Abstract
Vascular endothelial dysfunction develops with aging, as indicated by impaired endothelium-dependent dilation(EDD), and is related to increased cardiovascular disease risk. We hypothesized that short-term treatment with fenofibrate, a lipid-lowering agent with potential pleiotropic effects, would improve EDD in middle-aged and older normolipidemic adults by reducing oxidative stress. Brachial artery flow-mediated dilation (FMD), a measure of EDD, was assessed in 22healthy adults aged 50-77 years before and after 7days of fenofibrate (145 mg/d; n=12/7M) or placebo (n=10/5M). Brachial FMD was unchanged with placebo, but improved after 2 and 7 days of fenofibrate (5.1±0.7 vs. 2d: 6.0±0.7 and 7d: 6.4±0.6 %Δ; both P<0.005). The improvements in FMD after 7 days remained significant (P<0.05) after accounting for modest changes in plasma total and LDL-cholesterol. Endothelium-independent dilation was not affected by fenofibrate or placebo (P>0.05). Infusion (i.v.) of the antioxidant vitamin C improved brachial FMD at baseline in both groups and during placebo treatment (P<0.05), but not after 2 and 7 days of fenofibrate (P>0.05). Fenofibrate treatment also reduced plasma oxidized LDL, a systemic marker of oxidative stress, compared with placebo (P<0.05). In vascular endothelial cells sampled from peripheral veins of the subjects, endothelial nitric oxide synthase (eNOS) protein expression was unchanged with placebo and after 2 days of fenofibrate, but was increased after 7 days of fenofibrate (0.54±0.03 vs. 2d: 0.52±0.04 and 7d: 0.76±0.11 intensity/HUVEC control; P<0.05 7d). Short-term treatment with fenofibrate improves vascular endothelial function in healthy normolipidemic middle-aged/older adults by reducing oxidative stress and induces increases in eNOS.
Keywords: aging, endothelial-dependent dilation, flow-mediated dilation
Cardiovascular disease risk increases progressively with advancing age, at least in part, from the development of vascular endothelial dysfunction.1, 2 Indeed, vascular endothelial function, most commonly assessed by endothelium-dependent dilation (EDD), is impaired in middle-aged/older adults even in the absence of clinical disease.3, 4 As such, it is clinically important to identify potential treatment strategies that can improve vascular endothelial function in healthy middle-aged/older adults.
Fenofibrate is a commonly prescribed lipid-lowering agent that can exert pleiotropic effects beyond lipid lowering by activation of peroxisome proliferator-activated receptor α (PPARα).5 In old rats, fenofibrate improves EDD in the microcirculation (i.e., small mesenteric arteries).6 However, it is unknown if fenofibrate treatment can improve EDD in middle-aged/older humans.
Age-related impairments in EDD are a result of increased oxidative stress and decreased bioavailability of the vascular protective vasodilator, nitric oxide (NO).3, 7 In old rats, improvements in EDD with fenofibrate are mediated by reductions in oxidative stress,6 but this mechanism has not been established in humans. In cultured endothelial cells8 and the aorta of rats,9 fenofibrate also induces increases in endothelial nitric oxide synthase (eNOS), the enzyme that synthesizes NO in the endothelium. However, the effect of fenofibrate on eNOS expression in humans has not been investigated.
We hypothesized that fenofibrate would improve EDD, as measured by brachial artery flow-mediated dilation (FMD), in middle-aged/older adults and that reduced oxidative stress is an important mechanism for these improvements. We also hypothesized that fenofibrate might increase eNOS protein expression in endothelial cells in these subjects. To test these hypotheses, we performed a prospective, randomized, double-blind study of fenofibrate vs. placebo. To minimize the potential influence of marked and sustained reductions in plasma lipids, we studied only normolipidemic adults and used short-term (7 days) administration of fenofibrate with outcomes assessed at an early time point (2 days), as well as end-intervention.
Methods
Subjects
Twenty-four healthy middle-aged/older (50-77 years) men and women were enrolled in this study. All subjects had total cholesterol <240 mg/dl, LDL-cholesterol <160 mg/dl, triglycerides <200 mg/dl, fasting blood glucose <110 mg/dl, resting blood pressure <140/90 mmHg, body mass index (BMI) <35 kg/m2 and were nonsmokers and otherwise free of clinical diseases as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG. All subjects were sedentary, defined as no regular exercise (<30 min/day, <2 days/week) during the previous 2 years. Subjects were not taking medications and refrained from consumption of antioxidants (e.g., vitamins C and E) and aspirin within 2 weeks of the start of study involvement. All procedures were approved by the Institutional Review Board of the University of Colorado at Boulder. The nature, benefits and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Procedures
All measurements were performed at the University of Colorado at Boulder Clinical and Translational Research Center after a 12-hour fast and 24-hour abstention from alcohol and physical activity.
Subject Characteristics and blood analyses
For details on the measurement of subject characteristics and blood analyses, please see the online supplement.
EDD and endothelium-independent dilation
EDD (brachial FMD), endothelium-independent dilation (response to sublingual glyceryl trinitrate, GTN) and shear rate were assessed in the supine position using duplex ultrasonography (Power Vision 6000, Toshiba) with a linear array transducer, as previously described by our laboratory.3, 10 FMD and GTN responses are expressed as percent change from baseline and absolute change (mm) in diameter, per recent recommendations.11 FMD was measured first during saline infusion (control) and then during supraphysiological intravenous infusion of vitamin C as previously described.3 Further details can be found in the online supplement.
Endothelial cell protein expression
Venous endothelial cells collected from an antecubital vein were analyzed for eNOS expression, as described in detail previously by our laboratory.12, 13 For details of the methodology, see the online supplement.
Fenofibrate administration
In a double-blind parallel group design, subjects were assigned to receive 7 days of fenofibrate (145mg/day, TriCor, Abbott Laboratories, Abbott Park, IL) or placebo by block randomization. Subjects were instructed to take fenofibrate with their evening meal. Experimental visits (EDD and EID measurements, blood and endothelial cell collection) were conducted the morning before the 1st dose was taken (“baseline”) and the morning after the 2nd and 7th doses. Originally, 7 men were randomized to the placebo group, but 2 of these men withdrew before completing the final experimental visits and their data are not included in the analyses.
Data analysis
Statistical analyses were performed with IBM SPSS (version 20, Armonk, NY). Differences in subject characteristics between groups were assessed by t-tests for independent sample comparisons. A 2×2 repeated-measures ANOVA was performed to identify a group (fenofibrate or placebo) × time (baseline, 2 days, 7 days) interaction for FMD and other variables. In the case of significant interactions, a paired t-test for within group contrast and independent t-test for between group comparisons was performed with Bonferonni correction. The differences in FMD within subjects during saline infusion vs. vitamin C infusion on the same day were analyzed by paired t-test. Pearson correlation analysis was used to assess bivariate relations between the change in %FMD and the change in variables that could influence FMD. Multiple linear regression was used to determine the effect of fenofibrate on FMD while controlling for potentially confounding variables. Significance was set at P<0.05. Values are mean ± SE.
Results
Clinical characteristics
At baseline, subjects randomized to the fenofibrate or placebo groups did not differ in age, body mass index, waist:hip ratio, blood pressure, lipids or fasting glucose (Table 1). The response to short-term fenofibrate treatment was not different than the response to placebo for arterial blood pressure, fasting blood glucose, insulin, HOMA-IR, plasma HDL-cholesterol, triglycerides or C-reactive protein (CRP)(Table 2). Fenofibrate lowered plasma total cholesterol at 2 and 7days and LDL-cholesterol at 7days (P<0.05). No changes were observed in the placebo group.
Table 1. Group subject characteristics.
| Characteristic | Fenofibrate | Placebo |
|---|---|---|
| N (m/f) | 12 (7/5) | 10 (5/5) |
| Age, yr | 63 ± 2 | 63 ± 2 |
| Body mass index, kg/m2 | 24 ± 1 | 27 ± 1 |
| Waist:Hip Ratio | 0.84 ± 0.02 | 0.88 ± 0.03 |
| SBP, mmHg | 119 ± 3 | 122 ± 3 |
| DBP, mmHg | 76 ± 3 | 77 ± 1 |
| Total cholesterol, mg/dl | 207 ± 10 | 205 ± 9 |
| LDL-C, mg/dl | 128 ± 9 | 126 ± 5 |
| HDL-C, mg/dl | 57 ± 3 | 58 ± 8 |
| Triglycerides, mg/dl | 108 ± 10 | 105 ± 11 |
| FBG, mg/dl | 94 ± 2 | 92 ± 3 |
Data are mean±SE. SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; FBG, fasting blood glucose.
Table 2. Clinical characteristics.
| Characteristic | Fenofibrate | Placebo | RM ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Day 2 | Day 7 | Baseline | Day 2 | Day 7 | P value | |
| SBP, mmHg | 117 ± 5 | 116 ± 5 | 113 ± 4 | 112 ± 3 | 112 ± 4 | 113 ± 3 | 0.20 |
| DBP, mmHg | 72 ± 3 | 69 ± 3 | 68 ± 3 | 69 ± 2 | 69 ± 2 | 70 ± 2 | 0.13 |
| Total cholesterol, mg/dL | 192 ± 9 | 183 ± 9 * | 167 ± 6 * | 184 ± 11 | 189 ± 10 | 187 ± 9 | 0.001 |
| LDL-C, mg/dl | 119 ± 8 | 117 ± 8 | 103 ± 5 * | 115 ± 7 | 120 ± 7 | 118 ± 6 | 0.007 |
| HDL-C, mg/dl | 52 ± 3 | 51 ± 4 | 50 ± 4 | 47 ± 7 | 49 ± 6 | 49 ± 5 | 0.58 |
| Triglycerides, mg/dL | 108 ± 15 | 75 ± 8 | 71 ± 11 | 106 ± 12 | 99 ± 11 | 101 ± 16 | 0.12 |
| FBG, mg/dL | 92 ± 1 | 92 ± 2 | 92 ± 2 | 98 ± 3 | 97 ± 3 | 96 ± 3 | 0.46 |
| Insulin, μU/L | 10 ± 1 | 10 ± 1 | 11 ± 2 | 11 ± 2 | 12 ± 1 | 11 ± 1 | 0.28 |
| HOMA-IR | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.4 ± 0.2 | 2.8 ± 0.5 | 2.9 ± 0.4 | 2.7 ± 0.3 | 0.44 |
| oxLDL, mg/dL | 56 ± 5 | 53 ± 5 | 45 ± 3 * | 58 ± 3 | 55 ± 4 | 53 ± 3 * | 0.04 |
| CRP, mg/L | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 | 1.0 ± 0.2 | 1.3 ± 0.5 | 1.0 ± 0.2 | 0.60 |
Data are mean±SE. RM, repeated measures; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; FBG, fasting blood glucose; HOMA-IR, homeostasis model assessment-insulin resistance; oxLDL, oxidized LDL; CRP, C-reactive protein.
P<0.01 vs. baseline within group
EDD
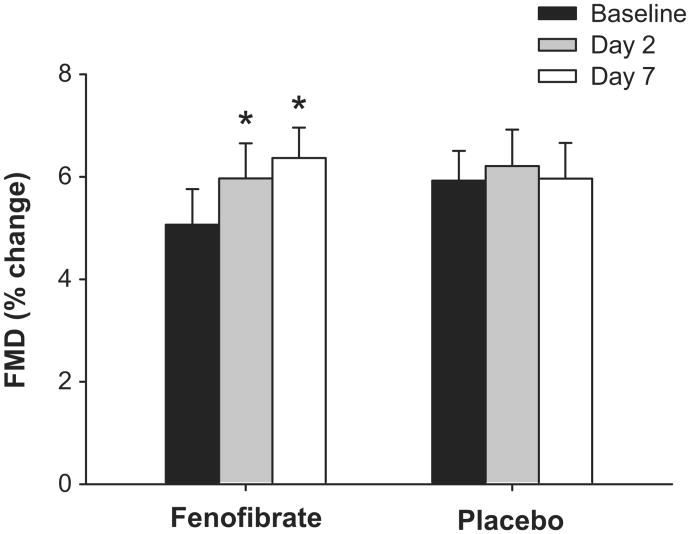
At baseline, brachial FMD did not differ between the fenofibrate and placebo group (%ΔFMD: P=0.35; mmΔFMD: P=0.17). Brachial FMD improved by ∼20% after 2 days of fenofibrate (P<0.005) and by ∼30% after 7 days of fenofibrate (P≤0.001) (Figure 1, Table 3). There was no change in brachial FMD after 2 or 7 days of placebo. Baseline brachial artery diameter, peak shear rate after cuff release and brachial artery dilation to GTN(endothelium-independent dilation) did not differ between groups or within subjects over time (Table 3).
Figure 1.
Flow-mediated dilation (FMD, percent change in diameter) at baseline and after 2 and 7 days of fenofibrate or placebo. Values are mean±SE. *P<0.05 vs. baseline for same group.
Table 3. Brachial artery characteristics.
| Characteristic | Fenofibrate | Placebo | RM ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Day 2 | Day 7 | Baseline | Day 2 | Day 7 | P value | |
| BA diameter, mm | 3.65 ± 0.23 | 3.66 ± 0.23 | 3.65 ± 0.23 | 3.74 ± 0.18 | 3.66 ± 0.18 | 3.66 ± 0.17 | 0.28 |
| FMD, mm | 0.17 ± 0.02 | 0.21 ± 0.02 * | 0.23 ± 0.02 * | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.03 | 0.02 |
| GTN, % change | 23 ± 2 | 24 ± 2 | 23 ± 2 | 27 ± 1 | 27 ± 2 | 26 ± 2 | 0.77 |
| GTN, mm change | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.41 |
| Peak SR, 1/s | 904 ± 101 | 979 ± 138 | 955 ± 123 | 981 ± 115 | 969 ± 150 | 977 ± 95 | 0.28 |
Data are mean±SE. RM, repeated measures; BA, brachial artery; FMD, flow-mediated dilation; GTN, dilation to glyceryl trinitrate; SR, shear rate.
P<0.005 vs. baseline within group.
Oxidative Stress
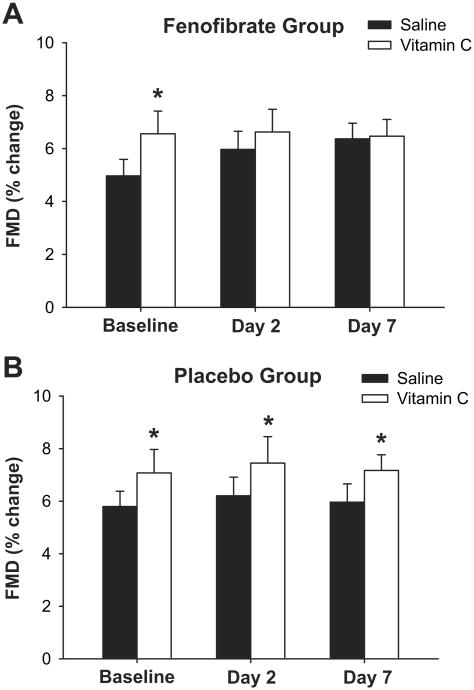
Intravenous infusion of the antioxidant vitamin C improved brachial FMD at baseline for both groups by ∼25% (P<0.05, Figure 2, Table S1,2). After 2 and 7 days of fenofibrate, brachial FMD was no longer changed in response to vitamin C (P>0.05, Figure 2A, Table S1). In contrast, the improvement in brachial FMD with vitamin C seen at baseline was maintained in the placebo group after 2 and 7 days (P<0.05)(Figure 2B, Table S2). Baseline brachial artery diameter and peak shear rate were not affected by vitamin C in either group at any time point (Table S1,2).
Figure 2.
Flow-mediated dilation (FMD, percent change) during intravenous saline and vitamin C infusion at baseline and after 2 and 7 days of fenofibrate (A) or placebo (B). Values are mean±SE. *P<0.05 vs. saline infusion on same day.
Oxidized LDL (oxLDL), a circulating (systemic) marker of oxidative stress, was not different between groups at baseline (Table 3). There was no change in oxLDL after 2 days of fenofibrate or placebo (Table 3). After 7 days of fenofibrate, oxLDL decreased by 21% (P<0.005, Table 3), which was significantly greater than the slight decrease observed with placebo(8%, ANOVA interaction P<0.05).
Endothelial cell eNOS
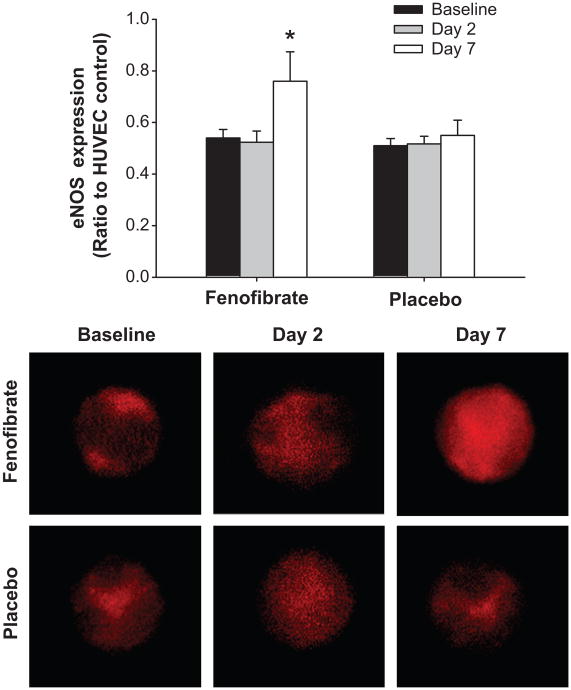
Endothelial cell expression of eNOS protein did not differ between groups at baseline (Figure 3). Endothelial eNOS did not change after 2 days of fenofibrate, but increased by 41% after 7 days of treatment (P<0.05). There was no change in eNOS after 2 or 7 days of placebo.
Figure 3.
Endothelial nitric oxide synthase (eNOS) protein expression in venous endothelial cells collected at baseline and after 2 and 7 days of fenofibrate or placebo. Values are fluorescent intensity normalized for human umbilical vein endothelial cell (HUVEC) control intensity. Representative images shown below. Values are mean±SE. *P<0.05 vs. baseline for same group.
Potential correlates of EDD
After 2 days, the change in brachial FMD in response to fenofibrate was related to only the change in total cholesterol (r=-0.62, P<0.05), and accounting for changes in total cholesterol rendered the effect on brachial FMD non-significant (P=0.22). After 7 days, the change in brachial FMD in response to fenofibrate was related to only the change in systolic blood pressure (r=-0.59, P<0.05) and diastolic blood pressure (r=-0.71, P<0.05). However, multiple linear regression analysis revealed that fenofibrate treatment significantly affectedthe change in brachial FMD (P<0.05), whereas systolic and diastolic blood pressure did not (P>0.05).
The potential impact of baseline brachial FMD, although not significant between groups, also was addressed with subgroup comparisons. In a subset of subjects matched for baseline FMD (fenofibrate: 5.65%, placebo: 5.60%, n=9/group), the change in brachial FMD over time was still significantly greater for the fenofibrate, compared to the placebo, treated group (repeated measures ANOVA interaction: P<0.01).
Discussion
We demonstrate for the first time that fenofibrate improves EDD in healthy normolipidemic middle-aged/older men and women, and that the improvements are mediated by reduced oxidative stress. We also show for the first time in humans that fenofibrate induces increased endothelial cell eNOS protein expression. These results indicate that fenofibrate may be an effective strategy to treat vascular endothelial dysfunction even in middle-aged/older adults with normal plasma lipids.
EDD
The improvements in brachial FMD in this study were specific to the vascular endothelium, as there were no changes in endothelium-independent dilation with fenofibrate treatment. Fenofibrate improvesEDD in individuals with type 2 diabetes14, 15 and hyperlipidemia,16-19 and we now extend these findings to show that fenofibrate improves endothelial function in healthy normolipidemic middle-aged/older adults. Ourresults here in conduit arteries of middle-aged/older humans also are consistent with earlier observations that fenofibrate improves EDD in mesenteric resistance arteries of old rats.6
Oxidative stress
Beyond its lipid lowering influence, fenofibrate is believed to exert pleiotropic effectson other potentially adverse factors in patients with hyperlipidemia. Here we demonstrate that fenofibrate lowers plasma oxLDL, a marker of systemic oxidative stress, in normolipidemic middle-aged/older adults, in agreement with a previous report in patients with hypertriglyceridemia.20 In the present study,however, we extend these findings by showing that vitamin C improves brachial FMD at baseline, but not after fenofibrate treatment. This indicates that oxidative stress impaired EDD in our subjects at baseline and that fenofibrate improved EDD by reducing the tonic suppression of endothelial function by oxidative stress. The present results also translationally extend previous observations that administration of antioxidants improves EDD assessed ex vivo in vehicle treated old rats, but not in old rats receiving fenofibrate.6
eNOS
eNOS is the key enzyme that produces NO in the vascular endothelium in healthy adults.21 In the present study, we report for the first time that endothelial cell eNOS protein is increased in humans with fenofibrate treatment. This finding is consistent with previous data showing that fenofibrate increases eNOS protein expression in cultured endothelial cells8, 22 and the aorta of rodents.9 In contrast, eNOS protein expression was reported not to change and NO was reported not contribute to the improvements in dilation in response to acetylcholinein the small mesenteric arteries of old rats with fenofibrate treatment.6 It is possible that fenofibrate may influence eNOS and the NO contribution to acetylcholine-induced dilation of small resistance arteries differently than in conduit arteries of humans in response to flow (present study), or in aorta or cell culture.8, 22 We were unable to measure the NO contribution to dilation using the brachial FMD model, and thus cannot determine if the increased eNOS expression with fenofibrate had functional benefit.
Potential modulating factors
For this study, we were interested in the pleiotropic effects of fenofibrate and sought to minimize and/or account for the potential influence of changes in clinical characteristics that could influence EDD. In particular we attempted to avoid the effects of marked, long-term reductions in plasma lipids by studying only normolipidemic individuals treated with fenofibrate for a short period. Even though total and LDL-cholesterol decreased modestly with treatment, the improvements in brachial FMD at 7 days were not related to plasma lipids. Although our results suggest that improvements in endothelial function after one week of fenofibrate were independent of changes in lipids, we cannot completely discount an influence of the latter on brachial FMD as it may have been masked by the variability associated with plasma lipid measurements, particularly triglycerides. It also is possible that the relation between changes in vascular function and plasma lipids with fenofibrate treatment differ in normolipidemic adults and patients with hyperlipidemia. However, in patients with hypertriglyceridemia or diabetes, improvements in EDD with fenofibrate have been reported to be both related to15, 17 and independent of19 reductions in lipids. Moreover, in patients with normal plasma lipids, the reduction in CVD events with fibrate (gemfibrozil) treatment is only weakly related to changes in lipids.23 Thus, our results after 7 days of fenofibrate administration are consistent with the range of observations in previous studies, but provide potential support for the non-lipid lowering effects of fenofibrate in mediating its vascular health-promoting influence in humans.
With regard to arterial blood pressure, the effect of fenofibrate is inconsistent, with reports of no effect24 or decreases in diastolic blood pressure25, 26 in disease populations, and even evidence for elevations in young healthy subjects.27 Although blood pressure was not significantly reduced with fenofibrate in the present study, there was a relation between changes in systolic and diastolic blood pressure and improvements in brachial FMD after 7 days of fenofibrate among individual subjects. However, results of our regression analysis indicate that the improvements in brachial FMD were independent of changes in blood pressure.
The actions of fenofibrate are thought to be mediated primarily via activation of PPARα, a nuclear transcription factor that increases fatty acid oxidation and can suppress oxidative stress and inflammatory signaling.5 Assessment of PPARα activity requires a reporter assay or a measure of DNA binding that cannot be performed with the limited number of cells available from endothelial biopsy in human subjects. However, 7 days of fenofibrate treatment in rodents is sufficient to increase PPARα activity in cardiac and liver tissue28, 29 and studies utilizing PPARα knockout mice demonstrate that the vascular and oxidative stress-related effects of fenofibrate are PPARα–dependent.30-32 In contrast, a recent study found that a brief (25 minute) ex vivo incubation with PPARα agonist GW7647 improved EDD in mesenteric arteries from adults <60years of age with cardiovascular risk factors, but not in older adults, and postulated that the latter might be due to a reduction in PPARα receptor expression at older ages.33 Thus, PPARα activation may have contributed to the improvements in EDD, reductions in oxidative stress and increases in eNOS expression in the middle-aged/older adults treated with fenofibrate in the present study, but this remains to be proven. The beneficial effects of fenofibrate also may have been the result of PPARα-mediated reductions in inflammatory signaling. Fenofibrate treatment did not reduce CRP, but CRP is a circulating inflammatory marker that does not necessarily reflect vascular inflammatory status. Thus, it is possible that reduced vascular inflammatory signaling by PPARα activation contributed to the improvements in endothelial function observed. PPARα protein expression decreases with aging in a number of tissues34-36 and targeting this pathway with medications such as the fibrates could be important in preventing/treating many age-related dysfunctions.
Lipid lowering medications in normolipidemic individuals
The use of lipid lowering medications in normolipidemic individuals is controversial. Use of these agents in healthy normolipidemic adults is supported by the observations that optimal LDL-C is lower than current guidelines37 and, thus, lipid lowering agents could be used to attain optimal LDL-C targets, and because medications such as statins and fibrates have pleiotropic benefits beyond lipid lowering. For example, in the JUPITER trial, rosuvastatin decreased cardiovascular events in normolipidemic individuals with high CRP.38 However, the potential adverse side-effects of statins and fibrates argue that their use be limited to specific populations with demonstrated benefit, such as normolipidemic adults with high CRP or possibly of older age, in addition to patients with clinical dyslipidemia.
Limitations
There are a few limitations of the present study. We only assessed conduit artery function and can only speculate on if improvements might also occur in resistance arteries. However, fenofibrate does improve EDD in mesenteric arterioles of old rats.6 In addition, we assessed eNOS in endothelial cells obtained from venous sampling because the non-invasive vascular measurements and short-term intervention period did not support repeated placements of arterial catheters over the 7-day treatment period. We have previously demonstrated that endothelial cell samples obtained from peripheral veins and arteries of the same subjects show good agreement for eNOS protein (r=0.81).12 Lastly, we performed a short-term fenofibrate treatment and do not know the effect of long-term fenofibrate treatment in this subject population.
Perspectives
Here we demonstrate for the first time that short-term treatment with fenofibrate improves vascular endothelial function in healthy normolipidemic middle-aged/older adults. The improvements in EDD were mediated, at least in part, by reduced vascular oxidative stress and are concomitant with relatively sizeable reductions in plasma oxLDL, a marker of systemic oxidative stress. After 7 days of fenofibrate, the improvements in EDD are not related to changes in plasma lipids, but are associated with increases in eNOS protein expression. Thus, fenofibrate has pleiotropic effects beyond lipid-lowering that enhance vascular endothelial function even in healthy normolipidemic middle-aged/older adults. Fenofibrate and other strategies that target PPARα-related signaling pathways may hold promise for the treatment of age-related vascular dysfunction and prevention of cardiovascular disease.
Supplementary Material
Table S1: Brachial artery characteristics in response to vitamin C in fenofibrate treatment group.
Data are mean±SE. BA, brachial artery; FMD, flow-mediated dilation; SR, shear rate. *P<0.05 vs. saline within day.
Table S2: Brachial artery characteristics in response to vitamin C in placebo treatment group.
Data are mean±SE. BA, brachial artery; FMD, flow-mediated dilation; SR, shear rate. *P<0.05 vs. saline within day.
Novelty and Significance.
What is New?
This is the first study to demonstrate a beneficial effect of fenofibrate on vascular endothelial function in healthy, normolipidemic middle-aged and older adults.
For the first time, a direct connection is established between improved vascular function with fenofibrate treatment and reduced oxidative stress in humans.
This is the first study to collect vascular endothelial cells and assess changes in protein expression following fenofibrate treatment. We were able to show for the first time that fenofibrate induces an increase in protein expression of endothelial nitric oxide synthase (eNOS) in humans.
What is Relevant?
Older individuals have a greater risk for cardiovascular diseases, at least in part, as a result of reduced vascular endothelial function. Thus, it is important to identify potential treatment strategies to improve vascular endothelial function in older adults.
This study demonstrates that fenofibrate and strategies that target related signaling pathways may hold promise for the treatment of age-related vascular dysfunction and prevention of cardiovascular disease.
Summary
Short-term fenofibrate treatment improves vascular endothelial function in healthy, normolipidemic middle-aged and older adults. These improvements are independent of changes in lipids and are a result of reduced oxidative stress. Protein expression of eNOS, the key enzyme synthesizing nitric oxide in the endothelium, increased in endothelial cells collected from middle-aged and older adults following fenofibrate treatment.
Acknowledgments
We would like to thank Eric Chung, Amber Hull and Livia Tsien for technical assistance.
Sources of Funding: This work was supported by National Institutes of Health awards AG031617, AG006537, AG013038, AG022241, AG000279, and RR00051 and American Heart Association 0715735Z.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A “Set up” For vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 5.Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor-alpha activators. Hypertension. 2005;46:1086–1092. doi: 10.1161/01.HYP.0000187900.36455.4c. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez de Sotomayor M, Mingorance C, Andriantsitohaina R. Fenofibrate improves age-related endothelial dysfunction in rat resistance arteries. Atherosclerosis. 2007;193:112–120. doi: 10.1016/j.atherosclerosis.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of no availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 8.Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, Saito H, Kouhara H, Kasayama S, Kawase I. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–663. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- 9.Blanco-Rivero J, Marquez-Rodas I, Xavier FE, Aras-Lopez R, Arroyo-Villa I, Ferrer M, Balfagon G. Long-term fenofibrate treatment impairs endothelium-dependent dilation to acetylcholine by altering the cyclooxygenase pathway. Cardiovasc Res. 2007;75:398–407. doi: 10.1016/j.cardiores.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Playford DA, Watts GF, Best JD, Burke V. Effect of fenofibrate on brachial artery flow-mediated dilatation in type 2 diabetes mellitus. Am J Cardiol. 2002;90:1254–1257. doi: 10.1016/s0002-9149(02)02847-3. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton SJ, Chew GT, Davis TM, Watts GF. Fenofibrate improves endothelial function in the brachial artery and forearm resistance arterioles of statin-treated type 2 diabetic patients. Clin Sci (Lond) 2010;118:607–615. doi: 10.1042/CS20090568. [DOI] [PubMed] [Google Scholar]
- 16.Capell WH, DeSouza CA, Poirier P, Bell ML, Stauffer BL, Weil KM, Hernandez TL, Eckel RH. Short-term triglyceride lowering with fenofibrate improves vasodilator function in subjects with hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2003;23:307–313. doi: 10.1161/01.atv.0000046230.02211.b4. [DOI] [PubMed] [Google Scholar]
- 17.Koh KK, Han SH, Quon MJ, Yeal Ahn J, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005;28:1419–1424. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 18.Kon Koh K, Yeal Ahn J, Hwan Han S, Kyu Jin D, Sik Kim H, Cheon Lee K, Kyun Shin E, Sakuma I. Effects of fenofibrate on lipoproteins, vasomotor function, and serological markers of inflammation, plaque stabilization, and hemostasis. Atherosclerosis. 2004;174:379–383. doi: 10.1016/j.atherosclerosis.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Malik J, Melenovsky V, Wichterle D, Haas T, Simek J, Ceska R, Hradec J. Both fenofibrate and atorvastatin improve vascular reactivity in combined hyperlipidaemia (fenofibrate versus atorvastatin trial--fat) Cardiovasc Res. 2001;52:290–298. doi: 10.1016/s0008-6363(01)00382-0. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Steffen BT, Cao J, Tsai AK, Ordovas J, Straka R, Zhou X, Kabagambe E, Hanson NQ, Arnett D, Tsai MY. Effects of fenofibrate on plasma oxidized ldl and 8-isoprostane in a sub-cohort of goldn participants. Atherosclerosis. 2011;214:422–425. doi: 10.1016/j.atherosclerosis.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming I, Busse R. No: The primary edrf. J Mol Cell Cardiol. 1999;31:5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Lu C, Li F, Wang H, He L, Hao Y, Chen AF, An H, Wang X, Hong T, Wang G. Ppar-alpha agonist fenofibrate upregulates tetrahydrobiopterin level through increasing the expression of guanosine 5′-triphosphate cyclohydrolase-i in human umbilical vein endothelial cells. PPAR Res. 2011;2011:523520. doi: 10.1155/2011/523520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: Va-hit: A randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 24.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: The diabetes atherosclerosis intervention study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 25.Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop PH, Taskinen MR. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: The field helsinki substudy. Diabetes Care. 2010;33:215–220. doi: 10.2337/dc09-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chew GT, Watts GF, Davis TM, Stuckey BG, Beilin LJ, Thompson PL, Burke V, Currie PJ. Hemodynamic effects of fenofibrate and coenzyme q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31:1502–1509. doi: 10.2337/dc08-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian S, DeRosa MA, Bernal-Mizrachi C, Laffely N, Cade WT, Yarasheski KE, Cryer PE, Semenkovich CF. Pparalpha activation elevates blood pressure and does not correct glucocorticoid-induced insulin resistance in humans. Am J Physiol Endocrinol Metab. 2006;291:E1365–1371. doi: 10.1152/ajpendo.00230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Vilarrupla A, Lavina B, Garcia-Caldero H, Russo L, Rosado E, Roglans N, Bosch J, Garcia-Pagan JC. Pparalpha activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56:1033–1039. doi: 10.1016/j.jhep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Diep QN, Benkirane K, Amiri F, Cohn JS, Endemann D, Schiffrin EL. Ppar alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin ii-infused rats. J Mol Cell Cardiol. 2004;36:295–304. doi: 10.1016/j.yjmcc.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Liu X, Guo Q, Namura S. Chronic treatment with fibrates elevates superoxide dismutase in adult mouse brain microvessels. Brain Res. 2010;1359:247–255. doi: 10.1016/j.brainres.2010.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2010;30:70–78. doi: 10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angulo J, Vallejo S, El Assar M, Garcia-Septiem J, Sanchez-Ferrer CF, Rodriguez-Manas L. Age-related differences in the effects of alpha and gamma peroxisome proliferator-activated receptor subtype agonists on endothelial vasodilation in human microvessels. Exp Gerontol. 2012;47:734–740. doi: 10.1016/j.exger.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappab signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 35.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M, Yamaguchi I. Aging-induced decrease in the ppar-alpha level in hearts is improved by exercise training. Am J Physiol Heart Circ Physiol. 2002;283:H1750–1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 36.Sung B, Park S, Yu BP, Chung HY. Modulation of ppar in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]
- 37.O'Keefe JH, Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: Lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–2146. doi: 10.1016/j.jacc.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Brachial artery characteristics in response to vitamin C in fenofibrate treatment group.
Data are mean±SE. BA, brachial artery; FMD, flow-mediated dilation; SR, shear rate. *P<0.05 vs. saline within day.
Table S2: Brachial artery characteristics in response to vitamin C in placebo treatment group.
Data are mean±SE. BA, brachial artery; FMD, flow-mediated dilation; SR, shear rate. *P<0.05 vs. saline within day.