Abstract
Regulatory T cells (Treg) suppress autoreactive immune responses and limit the efficacy of tumor vaccines; however, it remains a challenge to selectively eliminate or inhibit Treg. In this study, A20, a negative regulator of the TLR and TNFR signaling pathways, was found to play a critical role in controlling the maturation, cytokine production, and immunostimulatory potency of dendritic cells (DC). A20-silenced DCs with the spontaneous and enhanced expression of costimulatory molecules and proinflammatory cytokines have contrary effects on T cell subsets: inhibiting Treg and hyperactivating cytotoxic T lymphocytes and T-helpers that produced IL-6 and TNFα, infiltrated tumors, and were refractory to Treg-mediated suppression. Hence, this study not only identifies A20 as a critical antigen presentation attenuator in control of antitumor immune responses during both the priming and effector phases, but also provides a novel strategy to supersede Treg-mediated suppression in an antigen-specific manner, reducing the need to directly target Treg.
Introduction
Tumor vaccines, including DC vaccines1, can activate potent cytotoxic T lymphocyte (CTL) responses against self tumor-associated antigens (TAA); however, they have been largely ineffective in causing tumor regression in the clinic2-4. Moreover, autoimmune pathologies are rarely observed in the immunized patients, indicating that peripheral tolerance is still maintained at the host level5. CD4+CD25+Foxp3+ regulatory T cells (Treg) play an essential role in suppressing autoimmune responses, including those against TAA6,7. Treg were found to migrate to and accumulate inside tumors, where they inhibit the effector function of infiltrating immune cells8-11. Therefore, overcoming Treg-mediated immune suppression is required for the induction of effective anti-tumor immunity.
A direct means to overcome Treg-mediated immune suppression is to inhibit or deplete CD4+CD25+Foxp3+ Treg by using anti-CD25 antibodies or IL-2-toxin fusion proteins12-14. CD25 molecules, however, are highly expressed on activated effector T cells, and so far no unique surface marker expressed on Treg has been identified6,7. Alternatively, antagonist anti-CTLA4 or agonist anti-GITR antibodies can overcome Treg-mediated suppression by overactivating CTLs15,16. However, the non-specific overactivation of self-reactive T cells, unsatisfied clinical efficacy and pathological autoimmune toxicity, as observed in anti-CTLA4 and anti-CD28 clinical trials17-19, may restrict the clinical usefulness of these strategies. Thus, it is desirable to develop an approach that could override Treg-mediated suppression without compromising effector T cells and in an antigen-specific manner.
A20 is a zinc-finger ubiquitin-modifying enzyme and negatively regulates the TNFR and Toll-like receptor (TLR) signaling pathways20-23. A20 regulates the degradation of several key molecules, such as receptor interacting protein (RIP) and TNFR-associated factor (TRAF) 6, which are critically involved in NF-κB activation, via its dual functions of ubiquitination and deubiquitination20-24. A20-deficient mice show severe inflammation in multiple organs, and are neonatally lethal and hypersensitive to endotoxin shock and A20-deficient macrophages produce elevated levels of pro-inflammatory cytokines following stimulation with TLR ligands20-22, indicating an essential role for A20 in the maintenance of self-tolerance. In this study, we reveal a critical regulatory role of A20 in antigen presentation by DC. We demonstrate that A20 silencing endows DCs with unique abilities to hyperactivate CTLs and Th and to inhibit Treg, providing a novel strategy to supersede Treg-mediated suppression of antitumor immunity in an antigen-specific manner.
Results
A20 negatively regulates DC maturation and cytokine production
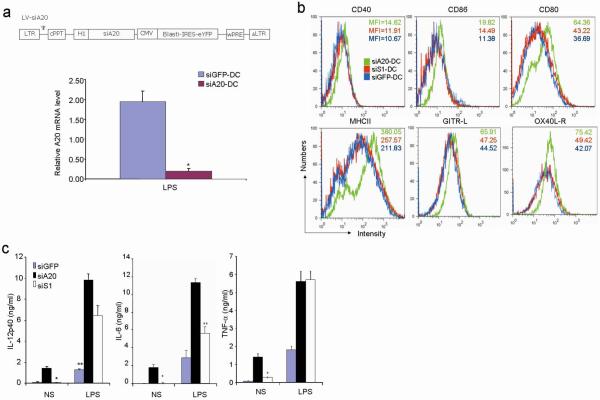
To investigate the possible role of A20 in regulating antigen presentation, we first examined the expression of A20 mRNA in myeloid DCs derived from mouse bone marrow (BM). Quantitative RT-PCR assays showed that the levels of A20 mRNA in BM-DCs were drastically induced by a TLR agonist (Supplementary Fig. 1). We generated a recombinant lentiviral vector (LV) expressing a short hairpin interfering RNA (siRNA) that effectively silences mouse A20 mRNA in DCs (Fig. 1a). To examine the role of A20 in regulating DC maturation, we used flow cytometric assays to analyze surface levels of various molecules related to antigen presentation. siA20-BM-DC showed significantly higher levels of CD80, CD86, CD40, and MHC class II than siGFP-BM-DC (Fig. 1b). The expression of glucocorticoid-induced TNFR-related protein (GITR) ligand (L) and OX40L receptor (R) was also significantly enhanced on siA20-DC (Fig. 1b). Importantly, we found that siA20-DC spontaneously produced high levels of proinflammatory cytokines such as IL-6, TNF-α, and IL-12p40 (Fig. 1c). In contrast, siGFP-DC spontaneously produced undetectable or very low levels of these cytokines. Furthermore, siA20-DC produced higher levels of these proinflammatory cytokines in response to the stimulation of TLR agonists than did siGFP-DC (Fig. 1c). LV-siA20-DC and LV-siGFP-DC had comparable viability in cell culture in the presence or absence of a TLR agonist (Supplementary Fig. 2), despite the reported anti-apoptotic role of A20 in other types of cells20. Collectively, these results indicate that A20 negatively regulates the maturation and cytokine production of DCs and that DCs with A20 knockdown are spontaneously activated and hyperactivated in response to TLR agonists.
Figure 1. A20 controls the maturation and cytokine production of BM-DCs.
a. Q-RT-PCR analysis of A20 mRNA levels of YFP+ LV-siA20-DC and LV-siGFP-DCs after stimulation with LPS for 12 hr. Experiments were repeated twice with similar results. *P < 0.01, vs. siGFP-DC. Schematic diagram of the recombinant lentiviral vector LV-siA20 (top panel) is presented. H1, H1 RNA promoter; cPPT, central polypurine tract sequence; IRES, internal ribosome entry site; wPRE, Woodchuck post-transcriptional regulatory element sequence; and siA20, A20 small hairpin interfering RNA.
b. Surface expression of molecules on CD11c+/YFP+ gated BM-DC 48 h after viral transfection using FACS analysis. Experiments were repeated three times with similar results.
c. ELISA measurement of the levels of cytokines secreted by transfected DCs (5×105 cells/ml) in response to stimulation with LPS (100 ng/ml) for 16h from one representative experiment of three. *P < 0.01, vs. siA20-DC.
A20-silenced DCs prime enhanced T cell responses in vivo
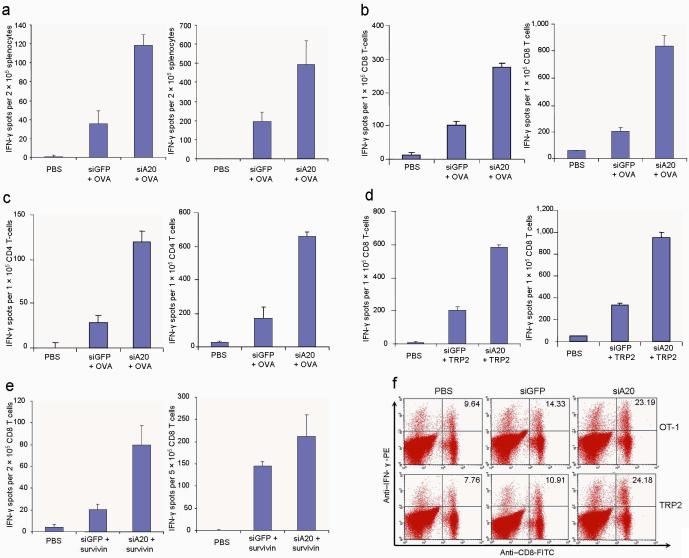
We tested whether the immunostimulatory potency of DCs was regulated by A20. Mice were immunized with ovalbumin (OVA)-pulsed, LV-DCs ex vivo matured with LPS, and the functional status of T cells was then evaluated using interferon (IFN)-γ ELISPOT assays and intracellular cytokine staining (ICS). Figs. 2a-f show that siA20-DC mice had higher numbers/frequencies of IFNγ+ CD4+ and CD8+ T cells, compared to siGFP-DC mice. Moreover, antigen-specific T-cell responses were also enhanced in mice immunized with OVA-pulsed DCs transfected with siA20 oligonucleotide duplexes (Supplementary Fig. 3). We also observed that in vivo TLR ligand (polyI:C) stimulation of mice that had been immunized with DCs 1 day earlier more effectively boosted OVA-specific CTL responses in siA20-DCs (Figs. 2a-c).
Figure 2. A20 negatively regulates the immunostimulatory potency of BM-DCs.
a-c. ELISPOT assays of CD4+ and CD8+ T cell responses against OVA. C57BL/6 mice were immunized with OVA-pulsed (100 μg/ml) LV-transduced DCs ex vivo matured with LPS followed by no (left panel) or in vivo stimulation with polyI:C (i.p.) (50 μg/mouse) (right panel) daily for 3 consecutive days. Splenocytes pooled from immunized mice (2-3/group) (a) or isolated CD8+ T cells (b) or CD4+ T cells (c) were subjected to IFNγ ELISPOT assays. Experiments were repeated three times with similar results. P < 0.05, siGFP-DC compared with siA20-DC.
d&e. ELISPOT assays of CD8+ T cell responses against self TRP-2 (d) and survivin (e). C57BL/6 or BALB/c mice were immunized with TRP2 or survivin peptide-pulsed DCs followed by no (left panel) or in vivo stimulation with polyI:C (i.p.) (right panel). IFNγ ELISPOT assays of CD8+ or CD4+ T cells isolated from pooled splenocytes (2-3 mice/group) are presented from one of three repeated experiments. P < 0.05, siGFP-DC compared with siA20-DC.
f. IFNγ intracellular staining of pooled splenocytes of mice (2-3/group) immunized with different transduced DCs followed by in vivo stimulation with PolyI:C was performed after 4 hr in vitro restimulation. Experiments were repeated twice with similar results.
We then tested whether siA20-DCs can enhance the immune response against self tumor-associated antigens, such as tyrosinase-related protein (TRP) 225,26. Figs. 2d&f show that siA20-DCs induced more potent antigen-specific CD8+ T-cell responses against mouse TRP2 and in vivo polyI:C stimulation more effectively boosted siA20-DC immunization. In agreement, CTL responses against another self antigen, survivin27,28,were also significantly enhanced in the siA20-DC mice (Fig. 2e). These data demonstrate a critical role for A20 as an antigen presentation attenuator (APA) in control of the immunostimulatory potency of DCs and the magnitude of T-cell responses.
A20-silenced DC immunization supersedes Treg-mediated suppression in vivo
We investigated whether A20-silenced DCs may induce more potent antitumor immunity with the ability to control the growth of pre-established tumors. Immunization with OVA-pulsed siA20-DC inhibited the growth of pre-established B16-OVA tumors, in contrast to the modest reduction of tumor growth in mice given OVA-pulsed siGFP-DC (Supplementary Fig. 4). Moreover, immunization with OVA-loaded DCs transfected with siA20 oligonucleotide duplexes also induced enhanced antitumor activity in an EG.7 tumor model (Supplementary Fig. 5).
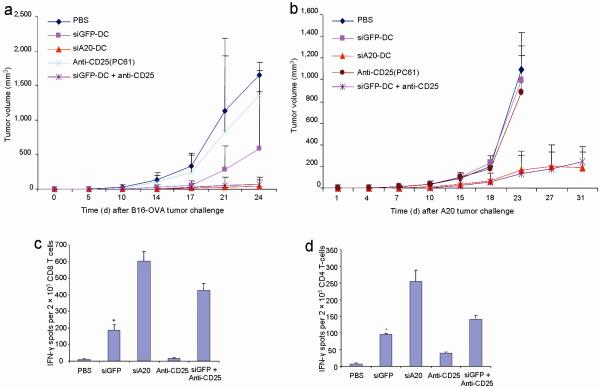
The enhanced ability to control tumor growth in wild-type mice by A20-silenced DCs prompted us to investigate whether the antitumor response induced by siA20-DCs is less sensitive to Treg-mediated suppression. We compared the antitumor potency of siA20-DC immunization with that of an anti-CD25 monoclonal antibody (clone PC61) depletion of CD25+ Treg cells or a combined siGFP-DC immunization and anti-CD25 depletion treatment. C57BL/6 mice were inoculated with B16-OVA cells and three days later treated with OVA-pulsed siA20-DC or siGFP-DC twice at a weekly interval. Anti-CD25 antibodies were administered (i.p.) into some groups of mice on days 1 and 5 prior to DC immunization, due to the high expression of CD25 on activated CD4+ and CD8+ T cells and impaired antitumor efficacy by anti-CD25 depletion after tumor vaccination29,13. A single administration of anti-CD25 antibodies was found to deplete more than 90% of CD4+CD25+ T cells for a week, which is consistent with a previous report30. Fig. 3a shows that siGFP-DCs or anti-CD25 antibody treatment alone only had moderate or marginal inhibitory effects on the growth of B16-OVA tumors. In contrast, OVA-pulsed siA20-DC immunization alone effectively inhibited the growth of pre-established B16-OVA tumors. Importantly, siA20-DC immunization only was as effective as the combined siGFP-DC and anti-CD25 treatment in controlling the growth of pre-established B16-OVA tumor. We also examined systemic T cell responses in B16-OVA tumor-bearing mice that were immunized with different regimens, as described above. Fig. 3c&d show that the frequencies of OVA-specific CD8+ T cells in spleens of the siA20-DC mice were significantly higher than those in siGFP-DC mice and in anti-CD25-treated mice.
Figure 3. Comparison of antitumor activity with anti-CD25 antibodies.
a&b. Comparison of antitumor activity against pre-established tumors. C57BL/6 or BALB/c mice were inoculated s.c. with B16-OVA (a) or A20 (b) tumor cells (2.5×105) and three days later were immunized with 1 ×106 OVA-pulsed siGFP-DC or siA20-DCs ex vivo matured with LPS twice at a weekly interval, followed by in vivo polyI:C stimulation i.p. after each DC immunization. Anti-CD25 antibodies (100 ng/mouse) were administered into some groups of mice twice on days 1 and 5 prior to DC immunization. Tumor growth curves (n=6 mice/group) represent one of two repeated experiments. P < 0.01, siGFP-DC compared with siA20-DC.
c&d. Comparison of antigen-specific T cell responses. CD8+ (c) and CD4+ (d) T-cells isolated from the pooled splenocytes of different groups of mice (2-3/group) immunized with OVA-pulsed transduced DCs with or without in vivo anti-CD25 antibody administration were subjected to IFNγ ELISPOT assays in vitro restimulated with OT-I or OT-II peptide-pulsed DCs (10 μg/ml). *P < 0.01, vs. siA20-DC.
We further compared the potency of these immunization regimens in a different tumor model, the mouse A20 B-cell lymphoma that naturally expresses survivin27. We observed that anti-CD25 treatment or mouse survivin peptide-pulsed siGFP-DC immunization alone had no apparent inhibitory effect on A20 tumor growth (Fig. 3b). The inability of anti-CD25 depletion of Treg alone to control pre-established tumors was previously reported13. In contrast, survivin-pulsed siA20-DCs, as well as the combined anti-CD25 and survivin-pulsed siGFP-DC treatment effectively inhibited the growth of pre-established A20 tumors. Taken together, these results indicate that A20-silenced, hyperactivated DCs induce an effective antitumor response capable of superseding Treg-mediated immune suppression in wild-type mice.
Superior immunostimulatory potency and antitumor activity
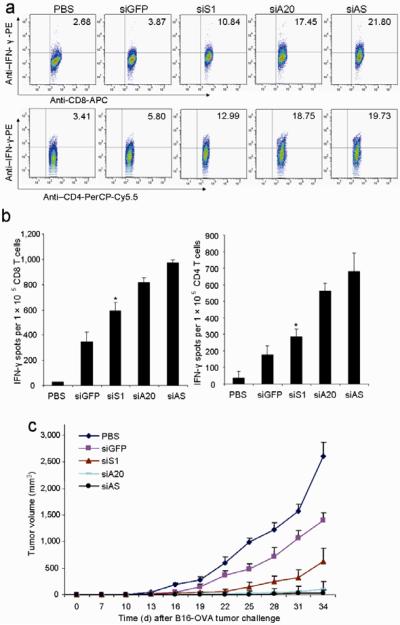
In our previous study, we demonstrated that inhibition of the suppressor of cytokine signaling (SOCS) 1, a key negative regulator of JAK/STAT signaling, enhanced the immunostimulatory potency of DCs31,32. To investigate whether siA20-DCs are superior to siSOCS1(siS1)-DCs, we immunized groups of mice with the same number of different transduced DCs pulsed with OVA protein. Figs. 4a&b show that siA20-DCs more efficiently induced antigen-specific CD8+ CTLs and CD4+ Th responses than did siS1-DCs. Since A20 and SOCS1 negatively regulate different signal transduction pathways, we investigated whether silencing of both A20 and SOCS1 has a synergistic effect on the immunostimulatory potency of DCs. It was found that DCs cotransfected with siA20 and siS1 (siAS) were slightly more potent than siA20-DCs and siS1-DCs in inducing T cell responses (Figs. 4a&b). Furthermore, siA20-DC immunization more effectively inhibited the growth of pre-established tumors than did siS1-DCs (Fig. 4c).
Figure 4. Comparison of antitumor activity with siS1-DCs.
a&b. Intracellular IFN-γ staining and ELISPOT assays. C57BL/6 mice were immunized once with OVA-pulsed, LV-transduced DCs ex vivo matured with LPS followed by in vivo stimulation with polyI:C (i.p.) once. Lymphocytes of pooled draining LN from immunized mice were subjected to intracellular staining after 4 hr in vitro restimulation (a). CD8+ T cells or CD4+ T cells isolated from pooled splenocytes of immunized mice were subjected to IFNγ ELISPOT assays (b). Experiments were repeated three times with similar results. *P < 0.05, siS1-DC vs. siA20-DC.
c. Antitumor activity against pre-established tumors. Groups of C57BL/6 mice were inoculated s.c. with B16-OVA tumor cells (2.5×105) and five days later, were immunized via the rear footpad with 1 ×106 OVA-pulsed, LV-transduced DCs with ex vivo LPS maturation (100 ng/ml). One day after DC transfer, in vivo polyI:C was administered i.p. once. Tumor growth curves (n=6 mice/group) represent one of three independent experiments. P < 0.05, siS1-DC compared with siA20-DC mice.
Contrary effects of siA20-DCs on inhibiting Treg and hyperactivating CTL/Th
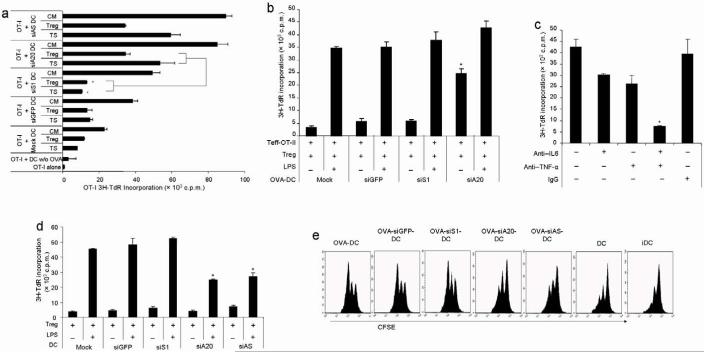
To investigate the superior immunostimulatory potency of siA20-DC, we first in vitro examined the effects of siA20-DCs on CD8+ CTLs and CD4+CD25- Th. siA20-DCs, siS1-DCs, siAS-DCs and siGFP-DCs loaded with OVA-I peptide and matured with LPS were co-cultured with CD8+ OT-I cells isolated from transgenic mice expressing T-cell receptor (TCR) specific for OVA MHC class I peptide in the presence or absence of Treg or B16 tumor lysate supernatant (TS) that contains various tumor-derived suppressive factors33. Fig. 5a shows that siA20-DCs more potently stimulated the proliferation of OT-I cells than siS1-DCs. Importantly, siA20-DCs that were pretreated with Treg cells or B16 tumor lysate were still able to effectively stimulate the proliferation of OT-I cells. In contrast, the ability of siS1-DCs to stimulate the proliferation of OT-I cells was more severely inhibited by Treg or B16 tumor lysate (Fig. 5a). In agreement, siA20-DCs were also more potent in stimulating the proliferation of CD4+CD25- OT-II isolated from transgenic mice expressing OVA MHC class II peptide-specific TCR (data not shown). These results demonstrate the superior immunostimulatory potency of A20-silenced DCs to activate CTL and Th cells even in immune suppressive environments.
Figure 5. Contrary effects of siA20-DC on CTLs and Treg.
a. Stimulatory effect on CD8+ OT-I T-cell proliferation. CD8+ OT-I T-cells (5×104/well) isolated from the spleens of OT-I transgenic mice in triplicate were co-cultured with irradiated LPS-matured transduced DCs pulsed with H-2Kb/OVA-I peptide (5×103/well). DCs were pretreated with CD4+CD25+ Treg cells isolated from naïve C57BL/6 mice (at the ratio of Treg:DC = 1:1), tumor lysate supernatant (TS) of surgically removed B16-OVA tumors at a protein concentration of 120 μg/ml, or culture medium (CM) control. After incubation for 48 h, 3H thymidine incorporation was assessed after cultivation for additional 16-18 h. Data are represent means +/-SEM (n = 3) from one of three independent experiments. *P < 0.01, siS1-DC vs. siA20-DC.
b&c. Inhibitory effects on Treg suppressive activity. CD4+CD25- OT-II Th cells (5×104/well) isolated from the spleens of OT-II transgenic mice and CD4+CD25+ Treg (5×104/well) isolated from the splenocytes of naive mice were co-cultured with OVA-II peptide-loaded, transduced BM-DCs (5×103/well) in the presence or absence of LPS for 72 h (b). For some cocultures with siA20-DCs (c), anti-IL-6 (MP5-20F3, BD Pharmingen) or/and anti-TNFα (MP6-XT3, BD Pharmingen) antibodies, or IgG control were added at final concentrations of 2 ug/ml. 3H thymidine incorporation rates were then measured and are presented from one of three independent experiments. *P < 0.05, siS1-DC vs. siA20.
d. Effects on Treg proliferation in vitro. CD4+CD25+ Treg (5×10/well) from naïve mice were stimulated with anti-CD3 (2.5 μg/ml) in the presence of transduced BM-DCs (5×103/well) with or without LPS (100 ng/ml). After 72 h incubation, 3H thymidine incorporation rates of Treg were then measured and are presented from one of three independent experiments. *P < 0.05, siS1-DC vs. siA20.
e. Effects on Treg proliferation in vivo. CD25+CD4+ OT-II Treg isolated from transgenic mice were labeled with CFSE and then injected into naive C57BL/6 mice (1×107/mouse). 1 day later, groups of mice (3/group) were administrated with 1×106 of OVA-II peptide loaded, transduced DCs matured by LPS, control LPS-matured DCs without OVA-II pulsing or DCs without LPS maturation via footpads. 3 days after DC administration, pooled cells of draining lymph nodes were assessed by flow cytometery, gated on CD25+CD4+ T cells. Data are representative of three independent experiments.
We also examined the effect of A20-silenced DCs on the suppressive activity and proliferation of Treg cells. LPS-activated DCs were all shown to have an inhibitory effect on the suppressive activity of CD4+CD25+ Treg (Fig. 5b), which is in agreement with previous reports34-37. However, only siA20-DCs without LPS maturation possessed the inhibitory effect on the suppressive activity of Treg (Fig. 5b). To investigate the mechanisms for this unique suppressive ability of siA20-DCs, we compared the levels of representative proinflammatory cytokines secreted by siA20-DCs and siS1-DCs. Fig. 1c shows that siS1-DCs without stimulation only spontaneously produced undetectable or low levels of proinflammatory cytokines; in contrast to the high levels of IL-6, TNFα, and IL-12p40 at the concentrations of 1,800, 1,410 and 1,400 pg/ml, respectively, spontaneously produced by unstimulated siA20-DCs. The levels of the proinflammatory cytokines produced by siS1-DCs in response to a TLR agonist (LPS) were also lower than those produced by siA20-DCs, although siS1-DCs produced higher levels of these cytokines than did siGFP-DCs. Moreover, there were no apparent increases in the levels of various surface costimulatory molecules on siS1-DCs, compared to those on siGFP-DCs (Fig. 1b). In contrast, the surface levels of these costimulatory molecules on siA20-DCs were significantly enhanced. Given the enhanced production of IL-6 and TNFα by siA20-DCs and the reported ability of these proinflammatory cytokines to inhibit Treg 16,34,38-42, we used antibodies against IL-6 and TNFα to examined the role of these soluble factors on Treg. Fig. 5c shows that the addition of both anti-TNFα and anti-IL-6 antibodies significantly reversed the inhibitory effect of siA20-DCs on Treg suppressive activity, while anti-TNFα or anti-IL-6 antibody alone also moderately reversed the inhibitory effect of siA20-DCs on Treg. Moreover, we compared antitumor responses induced by wt DCs and the DCs derived from TNFα KO. When we used DCs from TNFα KO mice for immunization, TNFα KO siA20-DC loaded with OVA were no longer able to effectively inhibit the growth of B16-OVA tumors (Supplementary Fig. 6). In addition, siA20-DCs derived from TNFR-/- mice (TNFR-/- siA20-DC) also exhibited a substantially reduced ability to induce tumor regression (Supplementary Fig. 7), suggesting the importance of a TNFR signaling network initiated by TNFα produced by antigen-presenting siA20-DCs.
We further investigated the effect of siA20-DCs on Treg proliferation. In contrast to their enhanced immunostimulatory potency to CTLs and Th, siA20-DCs exhibited a reduced ability to stimulate CD4+CD25+ Treg proliferation, compared with siGFP-DCs (Fig. 5d). However, siS1-DCs did not exhibit such a feature. BM-DCs without LPS maturation were unable to stimulate CD4+CD25+ Treg in the presence of anti-CD3 antibodies, in agreement with a previous report32. To confirm this in vitro observation, we examined the ability of OVA-pulsed, LPS-matured BM-DCs on OT-II CD4+CD25+ Treg proliferation in vivo. Fig. 5e shows that siA20-DCs, but not siS1-DCs, also exhibited a reduced ability to stimulate the proliferation of adoptively transferred CFSE-labeled CD4+CD25+ OT-II Treg cells in vivo. Taken together, these results demonstrate the unique contrary effects of siA20-DCs on inhibiting Treg functions and hyperactivating CTLs and Th cells.
Enhanced resistance of tumor-infiltrating effector T cells to Treg during the effector phase
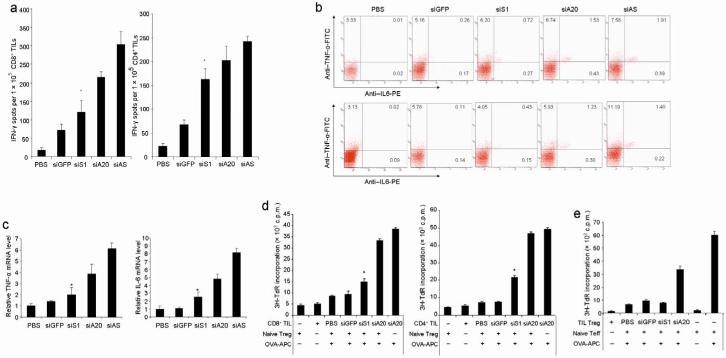
We also examined the effects of siA20-DCs and siS1-DCs on the effector phase of antitumor immune responses. C57BL/6 mice were inoculated with B16-OVA tumor cells and 7 days later immunized with OVA-pulsed modified DC, followed by in vivo PolyI:C stimulation. Tumor infiltration of activated IFNγ+ CD8+ and CD4+ T cells was enhanced in siA20-DC mice, as demonstrated by cytokine intracellular staining (data not shown). However, there was no apparent increase in Foxp3+ T cells in the TILs of siA20-DC mice. ELISPOT assays further showed higher frequencies of IFNγ-producing CD8+ and CD4+ T cells in the TIL population isolated from siA20-DC mice than those from siS1-DC mice (Fig. 6a). It was further observed that the frequencies of the CD8+ and CD4+ T cells that produced both IL-6 and TNFα in the TILs from siA20-DC mice were higher than those from siS1-DC mice (Fig. 6b). qRT-PCR assays confirmed the increased expression of IL-6 and TNFα in the TILs from siA20-DC mice, compared to those from siS1-DC mice (Fig. 6c).
Figure 6. Effects of siA20-DCs on effector T cells.
a. IFN-γ ELISPOT assays of CD8+ T cells or CD4+ T cells isolated from TILs of different groups of mice (6-8/group) 14 day after immunization with OVA-pulsed DCs or PBS control followed by in vivo stimulation with PolyI:C. Data are presented from one of three repeated experiments. *P < 0.05, siS1-DC vs. siA20-DC.
b. Intracellular staining of IL-6 and TNFα on the gated CD8+ or CD4+ T cells of TILs from different groups of mice from one of two independent experiments.
c. Q-RT-PCR assays of IL-6 and TNFα mRNA in TILs of different groups of immunized or PBS-treated mice from one of two independent experiments.
d. Enhanced proliferation of TILs in the presence of Treg. CD4+CD25- Th or CD8+ T cells isolated from the TILs of different immunized or PBS-treated groups of mice (6-8/group) (5×104/well) were co-cultured with OT-I or OT-II peptide loaded, irradiated splenic APCs (5×103/well) in the presence of CD4+CD25+ Treg (5×104/well) from naïve mice. After 3 days of coculture, the proliferation of TILs was then measured by [3H]thymidine incorporation. *P < 0.05, siS1-DC vs. siA20-DC mice.
e. Reduced suppressive activity of tumor-infiltrating Treg. CD4+ CD25- Th cells from naïve OT-II transgenic mice (5×104/well) were co-cultured with OT-II peptide-loaded, irradiated splenic APCs (5×103/well) in the presence of CD4+CD25+ Treg (5×104/well) from TILs of different DC-immunized or PBS-treated groups of mice. After 3 days of coculture, OT-II Th proliferation was then measured by [3H]thymidine incorporation. Data are presented from one of two repeated experiments. *P < 0.05, siS1-DC vs. siA20-DC.
We further examined the antigen-specific proliferative activity of these TILs in immune suppressive environments. Fig. 6d showed that both CD8+ CTL and CD4+CD25- Th isolated from TILs of siA20-DC B16-OVA-bearing mice more actively proliferated in response to OT-I or OT-II peptide-pulsed, irradiated syngeneic splenocytes as APCs even in the presence of CD4+CD25+ Treg cells isolated from naïve mice than those effector T cells isolated from siS1-DC B16-OVA-bearing mice. In these assay conditions, OT-I or OT-II peptide-pulsed, irradiated syngeneic APCs very poorly stimulated the proliferation of CD4+CD25+ Treg cells isolated from naïve mice (Fig. 6d). These results suggest the enhanced resistance of siA20-DC-activated effector T cells to Treg-mediated suppression. We also tested the suppressive activity of the tumor-infiltrating Treg isolated from TILs of different groups of mice. Fig. 6e shows that CD4+CD25+ Treg cells isolated from TILs of siA20-DC mice showed a reduced suppressive activity, compared to those from TILs of siS1-DC mice or siGFP-DC mice. Collectively, these data, together with other data (Figs. 5a-e), suggest that siA20-DCs hyperactivate effector CD4+ Th and CD8+ CTLs, leading to their enhanced tumor infiltration and resistance to Treg-mediated suppression, in part due to the enhanced production of proinflammatory cytokine such as IL-6 and TNFα by these hyperactivated effector T cells inside the tumors during the effector phase.
Discussion
The results of this study reveal that A20 negatively regulates the maturation, inflammatory cytokine production, and immunostimulatory potency of DCs, functioning as a critical antigen presentation attenuator (APA). This study further demonstrates the critical role of A20 in DCs in control of the magnitude of CD8+ and CD4+ T cell responses during both the priming and effector phases. Hence, this study provides new insights into the regulation of antigen presentation and adaptive immune responses.
This study provides a novel strategy to supersede Treg-mediated suppression of antitumor immunity in an antigen-specific manner via the hyper-activation of DCs by inhibiting A20. We demonstrated that hyperactivated A20-silenced DCs were almost as effective as the combined wt DCs and anti-CD25 depletion in controlling the growth of pre-established tumors. Further studies reveal that A20 silencing has contrary effects on DCs, endowing them with the unique ability to inhibit Treg and hyperactivate CTL and Th cells. The superior and unique immunostimulatory effect of siA20-DCs may be due to the broad roles of A20 in negative regulation of many proinflammatory signal transduction pathways20-24, as manifested by the spontaneous and higher surface expression of costimulatory molecules such as CD40, GITR-L and OX40L-R that were found to inhibit Treg’s suppressive activity16,38,43,44, and the spontaneous and stronger production of proinflammatory cytokines such as IL-6 and TNFα by A20-silenced DCs.
This study reveals the profound effect of A20-silenced DCs on the effector phase of antitumor responses. Tumor infiltration of antigen-specific, hyperactivated CTLs and Th was substantially increased upon siA20-DC immunization. These tumor-infiltrating CTL and Th in siA20-DC mice were more refractory to Treg-mediated suppression, which may be due to the increased production of IL-6 and TNFα by these TILs. Moreover, tumor-infiltrating Treg from siA20-DC mice had a reduced suppressive activity, suggesting an altered cytokine milieu inside the tumors. In supporting our findings, Korn et al. recently reported that functional Treg that accumulated in the inflammation tissues failed to inhibit pathogenic effector T cells that secreted IL-6 and TNFα, indicating that the inflammatory cytokine milieu may determine whether or not autoimmune inflammation can be controlled by Treg45. Several groups also reported the overriding of Treg suppression by overactivating CTLs15,16,38. Taken together, our study suggests that the inhibitory effects of hyperactivated siA20-DCs and tumor-infiltrating effector T cells on Treg may tip the balance from immune suppression to antitumor immunity as a collective outcome of interactive networks of pro- and anti- inflammatory factors and cells in tumor-bearing mice. This study also supports the notion that antigen-presenting DCs deficient in a critical and essential negative regulator of inflammatory signaling can hyperactivate an antitumor immune response to supersede Treg-mediated immune suppression.
Methods
Mice
C57BL/6, BALB/c, H-2Kb/OT-I-TCR (OT-I), and H-2Kb/OT-II-TCR (OT-II) transgenic mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and maintained in a pathogen-free mouse facility at the Baylor College of Medicine and University of Southern California according to institutional guidelines.
Peptides, proteins and cell lines
H2-Kb-restricted TRP2 (SVYDFFVWL) 26, H2-Kb-restricted OT-I (SIINFEKL) and OT-II (ISQAVHAAHAEINEAGR)32, and H2-Kd-restricted mouse survivin peptide (GWEPDDNPI and AFLTVKKQM)27 were synthesized and purified by HPLC to >95% purity by Genemed Synthesis Inc. (South San Francisco, CA, USA). All peptides were dissolved in DMSO before final dilution in endotoxin-free PBS (Sigma). Ovalbumin (OVA) protein was from Sigma. The mouse B-cell lymphoma A20 (H2-Kd), EG.7 thymoma (H2-Kb), B16-OVA melanoma (kindly provided by R. Dutton at Trudeau Inst.) (H2-Kb), and anti-CD25 antibody hybridoma (PC61) were obtained from American Type Culture Collection (Manassas, VA, USA).
Lentiviral vector construction, production and transduction
The HIV self-inactivating (SIN) vector used in this study was pTRIP-H1-BY-W modified from pTRIPΔU3 CMV eGFP32. Mouse A20 small hairpin interfering RNA sequence was inserted into pTRIP-H1-BY-W to generate pTRIP-siA20-BY-W (LV-A20-siRNA) that contains the A20 shRNA (5′-CTACCTGAGTTCCTTCCCCTTCAAGAGAGGGGAAGGAACTCAGGTAGTTTTT-3′). Recombinant pseudotyped lentiviral vectors were generated by co-transfection of three plasmids into 293 cells and concentrated by ultracentrifugation, as described previously46. The control LV-siGFP was generated previously32. Mouse BM-derived DCs were prepared and transduced as described in our previous study32.
Isolation of TILs, cytokine ELISA, and flow cytometric analysis
The method to isolate TILs is provided in the Supplementary Information. Levels of various cytokines were quantitated using the supernatant of cell cultures at the indicated time points using ELISA analysis (BD Biosciences) according to the manufacturer’s instructions. Cells were stained with antibody conjugates and analyzed on a FACSaria (Becton Dickinson) flow cytometer and FloJo software, as described previously31,32. The antibodies used were listed in Supplementary Information.
T cell proliferation and suppression assays
Spleens were harvested from mice, pooled, and disrupted to obtain a single cell suspension. T cells were purified by using the MACS CD8+, CD4+CD25-, or CD4+CD25+ T cell isolation kits (Miltenyi Biotec) from wt mice or OT-I or OT-II transgenic mice. For antigenic stimulation using TCR transgenic OT-I or OT-II cells and antigen-pulsed BM-DCs, a total of 5×104 purified T cells and 5×103 BM-derived DCs pulsed with OVA MHC class I or II peptides were placed in each well of a round-bottom 96-well microtiter plate in triplicate in 200 μl RPMI 1640 medium supplemented with 10% FCS. For stimulation of Treg proliferation, CD4+CD25+ Treg (5×104/well) isolated with magnetic beads from the spleens of mice were co-cultured with BM-DCs (5×103/well) in the presence of anti-CD3 (2.5 μg/ml) for 72 h35. For the suppression assays, 5×104 purified CD8+ or CD4+CD25- T cells were cocultured with 5×104 CD4+CD25+ Treg cells and 5×103 irradiated syngeneic splenic antigen-presenting cells in each well in the presence of OT-I or OT-II peptides for 72 hous45. Proliferation was measured under the indicated conditions after 2-3 days by addition of 1 μCi [3H] TdR per well for the last 16-18h of culture. Triplicate determinations were done and are representative of triplicate experiments. For in vivo proliferation, purified CD4+CD25+ OT-II Treg cells were labeled with 5 μM CFSE, and 1×107 T cell were injected i.v. into naïve mice. 1 day later, 1×106 OVA-pulsed or -unpulsed, LPS-matured BM-DCs were then injected via footpads. 3 days after DC administration, pooled cells of draining lymph nodes were assessed by flow cytometery, gated on CD25+CD4+ T cells.
DC immunization and tumor models
BM-derived DCs were transduced with LV at an MOI of 5 to 10. BM-DCs were then pulsed with peptides for 16h and after an additional 12-24h culture with LPS were washed with PBS, and then injected into mice (Jackson Laboratory) via a rear footpad. In the therapeutic model, B16-OVA or A20 tumor cells were injected subcutaneously (s.c.) into the right flank of syngeneic mice. The mice were then randomly divided into groups and injected with DCs or control. In some mice, poly(I:C) (50 μg; Invivogen) was administered intraperitoneally (i.p.) at indicated days. Tumor volumes were measured 2 or 3 times /week with an electronic caliper.
Statistical Analysis
For statistical analysis, we used Student’s t test, and a 95% confidence limit was taken to be significant, defined as p < 0.05. Results are typically presented as means ± standard errors (SE).
Additional methods
Detailed methodology is described in Supplementary Methods.
Supplementary Material
Acknowledgement
We thank Drs. Bangxing Hong, Bingya Liu, Melissa Aldrich, Natasha Lapteva, and Andrew Sharabi in the lab for technical assistance and valuable suggestions. We also thank Drs. M Brenner, H Heslop, C Rooney, RF Wang and other colleagues for helpful suggestions and assistance. This work was supported by grants from the National Institute of Health (R01CA90427, R01CA116677, and R01AI68472 to SYC, and R01 CA100841 to XFH), and the Leukemia and Lymphoma Society SCOR.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 4.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 5.Evel-Kabler K, Chen SY. Dendritic cell-based tumor vaccines and antigen presentation attenuators. Mol Ther. 2006;13:850–8. doi: 10.1016/j.ymthe.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 8.Woo EY, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with earlystage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 9.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 11.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 13.Sutmuller RP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dannull J, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GMCSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens GL, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 17.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 20.Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–4. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 22.Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–9. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 23.Sarma V, et al. Activation of the B-cell Surface Receptor CD40 Induces A20, a Novel Zinc Finger Protein That Inhibits Apoptosis. J. Biol. Chem. 1995;270:12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh T, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol. 2005;174:1507–12. doi: 10.4049/jimmunol.174.3.1507. [DOI] [PubMed] [Google Scholar]
- 25.Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J. Exp. Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Elsas A, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–9. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel S, Wagner A, Schmitz N, Zeis M. Induction of antitumour immunity using survivin peptide-pulsed dendritic cells in a murine lymphoma model. Br J Haematol. 2003;122:911–4. doi: 10.1046/j.1365-2141.2003.04535.x. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 29.Allouche M, et al. Phorbol myristate acetate induces both high affinity and low affinity interleukin 2-receptors on a pre-B leukemic cell line. Leukemia Research. 1990;14:353–61. doi: 10.1016/0145-2126(90)90163-4. [DOI] [PubMed] [Google Scholar]
- 30.Prasad SJ, et al. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. Journal of Immunology. 2005;174:90–8. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 31.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–53. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 33.Overwijk WW. Breaking tolerance in cancer immunotherapy: time to ACT. Curr Opin Immunol. 2005;17:187–94. doi: 10.1016/j.coi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 35.Kubo T, et al. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–58. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki S, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. Journal of Experimental Medicine. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. International Immunology. 2004;16:1769–80. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez-Montagut T, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–42. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 39.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King IL, Segal BM. Cutting edge: IL-12 induces CD4+CD25- T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–5. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 41.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 42.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 43.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.So T, Croft M. Cutting Edge: OX40 Inhibits TGF-beta- and Antigen-Driven Conversion of Naive CD4 T Cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 45.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroers R, Chen SY. Lentiviral transduction of human dendritic cells. Methods Mol Biol. 2004;246:451–9. doi: 10.1385/1-59259-650-9:451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.