Targeted therapeutics for cancer patients have evaded clinicians and researchers for decades. Since the identification of the first oncogene, Src, in 1970, new oncogenes have been discovered and have provided clues to the origin of oncogenesis and the potential cellular pathways that are deregulated. In the early 1990s, cyclin D1, a critical regulator of G1 to S phase transition, was identified to be a putative oncogene through experimentations in yeast and identification of cyclin D1 over-expression in various types of tumors.1
Although cyclin D1 is found to be overexpressed in many different types of human cancers, not all tumors have genetic lesions leading to chromosomal translocations or amplifications of the cyclin D1 gene. The overexpression of cyclin D1 leads to an increase in G1 to S phase transition promoting oncogenesis.
Cyclin D1 is important in the regulation of cell cycle, but was found to be inconsequential for the survival of cells. Genetic mouse models have demonstrated that cyclin D1 is not essential for the development of mice. The genetic knockout of cyclin D1 in mice were indeed viable, while transgenic mice overexpressing cyclin D1 in mammary cells led to the development of hyperplasias and neoplasias.2,3 These mouse models suggest that cyclin D1 can function in oncogene addiction. Oncogene addiction is a term used to describe the phenomenon where the over activity of a specific oncogene is required for cancer cells to survive and proliferate.4 These oncogenes are thus prime targets for drug development to block their oncogenic effects on cancer cells, while not affecting the viability of normal healthy cells. Cyclin D1 is a perfect candidate as a target for oncogene addiction; however the cause of the overexpression of cyclin D1 is not completely understood.
Recently it was uncovered that cyclin D1 is a very unstable protein with a short half-life. This was further supported by the identification of two E3 ubiquitin-protein ligases, SCFFBX4-αB crystalline and FBXW8, which were both capable of targeting cyclin D1 for ubiquitin-mediated proteasomal degradation.5,6 Interestingly, although the disruption of the E3 ligase activities led to the accumulation of cyclin D1, only the disruption of SCFFBX4-αB crystalline led to the increased speed of cell cycle progression, while the knockdown of FBXW8, prevented cyclin D1 from entering the nucleus and resulted in the slower rate of cell proliferation. Cyclin D1 accumulation is a clear example of oncogene addiction and provides a framework for the importance of ubiquitination on its oncogenic activity. In order to target the factors that specifically promote cyclin D1 degradation, it is essential to identify the endogenous deubiquitinating enzyme specific to cyclin D1. In a screen of potential deubiquitination enzymes, Shan et al. identified USP2 to specifically deubiquitinate cyclin D1, preventing the degradation of cyclin D1, and leading to the accumulation of cyclin D1.7 This accumulation induced a rapid progression of the cell cycle from the G1 to S phase. The knockdown of USP2 destabilized cyclin D1 levels in both human cancer cells and normal fibroblasts, but it only affected the cell growth and proliferation of cancer cells, demonstrating a specific effect on cancer cells. These data further supports the role of cyclin D1 in oncogene addiction and also presents USP2 as a novel drug target to block its activity and reduce the proliferation of cancer cells in a safe and non-toxic manner.
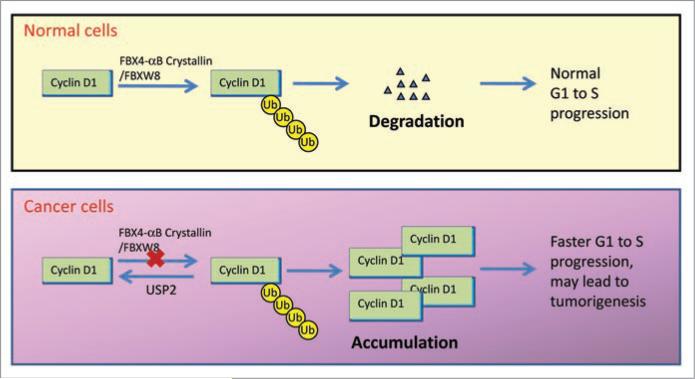
Cyclin D1 is overexpressed in an array of human cancers and has been demonstrated to be essential for the proliferation of many types of cancers. This dependence on cyclin D1 presents a novel example of oncogene addiction. The ability of USP2 to counteract the E3 ligase activity in regulating cyclin D1 stability suggests that USP2 is a critical regulator of cyclin D1-mediated oncogene addiction (Fig. 1). The ability to develop drugs that can specifically block the activity of USP2 will contribute greatly to a targeted therapeutic that is safe and effective in halting tumorigenesis.
Figure 1.
Cyclin D1 stability is regulated by ubiquitination-dependent proteasomal degradation. In normal cells, cyclin D1 degradation allows for normal G1 to S phase progression. In cancer cells, the cyclin D1 ubiquitination pathway is impaired by defects in the E3 ligase or upregulation of USP2, leading to cyclin D1 accumulation, accelerating G1 to S phase progression and increasing tumorigenesis.
References
- 1.Motokura T, Arnold A. Cyclin D and oncogenesis. Curr Opin Genet Dev. 1993;3:5–10. doi: 10.1016/s0959-437x(05)80334-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–71. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 3.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–72. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–66. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okabe H, Lee SH, Phuchareon J, Albertson DG, McCormick F, Tetsu O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS One. 2006;1:128. doi: 10.1371/journal.pone.0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan J, Zhao W, Gu W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell. 2009;36:469–76. doi: 10.1016/j.molcel.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]