berlin-Heiderberg, Germany: Springer-Verlag; 2008. pp. 1671–1695. [Google Scholar]
- 8.Suzuki Y, Yanagisawa M, Ariga T, Yu RK. Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development. J. Neurochem. 2011;116:874–880. doi: 10.1111/j.1471-4159.2010.07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009;50(Suppl):S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RG. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 11.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 12.Hakomori S, Handa K, Iwabuchi K, Yamamura S, Prinetti A. New insights in glycosphingolipid function: "glycosignaling domain," a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology. 1998;8:xi–xix. doi: 10.1093/oxfordjournals.glycob.a018822. [DOI] [PubMed] [Google Scholar]
- 13.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J. Lipid Res. 2008;49:1176–1186. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshikawa M, Go S, Takasaki K, Kakazu Y, Ohashi M, Nagafuku M, Kabayama K, Sekimoto J, Suzuki S, Takaiwa K, Kimitsuki T, Matsumoto N, Komune S, Kamei D, Saito M, Fujiwara M, Iwasaki K, Inokuchi J. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc. Natl. Acad. Sci. USA. 2009;106:9483–9488. doi: 10.1073/pnas.0903279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niimi K, Nishioka C, Miyamoto T, Takahashi E, Miyoshi I, Itakura C, Yamashita T. Impairment of neuropsychological behaviors in ganglioside GM3-knockout mice. Biochem. Biophys. Res. Commun. 2011;406:524–528. doi: 10.1016/j.bbrc.2011.02.071. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Itoh Mi M, Haraguchi M, Okajima T, Inoue M, Oishi H, Matsuda Y, Iwamoto T, Kawano T, Fukumoto S, Miyazaki H, Furukawa K, Aizawa S. b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 2002;277:1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- 17.Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, Sakae M, Kishikawa M, Shiku H, Aizawa S. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. USA. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiavegatto S, Sun J, Nelson RJ, Schnaar RL. A functional role for complex gangliosides: motor deficits in GM2/GD2 synthase knockout mice. Exp. Neurol. 2000;166:227–234. doi: 10.1006/exnr.2000.7504. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW, Schnaar RL. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc. Natl. Acad. Sci. USA. 1999;96:7532–7537. doi: 10.1073/pnas.96.13.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiura Y, Furukawa K, Tajima O, Mii S, Honda T. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience. 2005;135:1167–1178. doi: 10.1016/j.neuroscience.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Inokuchi J, Sugiura Y, Furukawa K. Reduced motor and sensory functions and emotional response in GM3-only mice: emergence from early stage of life and exacerbation with aging. Behav. Brain Res. 2009;198:74–82. doi: 10.1016/j.bbr.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Sugiura Y, Furukawa K. Dysfunction of muscarinic acetylcholine receptors as a substantial basis for progressive neurological deterioration in GM3-only mice. Behav. Brain Res. 2010;206:101–108. doi: 10.1016/j.bbr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Ohmi Y, Tajima O, Ohkawa Y, Yamauchi Y, Sugiura Y, Furukawa K. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 2011;116:926–935. doi: 10.1111/j.1471-4159.2010.07067.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohmi Y, Tajima O, Ohkawa Y, Mori A, Sugiura Y, Furukawa K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc. Natl. Acad. Sci. USA. 2009;106:22405–22410. doi: 10.1073/pnas.0912336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai H, Allende ML, Wada R, Kono M, Sango K, Deng C, Miyakawa T, Crawley JN, Werth N, Bierfreund U, Sandhoff K, Proia RL. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J. Biol. Chem. 2001;276:6885–6888. doi: 10.1074/jbc.C000847200. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita T, Wu YP, Sandhoff R, Werth N, Mizukami H, Ellis JM, Dupree JL, Geyer R, Sandhoff K, Proia RL. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc. Natl. Acad. Sci. USA. 2005;102:2725–2730. doi: 10.1073/pnas.0407785102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanagisawa, M. Stem Cell Glycolipids. Neurochem. Res. 2011 doi: 10.1007/s11064-010-0358-1. in press. [DOI] [PubMed] [Google Scholar]
- 28.Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS Lett. 2010;584:1694–1699. doi: 10.1016/j.febslet.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannagi R, Cochran NA, Ishigami F, Hakomori S, Andrews PW, Knowles BB, Solter D. Stage-specific embryonic antigens (SSEA-3 and −4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 33.Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 2010;20:78–86. doi: 10.1093/glycob/cwp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanagisawa M, Yoshimura S, Yu RK. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 2011;3:e00054. doi: 10.1042/AN20110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 37.Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–514. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- 38.Tchoghandjian A, Baeza N, Colin C, Cayre M, Metellus P, Beclin C, Ouafik L, Figarella-Branger D. A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol. 2010;20:211–221. doi: 10.1111/j.1750-3639.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaida K, Ariga T, Yu RK. Antiganglioside antibodies and their pathophysiological effects on Guillain-Barre syndrome and related disorders–a review. Glycobiology. 2009;19:676–692. doi: 10.1093/glycob/cwp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- 41.Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DC, Reinkensmeier G, Wang H, Wiznitzer M, Gurtz K, Verganelaki A, Pryde A, Patton MA, Dwek RA, Butters TD, Platt FM, Crosby AH. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004;36:1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- 42.Inokuchi J. Membrane microdomains and insulin resistance. FEBS Lett. 2010;584:1864–1871. doi: 10.1016/j.febslet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L, 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol. Aging. 2009;30:1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki K, Kato K, Yanagisawa K. Aβ polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta. 2010;1801:868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Ando S, Hirabayashi Y, Kon K, Inagaki F, Tate S, Whittaker VP. A trisialoganglioside containing a sialyl alpha 2–6 N-acetylgalactosamine residue is a cholinergic-specific antigen, Chol-1 alpha. J. Biochem. (Tokyo) 1992;111:287–290. doi: 10.1093/oxfordjournals.jbchem.a123751. [DOI] [PubMed] [Google Scholar]
- 46.Hirabayashi Y, Nakao T, Irie F, Whittaker VP, Kon K, Ando S. Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J. Biol. Chem. 1992;267:12973–12978. [PubMed] [Google Scholar]
- 47.Ariga T, Yanagisawa M, Wakade C, Ando S, Buccafusco JJ, McDonald M, Yu RK. Ganglioside metabolism in a transgenic mouse model of Alzheimer's disease: expression of Chol-1α antigens in the brain. ASN Neuro. 2010;2:e00044. doi: 10.1042/AN20100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee P, Faber AC, Shelton LM, Baek RC, Chiles TC, Seyfried TN. Ganglioside GM3 suppresses the pro-angiogenic effects of vascular endothelial growth factor and ganglioside GD1A. J. Lipid Res. 2008;49:929–938. doi: 10.1194/jlr.R800006-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanda T, Yoshino H, Ariga T, Yamawaki M, Yu RK. Glycosphingolipid antigens in cultured microvascular bovine brain endothelial cells: Sulfglucuronosyl paragloboside as a target of monoclonal IgM in demyelinative neuropathy. J. Cell Biol. 1994;126:235–246. doi: 10.1083/jcb.126.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svennerholm L, Brane G, Karlsson I, Lekman A, Ramstrom I, Wikkelso C. Alzheimer disease - effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dement. Geriatr. Cogn. Disord. 2002;14:128–136. doi: 10.1159/000063604. [DOI] [PubMed] [Google Scholar]
- 51.Klenk E. Über die Ganglioside, eine neue Gruppe von zuckerhaltigen Gehirnlipoiden. Hoppe-Seyler’s Z. Physiol. Chem. 1942;273:76–86. [Google Scholar]
- 52.Yamakawa T. A reflection on the early history of glycosphingolipids. Glycoconj. J. 1996;13:123–126. doi: 10.1007/BF00731485. [DOI] [PubMed] [Google Scholar]
- 53.Svennerholm L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- 54.IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Nomenclature of glycolipids. Pure Appl. Chem. 1997;69:2475–2487. [Google Scholar]
- 55.Wu G, Lu Z-H, Kulkarni N, Amin R, Ledeen RW. Mice lacking major brain gangliosides develop Parkinsonism. Neurochem. Res. 2011 doi: 10.1007/s11064-011-0437-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]