Abstract
Objective
To examine whether greater cognitive engagement during a marital conflict discussion, as evidenced by use of words that suggest thinking and meaning-making, results in attenuated proinflammatory cytokine increases to stress and wounding.
Design
Husbands and wives (N = 84 individuals) were observed during two separate 24-hr visits: each visit included a wounding procedure, which was followed by a nonconflictive marital discussion (first visit) and a conflictive marital discussion (second visit).
Main Outcome Measures
Serum proinflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).
Results
Individuals who used more cognitive processing words during the conflict discussion (but not the nonconflictive discussion) showed smaller increases in serum IL-6 and TNF-α over 24 hours; they also had lower levels of both cytokines 24 hours after baseline controlling for demographics, hostility, depressed mood, positive and negative interactions, and marital quality. Effects of word use were not mediated by ruminative thoughts after conflict. Although both men and women benefited from their own cognitive engagement, only husbands’ IL-6 patterns were affected by spouses’ engagement.
Conclusion
In accord with research demonstrating the value of cognitive processing in emotional disclosure, this research suggests that productive communication patterns may help mitigate the adverse effects of relationship conflict on inflammatory dysregulation.
Keywords: marital stress, proinflammatory cytokines, cognitive processing words, psychoneuroimmunology, gender
It is well established that psychological stress can dysregulate immune function and lead to adverse health changes (Glaser & Kiecolt-Glaser, 2005; Graham, Christian, & Kiecolt-Glaser, 2006b). Although severe and chronic stress appears to be particularly detrimental, even relatively acute and seemingly less severe stressors (e.g., public speaking and academic examinations) are associated with maladaptive changes (Glaser & Kiecolt-Glaser, 2005; Maes et al., 1998; Segerstrom & Miller, 2004). Recent research has highlighted the ability of psychological stress to dysregulate proinflammatory cytokines (Black, 2002; Kiecolt-Glaser et al., 2003), which are proteins synthesized by immune and other cell types that affect cell replication and function. Overproduction or chronically high levels of proinflammatory cytokines indicate a disruption of homeostasis and confer a substantial risk of earlier onset and faster progression of frailty and age-related diseases such as cardiovascular disease, Type II diabetes, arthritis, and certain cancers (Ershler & Keller, 2000; Hamerman, 1999; Raison & Miller, 2003a).
Although close relationships often provide support that can buffer the effects of stress (Cohen, 2004), relationships themselves are a common source of both acute and chronic stress, as epitomized by caregiving dynamics and marital conflict. Like other psychological stressors, relationship stress can dysregulate immune function (Graham, Christian, & Kiecolt-Glaser, 2006a; Kiecolt-Glaser et al., 1997; Kiecolt-Glaser & Newton, 2001), with outcomes of clear clinical relevance including impairment of wound healing (Kiecolt-Glaser et al., 2005) and increased risk for infectious illness (Cohen, 2005; Yang & Glaser, 2002). Stress from close relationships may also contribute to increased circulating (serum) levels of proinflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). For example, married individuals who demonstrated hostile, negative behavior during a monitored discussion in a laboratory setting showed higher circulating IL-6 and TNF-α levels 24 hours after a baseline observation, particularly if their hostility took place during a discussion about a topic of contention (Kiecolt-Glaser et al., 2005).
Relationship conflict discussions in a laboratory setting are typically associated with adverse physiological changes (Graham et al., 2006a). However, individual conflict discussions may be useful to the extent that they contribute to the resolution of an important area of dispute. Such discussions in a laboratory setting typically represent true topics of disagreement: issues that have taken place in the past and which may also arise in the future (Markman, 1991). Indeed, one of the reasons that laboratory-induced conflict discussions have such strong effects is because they often involve communication patterns that persist over time, particularly among married or long-term cohabiting couples (Malarkey, Kiecolt-Glaser, Pearl, & Glaser, 1994). Moreover, the awareness of being observed during laboratory-induced conflict discussions and their time-limited nature may alleviate some of the threat of delving into a conflictive topic, because the threat of escalation is reduced. Thus, in addition to providing a window into relationship dynamics, such discussions may provide a unique opportunity for individuals to engage in and work toward resolving problematic issues.
Research on emotional disclosure more generally indicates that there can be benefits of expressing negative emotion about difficult experiences (Pennebaker, Mayne, & Francis, 1997). The bulk of this research involves written emotional expression: although the strength and consistency of findings has been challenged, participants randomly assigned to write emotionally about stressful experiences have shown psychological and emotional health benefits in the months following writing (Frattaroli, 2006; Smyth, 1998). The majority of such studies focus on general health, mood, and outcomes relevant to specific disease processes. Emotional expression interventions have also resulted in immune changes relevant to cellular function and antibody response (Pennebaker, Kiecolt-Glaser, & Glaser, 1988; Petrie, Booth, Pennebaker, Davison, & Thomas, 1995; Petrie, Fontanilla, Thomas, Booth, & Pennebaker, 2004), although no such study has examined cytokine responses to our knowledge.
The mechanisms underlying the mood and health benefits of emotional disclosure are not well established. However, at least some effects appear to be accounted for by changes in cognitive processing, or the degree to which participants show evidence of complex or high-level thinking about their negative emotions and circumstances. One widely used method of assessing cognitive processing is to count the percentage of certain words used by participants. For example, words related to insight (e.g., realize, understand) and causation (e.g., because, why) have been strongly associated with benefits of emotion-focused writing (Pennebaker et al., 1997; Pennebaker, Mehl, & Niederhoffer, 2003). One of the only studies to relate use of cognitive words to immune function found that relatively high use of cognitive words predicted greater circulating lymphocyte counts over three days of writing (Petrie, Booth, & Pennebaker, 1998). It is widely believed that people who use more cognitive processing words in emotional expression interventions are more likely to resolve (or begin resolving) their difficult experiences and feelings. This work is thus related to research showing that individuals who make meaning from or find benefit in adversity show health benefits (Park & Blumberg, 2002).
The Present Research
Participants were observed during two separate overnight visits to a hospital research unit: first following a relatively neutral (nonconflictive) marital discussion and second following a conflictive marital discussion which required that spouses discuss an area of current marital contention (Kiecolt-Glaser et al., 2005). These two visits were about two months apart and were completely comparable in protocol except for the content of the discussion. We theorized that participants’ level of cognitive engagement would be particularly meaningful during the conflictive discussion due to the opportunities it offered for working toward resolving or cognitively resolving a conflict and because of the potential adverse consequences of not engaging in an important conversation.
Both visits included a wounding protocol as part of a larger study on the effects of stress on wound healing. Physical wounds typically increase local production of inflammatory cytokines such as IL-6 and TNF-α at the wound site over the inflammatory phase of healing (Christian, Graham, Padgett, Glaser, & Kiecolt-Glaser, 2006; Werner & Grose, 2003), with a less well-established effect on peripheral cytokines. Attributable particularly to the psychological stress of this procedure in addition to the psychological stressors of not knowing what to expect at the first visit and the conflict discussion at the second visit, all participants were expected to show considerable increases in peripheral IL-6 and TNF-α levels during the period of observation. However, we hypothesized that individuals who cognitively engaged more in the conflict discussion with their spouse, as evidenced by their using more cognitive processing words during the conflict discussion, would show an attenuated cytokine response by 24 hours after that discussion compared with those who did not. In contrast, we did not anticipate that the same individuals would have shown attenuated cytokine responses as part of the earlier and otherwise identical visit, or that cognitive word used during the nonconflictive marital discussion would attenuate cytokine responses following that discussion. The 24-hr time point was the latest time point for which we had blood data and was thus chosen to enable the examination of the effect of cognitive word use outside the acute effects of stress on cytokines; we expected that the effects of cognitive engagement would emerge more strongly over time as the partners experienced the evening and night together with or without having resolved or at least engaged in the conflict session.
The effects of cognitive word use were expected to be partially accounted for by fewer ruminative, intrusive thoughts about the conflict interaction, a stronger perception of having been understood, and a stronger perception of satisfaction with the conversation by participants who used more cognitive words than others. Ruminative thoughts may maintain physiological reactivity, and are associated with both depression and immunological responses (Brosschot, Gerin, & Thayer, 2006; Nolen-Hoeksema, 2000; Thomsen et al., 2004). We also theorized that an individual’s own cognitive words would be most strongly related to his or her own cytokine levels. However, participants were also expected to benefit from their partner’s level of cognitive engagement and the average couple engagement (total percent of cognitive words by both individuals). Although meaning-making is typically construed as a personal endeavor (Park & Blumberg, 2002), a partner’s words during a meaningful discussion may not only elicit or alter the other’s engagement but may represent a shared environment likely to influence that process.
Effects of cognitive word use on both an individual and couple level basis were expected to be independent of any effect of other key variables shown previously to be relevant to physiological responses from marital conflict: depressed mood, trait hostility, marital quality, and negative and positive marital behavior during the discussions. Effects were also examined in the context of age, body mass index, socioeconomic factors, and, for individual effects, of gender. On a more exploratory basis, the amount of sleep following the marital conflict discussion was examined as a possible behavioral mediator. A better night’s sleep was expected to lead to lower IL-6 and TNF-α responses the following morning (Vgontzas et al., 2004). We speculated that individuals who expressed themselves well or who were more engaged in the conflict conversation might sleep better knowing they had made progress toward resolving an important issue.
Method
Participants
Participants (N = 84) were 42 married heterosexual couples who participated in two 24-hr admissions in a hospital research unit, the Ohio State General Clinical Research Center (GCRC). As described in greater detail elsewhere (Kiecolt-Glaser et al., 2005), participants were recruited broadly from the community as well as from campus and were screened with stringent criteria that excluded 224 couples. Exclusion criteria included health problems and medications with an obvious immunological or endocrinological component or consequences for those systems (e.g., cancer, recent surgeries, strokes, diabetes mellitus, peripheral vascular disease, conditions such as asthma or arthritis that required regular use of anti-inflammatory medications, etc.). Couples were excluded if either spouse took blood pressure medication, smoked, or used excessive alcohol or caffeine.
The average age of participants was 37.52 (SD = 13.02), with a range from 22 to 77 years. The majority (84.5%) was employed, with a median household income of between $20–30K and a mode of $30K to $40K. Participants were predominately White (88.1%) and were well educated: 26.2% had additional postgraduate training, 40.5% were college graduates, 23.8% had some college training, and 9.5% were high school graduates. Participants had been married an average of 12.55 (SD = 11.01) years, with a range of 2 to 52 years.
Overall Protocol
Admissions
Participants were admitted for 24 hours on two separate occasions to the GCRC, visits which were approximately 2 months apart (M = 2.37, SD = 1.93 months). As noted above, the two admissions were very similar in most ways. On both occasions participants remained with their spouse in the same room for the entire 24-hr visit to assure consistent physical activity across dyads and admissions. We asked couples not to drink or eat anything after midnight before the admissions; all couples were served the same meals, controlling for dietary factors such as sodium. The overall timetable was also similar on both admissions. Couples were admitted to the GCRC at 7 a.m., fed a standard breakfast, and given questionnaires to complete. A heparin well was inserted in each subject’s arm, and the baseline blood sample for cytokine assays was obtained. As part of a larger study, blister wounds were raised on each participant’s arm starting at 9:15 a.m. (Kiecolt-Glaser et al., 2005). At approximately 10:45 a.m., couples were positioned in chairs facing each other in front of a curtain, at which point they completed several questionnaires. Shortly afterward, participants began a discussion as instructed, the content of which varied between the admissions as described below. The research team remained out of sight during all discussions.
During the first admission, participants engaged in two “non-conflictive” discussion tasks. First, each spouse was asked to discuss a personal characteristic or issue he or she wanted to change, for 10 minutes each, with the stipulation that the topic not be a source of marital contention (Pasch, Bradbury, & Sullivan, 1997). During these discussions it was typical for the speaker’s partner to offer supportive comments. However, this was not mandated during instructions, which asked simply that the partner “be involved in the discussion”. Next during this admission, couples were asked to tell the story of their relationship for 30 minutes, using the Relationship History Interview (Veroff, Sutherland, Chadiha, & Ortega, 1993). Based on participant open-ended thought-listings after the visit, this was typically perceived to be a neutral, even enjoyable, discussion.
The otherwise comparable second GCRC admission instead involved a conflictive discussion task. The experimenter first conducted a 10- to 20-min interview to identify the best topics for the discussion. Based on this interview and self-report from the Relationship Problem Inventory (Knox, 1971), couples were then asked to spend 30 minutes discussing and trying to resolve the marital issues that the interviewer judged to be the most conflict producing for each of them (e.g., money, communication, or in-laws).
After completion of all study protocols, participants were fed a standard dinner and remained in the room overnight. At 7 a.m. the next morning, 24 hours after the baseline blood draw, another peripheral blood sample was obtained for cytokine assays.
Measures
Plasma cytokine levels
Plasma IL-6 and TNF-α levels were assayed using Quantikine High Sensitivity Immunoassay kits (R&D systems, Mineapolis, MN), per kit instructions, as described elsewhere (Kiecolt-Glaser et al., 2003). Samples were run undiluted in duplicate, and all samples for a couple were run at the same time.
Cognitive word use
The discussions were transcribed according to Linguistic Inquiry and Word Count software (LIWC) guidelines (Pennebaker & Francis, 1999). The LIWC software uses a dictionary of 2,290 words and word stems to generate the percentage of words in a number of different categories. The Cognitive Processing category includes 49 words indicative of causal reasoning (e.g., because, effect, hence, why, and reason), 116 words indicative of insight (e.g., think, know, consider, realize, and understand), and other words related to thinking (e.g., should, ought), with 331 total words counted. The validity of words within these categories was rated and refined by independent judges (Pennebaker & Francis, 1996, 1999). All categories—including causal reasoning, insight, and the overall cognitive processing category—have been used in studies of the effects of emotional expression (Low, Stanton, & Danoff-Burg, 2006; Pennebaker et al., 2003) and the overall cognitive processing category has been most strongly associated with aspects of immune health (Petrie et al., 1998).
Mood
Negative mood was assessed with the 10-item subscale from the Positive and Negative Affect Schedule (PANAS) before and after the discussions as well as at 24 hours after each visit baseline.
Key Control Variables
Trait hostility and depressed mood
In a questionnaire mailed prior to the first admission, participants responded to the 50 true/false questions of the Cook Medley Hostility scale (Cook & Medley, 1954), which assesses trait tendencies toward cynical attitudes, aggression, and anger responses. Depressed mood at the beginning of the conflict admission was assessed with the Beck Depression Inventory-Short Form (BDI-SF; Beck, Steer, & Garbin, 1988). The 13 items in the BDI-SF describe affective, cognitive, and vegetative symptoms; participants rate their severity of each symptom on a scale from 0 to 3. A cut-off score of 5 is typically used to differentiate depressed from nondepressed older adults (Scogin, Beutler, Corbishley, & Hamblin, 1988).
Marital quality and marital interactions during conflict
At the beginning of the first admission, a measure of Positive Marital Quality was obtained via the subscale by that name from the Positive and Negative Marital Qualities scale (Fincham & Linfield, 1997); the subscale is formed by summing 3 items on a 0–10 scale and is distinguishable from general positive and negative affectivity (Fincham & Linfield, 1997). As described in more detail elsewhere (Kiecolt-Glaser et al., 2005), marital interactions were also videotaped and interaction behaviors were coded using the Rapid Marital Interaction Coding System (Heyman, 2004). Our tapes were coded by Richard Heyman, PhD, Stony Brook University. For this research we used an index summarizing negative behaviors across three categories (psychological abuse, distress-maintaining attributions, and hostility), and an index of positive behaviors (acceptance, relationship-enhancing attributions, and constructive problem discussion).
Possible Mediator Variables
Hours of sleep the night of the conflict discussion was self-reported the next morning. We also asked participants to rate on a 1-to-5 scale the degree to which they were satisfied with the discussion with their spouse, and the degree to which they felt that their spouse understood them after the discussion. Finally, participants completed 14 items from the Impact of Events scale (Horowitz, Wilner, & Alvarez, 1979), which assessed their degree of ruminative, intrusive thoughts about the conflict discussion (e.g., “I thought about it when I didn’t mean to”; 0 = not at all, 3 = often).
Data-Analysis
A few participants were missing either an IL-6 (n = 6) or TNF-α (n = 4) value at the 24-hr follow-up time point, because of difficulty with blood draws or assays. Because missing data was minimal and apparently missing at random, to maximize use of available data these missing IL-6 and TNF-α values were replaced using the linear interpolation replacement technique via SPSS, which is less likely to lower variable standard deviation than mean replacement (McKnight, McKnight, Sidani, & Figueredo, 2007). As is typical, IL-6 and TNF-α values were highly skewed; this was corrected by using log-transformed values of both cytokines in all analyses. Marital quality was also skewed, but nontransformed values were used after determining that using transformed values did not affect results.
Analyses were performed in SPSS Version 15 (SPSS, 2005). Although the continuous variable was used in preliminary analyses, high and low users of cognitive processing words were dichotomized into high and low use groups by median splits for two reasons: to enable comparison of the same individuals across the two visits and to facilitate interpretation of key analyses in the context of typical cognitive word use, with average cognitive word use in expressive writing studies used as a guideline to confirm validity of these groups (Pennebaker & Francis, 1999). Average couple cognitive mechanism word use was similarly dichotomized. The primary analyses were conducted with analysis of covariance (ANCOVA) procedures to examine 24-hr levels of serum IL-6 and TNF-α by high or low word use, controlling for the appropriate same-visit baseline level of the cytokine being predicted. These analyses effectively test for change in cytokines over time during each of the visits. Partial eta-squared ( ) values are a recommended measure of effect size with ANCOVA (Tabachnick & Fidell, 2006). Although ANCOVA can be used to control for multiple continuous covariates, considerable power is lost and the test is particularly sensitive to multicolinearity between covariates. Thus, analyses to control for possible confounding variables and to test for mediation were performed with hierarchical linear regression analyses, controlling for baseline levels in the first step and additional variables in later steps as specified.
Results
As expected, proinflammatory cytokine levels rose over the 24-hr period of both visits, with elevations in both serum IL-6 and TNF-α levels from baseline during the conflict visit, F(1, 83) = 113.41, p < .001 and F(1, 83) = 18.93, p < .001, respectively, and comparable increases in IL-6 and TNF-α levels during the non-conflict visit, F(1, 83) = 60.05, p < .001 and F(1, 83) = 22.67, p < .001, respectively. As reported elsewhere (Kiecolt-Glaser et al., 2005), self-reported negative mood at the beginning of the first GCRC visit (nonconflict) was higher than at the beginning of the second GCRC visit (conflict), but decreased after the nonconflict discussion; in contrast, negative mood increased from conflict baseline to after the conflict discussion. Table 1 shows means and SDs for cognitive word use and key control variables. On average, participants were very satisfied with their marriage (M = 27.55, SD = 3.24) and not clinically depressed based on their BDI-SF scores (M = 2.45, SD = 2.59). Participants reported a mean of 6.86 (SD = 1.35) hours of sleep the night after the conflict visit. As expected, husbands’ and wives’ use of cognitive processing words during the conflict session was correlated, r = .43, p < .01, but different enough to justify our planned examination of individual level effects.
Table 1.
Means, SDs, and Correlations of Cognitive Processing Word Use and Key Control Variables With Serum Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) at Conflict Baseline and 24 Hours Later
| IL-6
|
TNF-α
|
%
|
||||
|---|---|---|---|---|---|---|
| Baseline | 24 hr | Baseline | 24 hr | M | SD | |
| Cognitive word use | −.15 | −.35** | −.14 | −.22* | 8.66 | 1.40 |
| Gender (higher = female) | .20† | .06 | −.11 | −.23* | ||
| Body mass index | .28* | −.10 | −.09 | −.14 | 25.96 | 5.45 |
| Age | .19† | .06 | −.12 | −.06 | 37.52 | 13.02 |
| Marital quality | −.23* | −.01 | −.20† | −.18† | 27.55 | 3.24 |
| Negative interactions | .24* | .16 | −.06 | −.17 | 8.32 | 9.70 |
| Positive interactions | −.27** | −.05 | .14 | .13 | 17.75 | 9.94 |
| Depressed mood | −.01 | −.12 | −.01 | −.11 | 2.12 | 2.79 |
| Trait hostility | .15 | .09 | .04 | −.05 | 15.29 | 7.66 |
Note. The continuous variable for cognitive word use was used for correlation analyses and to present means and standard deviations.
p < .05.
p < .01.
p < .10.
Effects of Individual’s Cognitive Word Use
As shown in Table 1, cognitive word use (as a continuous variable) was not significantly associated with IL-6 and TNF-α levels at conflict baseline, but was negatively associated with both IL-6 and TNF-α levels 24 hours after conflict baseline, r = −.35, p < .01 and r = −.22, p < .05, respectively. As expected, cognitive word use during the nonconflict visit was not associated with IL-6 levels from the same visit at either baseline or 24 hours later, r = .09, p = .41 and r = .02, p = .89, or TNF-α levels from the nonconflictive visit at either baseline or 24 hours later, r = −.05, p = .66 and r = −.09, p = .40. Later analyses thus focus on cognitive word use during the conflict session. Correlations of key control variables with IL-6 and TNF-α during the conflict visit are also shown in Table 1.
The average percentage of overall cognitive processing words used in emotion-focused writing is 7.8, based on 43 studies with diverse samples (Pennebaker & Francis, 1999). Individuals categorized as low cognitive word users (n = 39) in this study based on their word use during the conflict discussion used fewer such words than the written emotional expression average, 7.59% (SD = .80, range = 5.65% to 8.60%), while high cognitive word users (n = 45) used considerably more, 9.69% (SD = .94, range = 8.65% to 12.50%). High and low cognitive word users did not significantly differ in terms of their total words used, t(82) = .52, p = .60, or words per sentence, t(82) = −.87, p = .39. Using similar categorizations, high and low cognitive word use groups were formed on the basis of word use during the nonconflict session.
Table 2 shows means and standard deviations of IL-6 and TNF-α (raw values) at both time points and for both visits, separated by low and high cognitive word use groups on the basis of word use during the conflict session. Although the high cognitive word use group had somewhat lower levels of IL-6 at conflict baseline, a t test revealed that neither this difference t(82) = 1.40, p = .17, nor other group differences in baseline cytokine levels were significantly different for either visit. Levels of both serum IL-6 and TNF-α were significantly higher at the end of the conflict visit among individuals who used relatively few cognitive words during the conflict discussion as compared to those using more cognitive words, t(82) = 3.34, p < .001 and t(82) = 2.08, p < .05, respectively. No significant differences were found in mean levels of either IL-6 or TNF-α at the end of the nonconflict visit based on this same grouping by cognitive word use, t(82) = −.47, p = .64 and t(82) = .45, p = .65. Comparison groups based on cognitive word use during the nonconflictive session similarly showed comparable cytokine levels at nonconflict baseline and 24 hours later. Furthermore, neither word use group showed a different pattern of negative mood, at either nonconflict or conflict, with similar mean levels of negative mood at 24 hours after conflict, t(82) = 1.39, p = .17.
Table 2.
Means and Standard Deviations of IL-6 and TNF-α at Baseline and 24 Hours Later During Non-Conflict and Conflict Visits
| IL-6
|
TNF-α
|
|||
|---|---|---|---|---|
| Baseline | 24-hr | Baseline | 24-hr | |
| Nonconflict visit | ||||
| Low cognitive word use | 1.75 (1.39) | 3.91 (3.61) | 1.52 (.72) | 2.34 (1.58) |
| High cognitive word use | 1.91 (1.78) | 4.71 (4.42) | 1.54 (.69) | 2.19 (1.43) |
| Conflict visit | ||||
| Low cognitive word use | 1.73 (1.34) | 5.35 (3.85) | 1.59 (.80) | 2.16 (1.32) |
| High cognitive word use | 1.39 (.74) | 3.29 (2.83) | 1.40 (.59) | 1.70 (.97) |
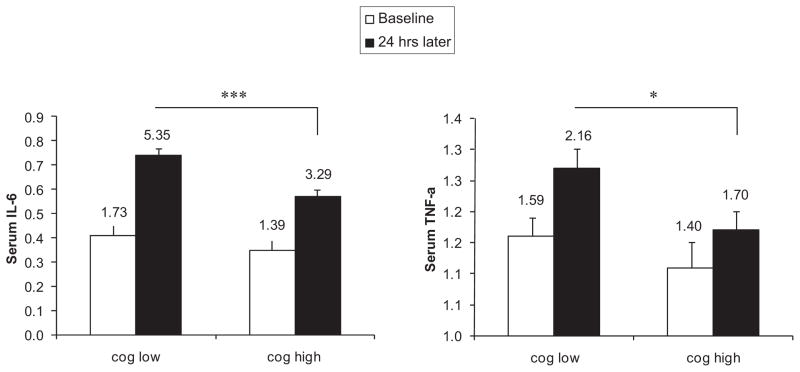
To further examine effects of cognitive word use during the conflict session, we next used ANCOVA to examine cytokine levels by group (low and high cognitive word users) at 24 hours after baseline controlling for baseline levels of the appropriate cytokine from the same visit. As expected, during the conflict discussion 24-hr IL-6 was significantly different between groups, with low users of cognitive words showing a steeper increase in IL-6 over time, F(1, 81) = 9.32, p < .01, . Furthermore, there was a similar and marginally significant group effect for cognitive word use and TNF-α, with low users of cognitive words showing steeper increases in TNF-α over time, F(1, 81) = 3.24, p = .08, . Results of all above analyses were comparable when using repeated measures analysis of variance (ANOVA), without controlling for cytokine levels, as illustrated in Figure 1.
Figure 1.
Increases in serum IL-6 and serum TNF-α (logged values) from baseline to end of conflict admission in participants with low cognitive word use compared to participants with high cognitive word use. Error bars represent standard errors of the mean (SEM). Raw values are shown above the error bars. Repeated measures ANOVA showed a significant group by time interaction for IL-6, F(1, 82) = 6.65, p < .01, and a marginally significant group by time interaction for TNF-α, F(1, 82) = 2.98, p = .09. Mean differences between groups at 24-hr time point were significant for both cytokines, as shown. * p < .05, *** p < .001.
Unique Impact of Individual’s Cognitive Word Use on Inflammatory Markers
According to a χ2 analysis, women were more likely to use cognitive words than men, χ2 = 3.88, p < .05. Neither education level nor dichotomized education level (high school vs. some college or more) was associated with cognitive word use, χ2 = 1.34, p = .72 and χ2 = 0.92, p = .34, although the tendency was for those with more education to be more likely to use cognitive words. We did not have enough ethnic diversity in the sample for detailed analyses, but Black participants did not differ significantly in cognitive word use from White participants, χ2 = 1.23, p = .27. Cognitive word use was not associated with age, body mass index, hostile or positive interactions during the conflict sessions, marital quality, ps > .23, or income, χ2 = 5.94, p = .55. However, high cognitive word users had higher depressed mood and marginally lower hostility, t(82) = −2.66, p < .01, and t(82) = 1.70, p = .09, respectively.
We used hierarchical linear regression analyses to examine the unique impact of cognitive word usage on both IL-6 and TNF-α levels at the end of the conflict visit (24-hr postbaseline). The baseline level of the appropriate inflammatory cytokine (i.e., TNF-α in analyses to predict TNF-α) was entered into the first step of these analyses. In the second step, biological risk factors were entered: gender, age, and body mass index. Although education level was not significantly associated with cognitive word use, dichotomized education level was entered in the third step because it is the marker of socioeconomic status most theoretically relevant to cognitive word use. Next, hostile interactions, positive interactions, and marital quality were entered in the fourth step; again, although marital quality, and positive and negative interactions were not associated with word use, they were entered into the equation due to their theoretical relevance and demonstrated impact on immune and inflammatory measures (Kiecolt-Glaser et al., 2005; Suarez, 2003). Depressed mood, and hostility were entered in the sixth step, with cognitive word use group (high vs. low cognitive word use) entered in the final step. As shown in Table 3, the effect of cognitive word use on neither IL-6 nor TNF-α was significantly diminished after controlling for all possible confounding variables. High use of cognitive words uniquely predicted lower IL-6, t(72) = −2.20, p < .05, β = −.26, and marginally predicted lower TNF-α, t(73) = −1.76, p = .09, β = −.18, after controlling for all potential confounds as well as baseline levels.
Table 3.
Summary of Hierarchical Regressions to Predict Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) 24-Hours After Baseline
| Variable | IL-6
|
TNF-α
|
||||||
|---|---|---|---|---|---|---|---|---|
| SE | β | ΔR2 | ΔF | SE | β | ΔR2 | ΔF | |
| Step 1 | .17*** | 15.64*** | .34*** | 38.12*** | ||||
| Baseline cytokine | .17 | .42*** | .12 | .58*** | ||||
| Step 2 | .05 | 1.48 | .04 | 1.58 | ||||
| Gender | .05 | .02 | .04 | −.17 | ||||
| Body mass index | .01 | −.23* | .00 | −.12 | ||||
| Age | .00 | .03 | .00 | .02 | ||||
| Step 3 | .00 | .03 | .00 | .42 | ||||
| Education | .10 | −.02 | .09 | .06 | ||||
| Step 4 | .02 | .67 | .04 | 1.70 | ||||
| Marital quality | .01 | .11 | .01 | −.20† | ||||
| Negative interactions | .00 | .15 | .00 | −.19† | ||||
| Positive interactions | .00 | .12 | .00 | .02 | ||||
| Step 5 | .03 | 1.13 | .03 | 1.83 | ||||
| Depressed mood | .01 | −.21 | .01 | −.19 | ||||
| Trait hostility | .00 | .13 | .00 | −.05 | ||||
| Step 6 | .05* | 4.83* | .02† | 2.75† | ||||
| Cognitive word use | .06 | −.26* | .05 | −.17† | ||||
Note. Each step also contains the variables above it, such that only effects above and beyond those variables are reported.
p < .05.
p < .001.
p < .10.
Mediation Analyses
We hypothesized that the effect of individual cognitive word use on changes in IL-6 or TNF-α would be mediated by cognitive changes. Specifically, we expected that individuals who used more cognitive words would have fewer intrusive, ruminative thoughts about the conflict interaction, would feel like their spouse better understood them, and would feel more satisfied after the conflict, and that these variables would account for the effects of cognitive word use on the inflammatory markers. Typically, to be considered a possible mediator a variable must be correlated with both the predictor and the criterion variable (Baron & Kenny, 1986). None of the proposed mediator variables above were significantly correlated with cognitive word use. The perception of being understood was marginally associated with lower IL-6 24 hours after baseline (r = −21, p = .07), but none of the other variables were associated with IL-6 or TNF-α. Moreover, controlling for perceptions of being understood as a separate step in the hierarchical linear regression analyses reported above did not alter the unique impact of cognitive word use group on IL-6. Our more exploratory behavioral mediator (hours of sleep during the conflict admission) was not associated with either IL-6 or TNF-α 24 hours after baseline.
Effects of Cognitive Word Use by Couple
Repeated measures ANCOVAs were used to examine cytokine changes for husbands and wives, based on either their spouses’ cognitive word use during the conflict session (high or low) or their average word use as a couple (high or low), controlling for baseline levels of the appropriate cytokine at baseline from the nonconflict discussion visit. Couple-average cognitive word use predicted husbands’ IL-6 change over time: There was a group by time interaction, with low-use couple conversations resulting in a steeper increase in IL-6 over time for husbands, F(1, 39) = 6.97, p < .01, . Spouse cognitive word use also predicted husband’s IL-6 change over time with a similar pattern, F(1, 39) = 5.56, p < .01, . Neither spouse nor couple-average cognitive word use significantly predicted changes in TNF-α over time of husbands, and neither spouse nor average couple-average cognitive word use predicted changes in wives’ IL-6 or TNF-α.
Using hierarchical linear regression to control for all variables shown in Table 3 other than gender, including baseline IL-6 levels, age, education, depressed mood, and hostility, both spouse and couple-average cognitive word use uniquely predicted husband’s IL-6 levels at the end of the conflict session, t(32) = −2.15, p < .05, β = −.36 and t(32) = −1.98, p < .05, β = −.35, respectively.
Discussion
Following each of two separate overnight admissions to a hospital research unit (GCRC) with protocols that differed only in the content of a marital discussion, levels of two proinflammatory cytokines—IL-6 and TNF-α—rose over a 24-hr period; these changes were attributed to a wounding procedure incorporated in the larger study as well as the psychological stress of not knowing what to expect (relevant particularly to the first, nonconflict discussion visit) and the psychological stress of the conflict visit tasks (second admission) (Kiecolt-Glaser et al., 2005). As reported elsewhere (Kiecolt-Glaser et al., 2005), those who engaged in negative, hostile behavior showed a significantly greater increase in IL-6 and TNF-α at 24 hours after baseline compared with those who did not, particularly if that hostile behavior was exhibited during the conflict session. In the present research, individuals who took the opportunity to more fully cognitively engage in the conflict discussion with their spouse, as evidenced by their greater use of cognitive processing words (words indicative of thinking, such as because, think, and ought), showed significantly attenuated cytokine responses following that discussion: In addition to showing less of an increase in both IL-6 and TNF-α over time, higher cognitive word users showed lower absolute levels of both cytokines 24 hours after baseline, a result which was not substantially affected by controlling for age, gender, body mass index, income, education, generally positive or negative interactions during the conflict discussion, marital quality, trait hostility, or depressed mood. In contrast, the same individuals did not show attenuated cytokine responses during the earlier nonconflict visit, illustrating that individuals categorized as “high cognitive word users” in this study were not consistently low responders. Similarly, the use of cognitive words during the nonconflict discussions was not significantly associated with cytokine responses following that discussion, indicating that the attenuation seen in the latter session was related to the dynamics of the conflict session specifically.
Results suggest that the act of cognitive immersion in a laboratory conflict marital discussion, as indicated by cognitive word use, had an anti-inflammatory effect: an attenuation of response to the multiple stressors that were part of the larger study. Although it may seem unusual that a focus on conflict seems beneficial given that conflict discussions typically result in increased blood pressure and have resulted in immune-related impairment among those who exhibit hostile behavior (Kiecolt-Glaser & Newton, 2001), this finding is in concordance with previous work showing that the engagement in marital conflict is adaptive and important for marital satisfaction (Markman, 1991; Sher & Weiss, 1991). Marital satisfaction, in turn, has been associated with a better response to influenza vaccine (Phillips et al., 2006). This is the first study to our knowledge to show a change in inflammatory responses using an analysis of specific word use. One advantage of such a technique is that it is not biased by conscious processes. Instead, examining word use in the context of an intimate conversation provides an unobtrusive window into individuals and the cognitive aspects of their relationships (Pennebaker et al., 2003; Sillars, Shellen, McIntosh, & Pomegranate, 1997). Rather than being indicative of a direct effect of word use on inflammation, however, it is likely that cognitive processes reflected in word use serve to change sympathetic-adrenal-medullary activity, which is a primary stimulus for stress-related inflammatory changes. Purely psychological stress can lead to cellular changes via catecholamine secretion in response to adrenergic activation (Bierhaus et al., 2003). Recent research has shown that anticipatory cognitive appraisals can affect these stress systems, as well as the expression of cytokines (Wirtz et al., 2007).
The pattern of results—with cognitive word use associated with cytokines following conflict but not nonconflict discussions—is also consistent with research showing benefits of cognitive processing in emotional expression about stressful events in particular (Pennebaker et al., 1997). In the context of a conflictive laboratory discussion, the high use of cognitive words suggests an active process of meaning making, a process that involves beginning to understand and perhaps even resolve conflict-producing issues of great personal relevance. We expected that greater cognitive engagement would lead to fewer intrusive, ruminative thoughts and that such cognitive factors would account for its benefits. However, neither ruminative thoughts the morning after the conflict discussion nor satisfaction with the discussion or perceptions of being understood mediated the effect of cognitive word use on cytokine responses. It is possible that more than a day is needed before perspective gained from cognitive engagement in a laboratory conflict session results in broader cognitive perceptions, or that participants were not yet able to verbalize their perceptions so soon after the event. It is also possible that the present research shows not so much a positive benefit of cognitive processing but rather some detriment in those who failed to take advantage of an opportunity to engage with their spouse about a topic of real-life conflict and who may therefore have found it stressful to sit in the same room with their spouse for many hours afterward. However, the evidence is consistent with at least some benefit of cognitive processing, perhaps with effects in both directions: the highest 24-hr IL-6 values across both visits were seen in the low cognitive word users post conflict, and the lowest IL-6 values were seen among the high cognitive word users post conflict.
We anticipated that individuals would benefit from their partner’s level of engagement and the aggregate level of engagement during the conflict discussion, albeit less strongly than from their own cognitive engagement. Both couple-average and spousal cognitive word use predicted husbands’ changes in IL-6 and uniquely predicted husbands’ absolute levels at 24 hours after baseline. Neither couple-average nor spousal cognitive word use predicted IL-6 patterns for wives, nor TNF-α patterns for either wives or husbands. It thus appears that wives’ level of cognitive engagement more strongly influenced husbands’ cytokine patterns in this study than vice versa, which is in concordance with research showing that husbands benefit more from marriage (Kiecolt-Glaser & Newton, 2001). Women in Western culture are often thought to be more relationship-directed than men and to take on more of the burden for resolving relationship conflict (Silverstein, Bass, Tuttle, Knudson-Martin, & Huenergardt, 2006) and may perhaps be more adept at cognitive engagement in such a setting or likely to influence their partners to think more broadly. In support of this possibility, wives were more likely than husbands to use cognitive words in the present study.
The changes in raw (nonlogged) values of IL-6 seen in this research suggest potential clinical significance: Average raw values of IL-6 at the end of the conflict visit were 5.35 pg/ml among low cognitive word users as compared with 3.29 pg/ml among high cognitive word users. Moreover, this research was conducted with relatively well-functioning and healthy couples and may thus underestimate the risk of relationship conflict. Because problematic communication patterns persist in close relationships and multiple episodes of transient stress may dysregulate proinflammatory responses more generally (McEwen, 1998; Raison & Miller, 2003b), the effects observed in the present research may have significant implications for health. It should be noted, however, that additional work is needed to establish causal connections between transient stress episodes and chronic dysregulation; for example, the repeated experience of acute marital stress may not necessarily lead to chronically high levels of inflammation.
Although it would have been ideal, counterbalancing between visit type (conflict or nonconflict) was not possible in this study. Several factors, however, make it unlikely that this issue accounted for our results. First, baseline levels of the cytokines were similar at each visit. Second, although participants reported greater negative mood at the onset of the first visit, negative mood levels were comparable by the end of each visit. Moreover, because the stress of the first visit coincided with the nonconflict visit, this issue is more likely to have made the two groups more similar and minimized rather than contributed to the results of this research. However, because we found differences between word use groups at the conflict visit, when participants were not experiencing the additional stress of a first visit (as evidenced by lower initial negative mood), this issue is unlikely to have accounted for our results.
Complementing literature indicating immune and health benefits of meaning making, benefit finding, and cognitive processing of stressful events (Park & Blumberg, 2002; Pennebaker et al., 1997; Urcuyo, Boyers, Carver, & Antoni, 2005), this research thus suggests that effective and productive communication patterns may help mitigate inflammatory responses to psychological and physical stress. Attempting to improve communication patterns between couples and examining longer term cytokine profiles to stress more broadly may prove a fruitful area of intervention and may further elucidate the dramatic effects of close relationships on health.
Acknowledgments
This research was supported in part by National Institutes of Health (NIH) Training grant T32AI55411, grants AG16321, DE13749, and MH18831, and General Clinical Research Center grant MO1-RR-0034, and Ohio State University Comprehensive Cancer Center Core grant CA16058.
Contributor Information
Jennifer E. Graham, Department of Biobehavioral Health, The Pennsylvania State University
Ronald Glaser, Institute for Behavioral Medicine Research and the Department of Molecular Virology, Immunology, and Medical Genetics, The Ohio State University College of Medicine.
Timothy J. Loving, Department of Human Development and Family Sciences, The University of Texas at Austin
William B. Malarkey, Institute for Behavioral Medicine Research and the Department of Molecular Virology, Immunology, and Medical Genetics, The Ohio State University College of Medicine
Jeffrey R. Stowell, Department of Psychology, Eastern Illinois University
Janice K. Kiecolt-Glaser, Institute for Behavioral Medicine Research and the Department of Psychiatry, The Ohio State University College of Medicine
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain, Behavior and Immunity. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. The American psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S. The Pittsburgh Common Cold Studies: Psychosocial predictors of susceptibility to respiratory infectious illness. International Journal of Behavioral Medicine. 2005;12:123–131. doi: 10.1207/s15327558ijbm1203_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Ershler W, Keller E. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fincham FD, Linfield KJ. A new look at marital quality: Can spouses feel positive and negative about their marriage? Journal of Family Psychology. 1997;11:489–502. [Google Scholar]
- Frattaroli J. Experimental disclosure and its moderators: A meta-analysis. Psychological Bulletin. 2006;132:823–865. doi: 10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Graham JE, Christian LM, Kiecolt-Glaser JK. Close relationships and immunity. In: Ader R, editor. Psychoneuroimmunology. Vol. 2. Burlington, MA: Elsevier, Inc; 2006a. pp. 781–798. [Google Scholar]
- Graham JE, Christian LM, Kiecolt-Glaser JK. Stress, age, and immune function: Toward a lifespan approach. Journal of Behavioral Medicine. 2006b;29:389–400. doi: 10.1007/s10865-006-9057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman D. Toward an understanding of frailty. Annals of Internal Medicine. 1999;130:945–950. doi: 10.7326/0003-4819-130-11-199906010-00022. [DOI] [PubMed] [Google Scholar]
- Heyman RE. Rapid marital interaction coding system (RMICS) In: Kerig PK, Baucom DH, editors. Couple observational coding systems. Mahwah, NJ: Erlbaum; 2004. pp. 67–94. [Google Scholar]
- Horowitz M, Wilner N, Alvarez WA. Impact of Events Scale: A measure of subjective stress. Psychosomatic Medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, et al. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosomatic Medicine. 1997;59:339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. Marriage happiness. Champaign, IL: Research Press; 1971. [Google Scholar]
- Low CA, Stanton AL, Danoff-Burg S. Expressive disclosure and benefit finding among breast cancer patients: Mechanisms for positive health effects. Health Psychology. 2006;25:181–189. doi: 10.1037/0278-6133.25.2.181. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De JR, Van GA, Kenis G, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Malarkey W, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosomatic Medicine. 1994;56:41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Markman HJ. Constructive marital conflict is NOT an oxymoron. Behavioral Assessment. 1991;13:83–96. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McKnight PE, McKnight KM, Sidani S, Figueredo AJ. Missing data: A gentle introduction. New York: The Guilford Press; 2007. [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Park CL, Blumberg CJ. Disclosing trauma through writing: Testing the meaning-making hypothesis. Cognitive Therapy and Research. 2002;26:597–616. [Google Scholar]
- Pasch LA, Bradbury TN, Sullivan KT. Social support in marriage: An analysis of intraindividual and interpersonal components. In: Pierce GR, Lakey B, Sarason IG, Sarason BR, editors. Sourcebook of social support and personality. New York: Plenum Press; 1997. pp. 229–256. [Google Scholar]
- Pennebaker JW, Francis ME. Cognitive, emotional, and language processes in disclosure. Cognition and Emotion. 1996;10:601–626. [Google Scholar]
- Pennebaker JW, Francis ME. Linguistic Inquiry and Word Count: LIWC. Mahwah, NJ: Erlbaum; 1999. [Google Scholar]
- Pennebaker JW, Kiecolt-Glaser JK, Glaser R. Disclosure of traumas and immune function: Health implications for psychotherapy. Journal of Consulting and Clinical Psychology. 1988;56:239–245. doi: 10.1037//0022-006x.56.2.239. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Mayne TJ, Francis ME. Linguistic predictors of adaptive bereavement. Journal of Personality and Social Psychology. 1997;72:863–871. doi: 10.1037//0022-3514.72.4.863. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Mehl MR, Niederhoffer KG. Psychological aspects of natural language use: Our words, our selves. Annual Review of Psychology. 2003;54:547–577. doi: 10.1146/annurev.psych.54.101601.145041. [DOI] [PubMed] [Google Scholar]
- Petrie KJ, Booth RJ, Pennebaker JW. The immunological effects of thought suppression. Journal of Personality and Social Psychology. 1998;75:1264–1272. doi: 10.1037//0022-3514.75.5.1264. [DOI] [PubMed] [Google Scholar]
- Petrie KJ, Booth RJ, Pennebaker JW, Davison KP, Thomas MG. Disclosure of trauma and immune response to a hepatitis B vaccination program. Journal of Consulting and Clinical Psychology. 1995;63:787–792. doi: 10.1037//0022-006x.63.5.787. [DOI] [PubMed] [Google Scholar]
- Petrie KJ, Fontanilla I, Thomas MG, Booth RJ, Pennebaker JW. Effect of written emotional expression on immune function in patients with human immunodeficiency virus infection: A randomized trial. Psychosomatic Medicine. 2004;66:272–275. doi: 10.1097/01.psy.0000116782.49850.d3. [DOI] [PubMed] [Google Scholar]
- Phillips A, Carroll D, Burns V, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain, Behavior, and Immunity. 2006;20:279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Depression in cancer: New developments regarding diagnosis and treatment. Biological Psychiatry. 2003a;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003b;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Scogin F, Beutler L, Corbishley A, Hamblin D. Reliability and validity of the short form Beck Depression Inventory with older adults. Journal of Clinical Psychology. 1988;44:853–857. doi: 10.1002/1097-4679(198811)44:6<853::aid-jclp2270440604>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher TG, Weiss RL. Negativity in marital communication: Where’s the beef? Behavioral Assessment. 1991;13:1–5. [Google Scholar]
- Sillars A, Shellen W, McIntosh A, Pomegranate M. Relational characteristics of language: Elaboration and differentiation in marital conversations. Western Journal of Communication. 1997;61:403–422. [Google Scholar]
- Silverstein R, Bass LB, Tuttle A, Knudson-Martin C, Huenergardt D. What does it mean to be relational? A framework for assessment and practice. Family Process. 2006;45:391–405. doi: 10.1111/j.1545-5300.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Smyth JM. Written emotional expression: Effect sizes, outcome types, and moderating variables. Journal of Consulting and Clinical Psychology. 1998;66:174–184. doi: 10.1037//0022-006x.66.1.174. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS 15.0. Chicago, IL: SPSS; 2005. [Google Scholar]
- Suarez EC. Plasma interleukin-6 is associated with psychological coronary risk factors: Moderation by use of multivitamin supplements. Brain, Behavior & Immunity. 2003;17:296–303. doi: 10.1016/s0889-1591(03)00059-x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Boston: Allyn & Bacon; 2006. [Google Scholar]
- Thomsen DK, Mehlsen MY, Hokland M, Viidik A, Olesen F, Avlund K, et al. Negative thoughts and health: Associations among rumination, immunity, and health care utilization in a young and elderly sample. Psychosomatic Medicine. 2004;66:363–371. doi: 10.1097/01.psy.0000127688.44363.fb. [DOI] [PubMed] [Google Scholar]
- Urcuyo KR, Boyers AE, Carver CS, Antoni MH. Finding benefit in breast cancer: Relations with personality, coping, and concurrent well-being. Psychology & Health. 2005;20:175–192. [Google Scholar]
- Veroff J, Sutherland L, Chadiha L, Ortega RM. Newlyweds tell their stories: A narrative method for assessing marital experiences. Journal of Social and Personal Relationships. 1993;10:437–457. [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. Journal of Clinical Endocrinology & Metabolism: Clinical and Experimental. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiological Reviews. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von Kanel R, Emini L, Suter T, Fontana A, Ehlert U. Variations in anticipatory cognitive stress appraisal and differential proinflammatory cytokine expression in response to acute stress. Brain, Behavior, and Immunity. 2007;21:851–859. doi: 10.1016/j.bbi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Yang EV, Glaser R. Stress-associated immunomodulation and its implications for responses to vaccination. Expert Review of Vaccines. 2002;1:453–459. doi: 10.1586/14760584.1.4.453. [DOI] [PubMed] [Google Scholar]