Abstract
Behavioural studies of children with specific language impairment (SLI) have reported long term growth outcomes across different dimensions of language. Genetic studies of children with SLI have identified candidate genes and putative associations of gene variants with SLI. The aims of this review are to summarize these two lines of investigation and to highlight the possible role of underlying growth timing mechanisms that influence the trajectory of language outcomes throughout childhood and into adolescence. Behavioural growth trajectories demonstrate that children with SLI have notable strengths in language acquisition, as well as limitations, across different dimensions of language. Language onset appears delayed, although the rate and pattern of change over time is similar to unaffected children. Growth rate decelerates early in adolescence for some dimensions of language. Genetic investigations reveal candidate genes that are known to influence neuronal development, and reveal possible gene interactions along a causal pathway. Epigenetic studies reveal other genetic influences implicated in the cognitive decline associated with aging. This review highlights possible parallels between underlying genetic mechanisms and characteristics of linguistic growth trajectories. The conclusion is that new developmental perspectives are needed to inform language intervention in ways that align nurture with nature.
Keywords: Genetics, language impairment, specific language impairment, language growth, language phenotypes, typical language development
Introduction
Children with language impairments are identified on the basis of language performance levels lower than expected relative to their age peers. Clinical services aim to increase their language performance to levels commensurate with age expectations. How language grows over time is a fundamental benchmark for clinical intervention, from children’s first words to the creative and productive sentences they generate by 5 years, to the range of linguistic abilities they demonstrate in adolescence as they move toward adult levels of language competence. Although cross-sectional studies have provided valuable generalizations about patterns of language acquisition for children of a given age, there has been relatively little documentation of how particular dimensions of language grow over time in individual children with and without language impairments. New findings provide some much needed clarification of how this process unfolds for children with language impairments compared to typically developing children.
This paper summarizes outcomes from a long-term longitudinal study that compares growth in different dimensions of language in a group of young children with language impairments compared to young children with typical language growth, a study directed by the author and funded by the National Institutes of Health (NIH) (DC001803). The study also aims to investigate genetic influences on language acquisition, in the context of a family-based candidate gene research design. The program of investigation, underway for 20 years, has revealed patterns of similarity and difference in language growth in children with and without Specific Language Impairment (SLI), outcomes that suggest new questions that lead us toward possible biological mechanisms of change under genetic regulation. This paper will summarize linguistic growth outcomes, relevant genetic findings, and new perspectives on gene regulatory mechanisms; portions of these sections are similar to a more detailed review paper (Rice, 2012). The paper concludes with a call for a new perspective on language growth in children with SLI, one that attempts to bridge the contributions of nature as well as nurture.
Children with Specific Language Impairment
Language impairments can appear in children with or without other disabilities. The children of interest here are those who meet criteria for SLI. A generic definition of the condition of SLI is the presence of a language disorder in the absence of other disorders, with no obvious cause. (see https://www.nidcd.nih.gov/health/voice/pages/specific-language-impairment.aspx). In the study summarized here, conventional research inclusionary and exclusionary criteria were used to identify children with SLI, who entered the study as probands (the first affected family member identified for family-based genetics study). The primary inclusionary criterion was a low level of language performance relative to age expectations on an age-appropriate omnibus language assessment, such as the Test of Language Development-Primary (TOLD-P) (Newcomer & Hammill, 1997). “Low level” was defined as one standard deviation or more below the age group mean, or approximately the bottom 15th percentile of the child’s age group. Another inclusionary criterion was monolingual language acquisition and exposure, in order to avoid possible confounds of bilingualism. The exclusionary criteria were general cognitive impairment (children with a nonverbal IQ of 85 or lower were not entered as probands), hearing loss, frank neurological impairment (such as epilepsy or cerebral palsy; children diagnosed with autism were also excluded as probands), speakers of dialectal variants that would confound the grammar assessments, and children with speech impairments that limit intelligibility in conversational speech samples and/or omit affixes –s, -sh, -t, -d needed for marking finiteness. The criteria are consistent with those developed by Tomblin and colleagues (J. B. Tomblin et al., 1997) in their epidemiologically ascertained sample of children with SLI. This sample yielded a widely adopted prevalence estimate of 7–8% of 5-year-old children, indicating that SLI is one of the most common childhood learning disabilities. In the context of a genetics study, child probands with SLI provide an opportunity to study genetic influences on language impairments not confounded with possible genetic influences on hearing loss or general intellectual impairment. Most of the probands were recruited from clinical caseloads when they were 3–7 years. Although they were receiving speech-language therapy at the outset of their participation in the study, ongoing monitoring of the services they were receiving shows that the children were likely to be dropped from services by mid-childhood, although they were likely to receive services for reading or other academic limitations.
A control group of children were recruited of the same age as the probands. Children in the control group met the exclusionary criteria at study entry, as well as a language standard score of 86 or above (roughly, 16th percentile or higher). Children whose language standard scores were above 120 were not included in the control group. The families of the probands and control children were recruited to the study for longitudinal behavioural assessments as well, providing a further source of children with and without SLI, defined according to the same inclusionary and exclusionary criteria as required for definition of SLI in the proband. Children and participating parents were assessed with a comprehensive language assessment protocol designed to capture growth over time for the children in multiple dimensions of language. The aims were to determine if the different dimensions of language grow in a similar way, how growth differs for different age levels, and when children arrive at an adult level of performance. Participating parents and children provided DNA samples to be analyzed in the candidate gene investigation component of the study. More than 200 probands and 90 control children, and families, are in the database. The total participant base with longitudinal direct behavioural assessments is more than 900. The child assessments encompass ages 2 to 26 years, with data collection every six months for children younger than nine years and annually for older children. Data are available for as many as 17 years for some of the probands; most children in the data reported here were studied for more than 5 years. Overall, the study is unique for the relatively large sample size of probands with SLI, controls, and siblings; well specified criteria for SLI; and extensive, detailed longitudinal data across multiple dimensions of language.
Growth patterns across dimensions of language for affected and unaffected children
Children with SLI identified when young are likely to persist in a low level of language performance on standardized instruments, relative to their age peers, as they move through childhood (Johnson et al., 1999; J. B. Tomblin, Zhang, Buckwalter, & O’Brien, 2003). Yet we have known little about the shape of growth over time because most of the longitudinal studies have used standard scores of omnibus language tests that are age adjusted and therefore not expected to change over time, (e.g., (Conti-Ramsden, St Clair, Pickles, & Durkin, 2012). Omnibus standard scores are not sensitive to details of growth across different dimensions of language
In the program of investigation reported here, child assessments for the growth analyses were designed to yield meaningful raw scores that track progress toward the adult grammar. The variables are intended to capture key components of language growth in the age span of 2 to 20 years. Multiple dimensions of language are assessed, because children with SLI have more difficulty with some properties of grammar compared to other areas of language acquisition (Rice, 2003; Rice, Redmond, & Hoffman, 2006; Rice & Wexler, 1996). Further, vocabulary and grammar seem to follow different paths (J. B. Tomblin & Zhang, 2006). One dimension is vocabulary growth, of interest because early word comprehension is one of the earliest reliable indicators of children’s language acquisition, and is a stable linguistic trait in childhood (Rice et al., 2006; Rowe, Raudenbush, & Goldin-Meadow, 2012). The second dimension is the mean length of utterance (MLU), a measure of early grammatical development in the age range of 2 to 8 years that can be viewed as an index of progress toward adult clause constructions (Rice et al., 2006; Rice et al., 2010). The third dimension is finiteness, a morphosyntactic property of the grammar. In English, finiteness is manifest in simple clauses by a small set of morphemes that interact with word order in the syntax: Auxiliary and copula BE, auxiliary DO (but not main verb DO (Rice & Blossom, 2013)), -s third person agreement marking, regular and irregular past tense marking. Young English-speaking children tend to omit finiteness markers in clauses where they are required, a phenomenon called an Optional Infinitives Stage (Wexler, 1994, 2003, 2011). For example, they say “Patsy walking” instead of “Patsy is walking” or “Patsy walk home” instead of “Patsy walked home” or “what he want” instead of “what does he want?” During this period of development they seem to know many of the adult grammar requirements for word order at the same time they may or may not use the required finiteness forms. Children with SLI show the same pattern of omissions, even more than younger children at equivalent MLU levels. This period of grammatical deficit is referred to as an Extended Optional Infinitive (EOI) stage, given that the pattern is highly similar to that of younger children in the OI stage, but this stage persists much longer for children with SLI (Rice & Wexler, 1996, 2001; Rice, Wexler, & Cleave, 1995; Rice, Wexler, & Hershberger, 1998; Rice, Wexler, Marquis, & Hershberger, 2000; Rice, Wexler, & Redmond, 1999). Because the EOI is likely to trail behind other delayed language properties, it is a strong clinical marker of SLI, with high levels of sensitivity and specificity for identifying affected children (Rice & Wexler, 2001). Recent investigations suggest this clinical marker may appear in other forms of language impairment, as well, including autism (Roberts, Rice, & Tager-Flusberg, 2004) and Fragile X (Sterling, Rice, & Warren, 2012).
The observed growth outcomes for children with and without SLI in the data archives of the ongoing longitudinal study are reported in a series of six figures, as depicted in a recent review (Rice, 2012).. The figures demonstrate growth in three dimensions of linguistic assessment across the age range of 2 to 20 years. Two elements of growth outcomes are noteworthy across the three dimensions of language: One is that children with SLI show, on average, a delayed start of language acquisition relative to age expectations, delayed onset which cascades across different ages. The second growth outcome is that once acquisition of a particular language dimension begins, the children with SLI follow, on average, the same growth trajectories as children without SLI. Across linguistic dimensions, statistical models confirm that at the first time of assessment performance is lower for the SLI group than the control children, with approximately a 2-year delay of expected onset of a given linguistic dimension. Note that the delay is even more pronounced for finiteness marking than vocabulary growth. The same growth models fit the data for the SLI and control groups, indicating that the groups do not differ in how language grows over time, over dimensions, once growth begins.
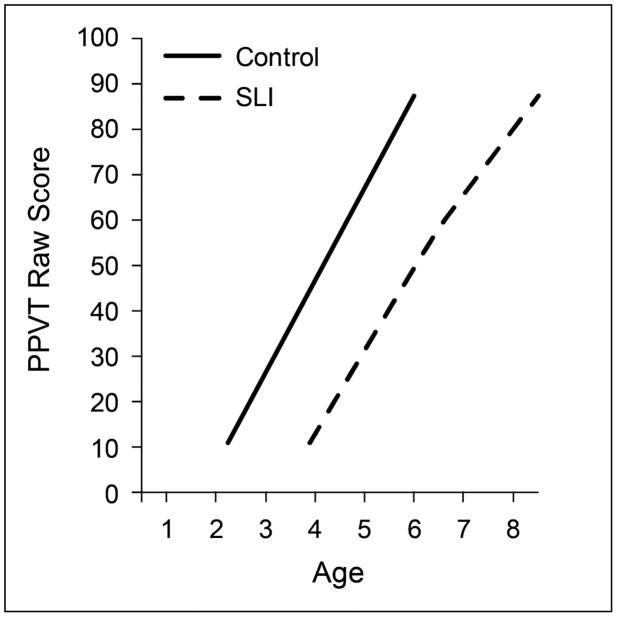
These generalizations are illustrated in the figures. Figure 1 depicts growth in vocabulary comprehension in the important period between 2 and 8 years of age, when children’s early vocabularies grow quickly. The data plotted in Figure 1 (Rice et al., 2006; Rice et al., 2010) are outcomes on the raw score of the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997), an index of children’s vocabulary comprehension. Note that at outset there is about a 2-year advantage for the earliest ages in which the control children comprehend words compared to the SLI children. Growth is mostly linear, with a relatively steep rate of growth in this age period. Note also that although they start later, once they begin to comprehend words on the test the children in the SLI group, on average, follow a similarly rapid rate of acquisition as the children in the control group. Also note that the children with SLI, although learning new words quickly, do not overcome the gap with the control group because the rate of change of the two groups does not differ. In this age period, it is not that the children with SLI are slow learners of new words; instead, they cannot learn faster than the control group.
Figure 1.
Growth of receptive vocabulary for controls and children with specific language impairment (SLI)
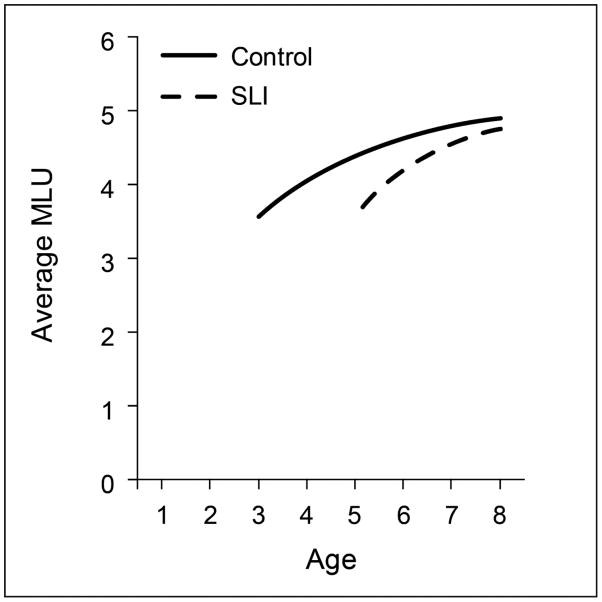
Figure 2 is an early index of grammatical abilities, as children begin to combine words and morphemes to generate phrases and clauses. The mean length of utterance (MLU) is calculated from transcripts of children’s spontaneous utterances as they interact with an adult in play with toys, a method developed in a seminal study by Roger Brown (Brown, 1973). As shown in the figure, in studies of children with and without SLI (Rice et al., 2006; Rice et al., 2010), this index captures growth from 2 to 8 years, growth with a natural leveling around an average level of 5 words or morphemes in the sampling situation. The MLU data also shows a 2-year difference between groups for the first time of measurement, where the controls achieve an average of about 3.5 morphemes at 3 years of age, a level observed for the SLI group at 5 years. The growth trajectory is not linear but instead grows quickly to the naturally constrained levels, a leveling achieved several years earlier for the control children than for the children in the SLI group. Growth models do not differentiate between the two groups, showing that once the children with SLI begin to increase their utterance length they do so at a similar rate and trajectory for change. As with vocabulary acquisition, the children with SLI rapidly increase their MLU levels from 5 to 8 years of age, although they do not catch up with their peers until they reach the upper level, a level achieved by their peers two years earlier.
Figure 2.
Growth of mean length of utterance (MLU) for controls and children with specific language impairment (SLI)
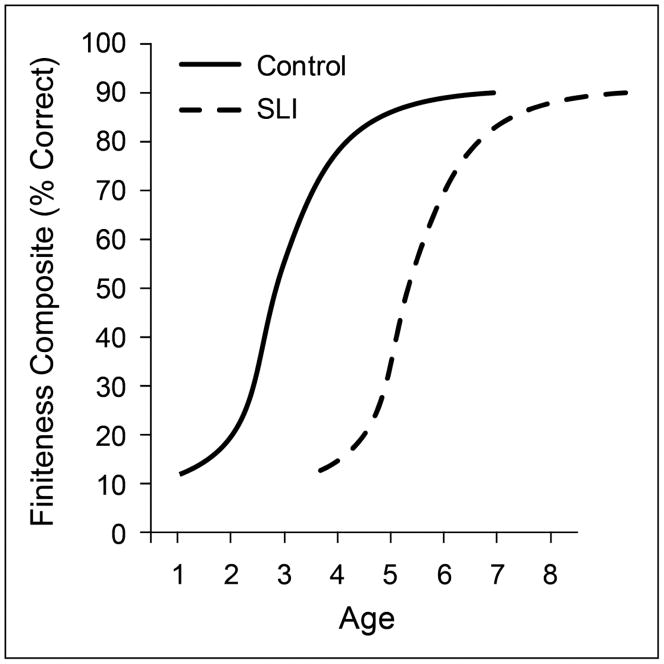
In Figures 3–5 the measures of language shift to age-appropriate indications of finiteness marking in simple clauses. Figure 3 reports the percentage of required finiteness markers in children’s spontaneous utterances. The measure is the composite percentage of use in obligatory simple declarative sentence contexts for the following morphemes: Copula and auxiliary BE, auxiliary DO, third person singular –s, regular and irregular past tense (Rice & Wexler, 2001). In English this obligatory property of grammar appears and becomes consistently marked in the age range of 2 to 5 years for children without language impairments. Other languages show faster acquisition and some languages have little overt evidence of this property, differences that are of considerable theoretical interest (Wexler, 2003, 2011). The growth trajectories in Figure 3, drawn from data reported elsewhere (Rice, Tomblin, Hoffman, Richman, & Marquis, 2004; Rice & Wexler, 2001; Rice et al., 1998; Rice et al., 2000), are not the same as in the first two figures, showing rapid change that levels off at the expected level of adult grammar, just above 90% in spontaneous samples. Even so, the two generalizations hold about group differences in the age at which change begins and the similarity in the rate of change over time once acquisition is underway. A noteworthy difference for finiteness marking is that the children with SLI are more than 2 years delayed (relative to controls) for finiteness acquisition, and this delay persists for years. Because the average level of finiteness marking for children with SLI is likely to be below the average level of children at equivalent MLU or vocabulary levels, this weakness is sometimes called a ‘deficit’ because the finiteness levels are not at commensurate levels of the other language dimensions of immature language (Rice, 2003). Even with this greater delay before finiteness marking acquisition is underway for children with SLI, once started the rate of change is equally strong for the SLI group as the control group. The same growth model fits both groups. It is as if the children with SLI are prepared to employ the same growth mechanisms as the control children once the system starts.
Figure 3.
Growth of the percentage of required finiteness markers in the utterances of controls and children with specific language impairment (SLI)
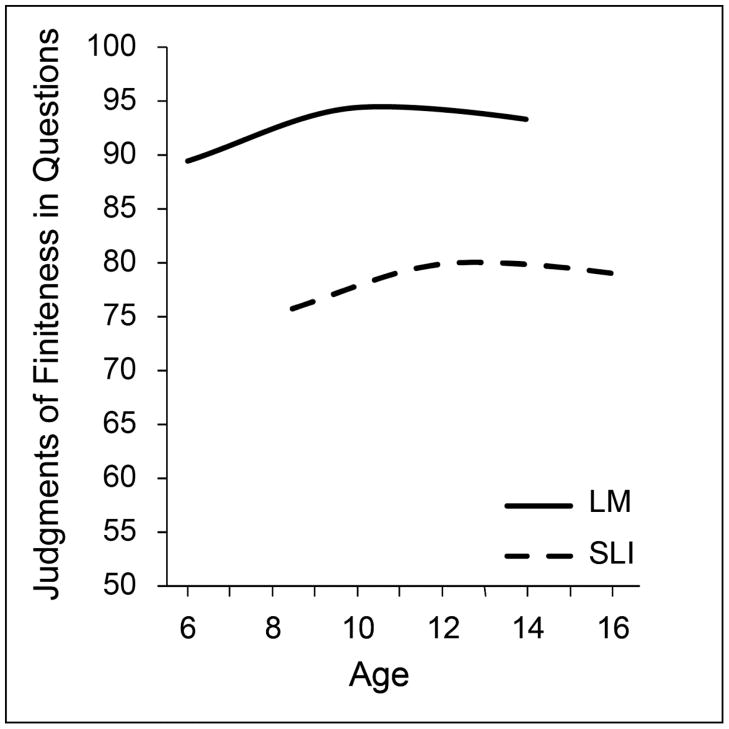
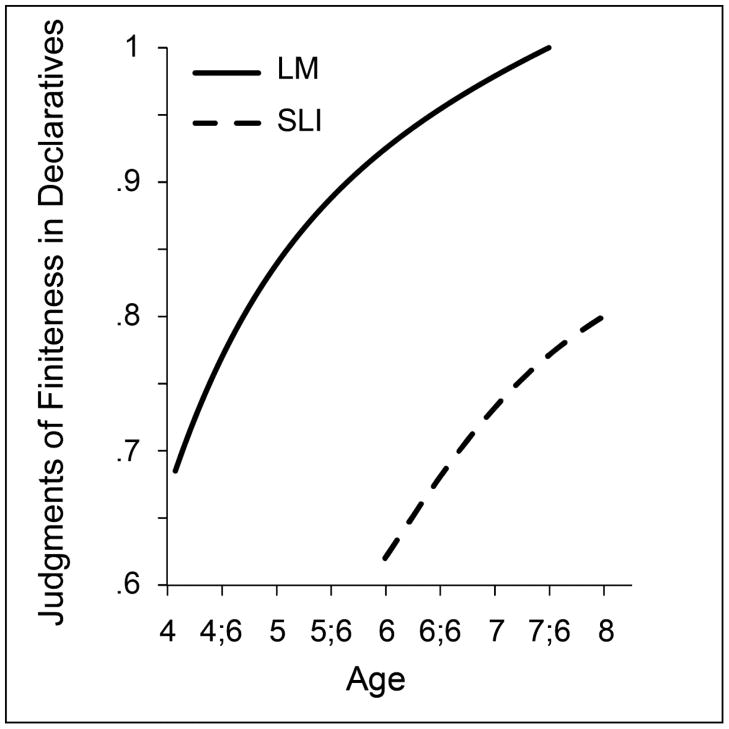
Figure 5.
Growth of the accuracy levels in judgments of finiteness marking in simple questions in controls and children with specific language impairment (SLI)
It is clear from Figure 3 that growth in finiteness marking in simple declarative clauses is complete by age 6 for the controls and age 8 for the children with SLI, on average. An experimental measure was developed for accessing continuing growth. This second-stage measure used a grammatical judgment task to assess children’s sense of grammatical well-formed-ness in a series of sentences such as: “The boy (is) happy”; “the girl (is) running”; “the dog run(s) home”; “the cat walk(ed) home”; “he run(ran) home.” The finiteness markers indicated in parentheses are likely to be omitted by young children and by children with SLI who are older. The judgment task was comprised of simple declarative clauses with or without omitted finiteness markers, intermingled across items. Children were required to judge each item as “good” or “not so good.” Figure 4 reports the longitudinal outcomes for two groups on the judgment task, for the age range of 4 to 8 years(Rice & Wexler, 2001; Rice et al., 1999). Once again, the two groups differ at the age at which they reach performance of 0.7 on the index, separated by more than 2 years. Yet once underway the growth curves do not differ, confirmed by statistical growth curve models. For this index it is clear that the control group arrives at an adult level of judgments although the SLI group is possibly leveling off before reaching that target.
Figure 4.
Growth of the accuracy levels in judgments of finiteness marking in simple declarative clauses by controls and children with specific language impairment (SLI)
Figure 5 raises the age range from 6 to 16 years, for another judgment task, this time for simple questions, which pose somewhat more difficulty for both groups of children. Examples are: “Where (does) he go?” “(Is) she ready to go?” “Where (is) he going?” “(Does) a dog like cat food?” The task again required detection of the ungrammaticality of omitted finiteness markers in a series of questions some of which were grammatically correct and some of which had a missing finiteness morpheme in a site in the question where finiteness is required to be overtly marked (Rice, Hoffman, & Wexler, 2009). Again, the level of performance differs at the first time of measurement, when the older children with SLI are markedly less proficient than the age controls. At 6 years of age the control group is near adult levels and persists at a high level of performance throughout (90–95% accuracy), whereas the 8-year-old SLI group seems to be ‘stuck’ at less than adult levels (75–80% accuracy) from 8 to 16 years. For this task, there is little indication of change over time. The SLI group does not ‘catch up’ to their age peers. Indeed, the data conform to the indication from Figure 4 that if the children with SLI do not ‘catch up’ with their peers by middle childhood they are unlikely to close the gap. Instead, their grammar remains at an immature level into adolescence and the adult period.
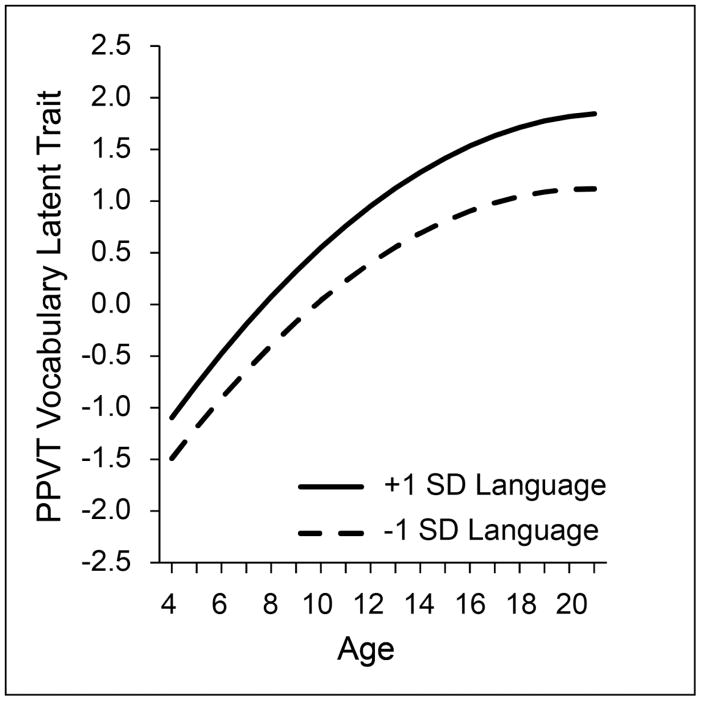
A similar suggestion of leveling at upper childhood is evident in vocabulary acquisition, as shown in Figure 6. This figure reports analysis of PPVT receptive vocabulary scores for the age range of 4 to 20 years, calculated on the basis of item level responses(Hoffman, Templin, & Rice, 2012). The calculations yield a vocabulary trait index allowing for a continuous estimate over time and different versions of the test. The findings can be interpreted as similar to, but not exactly the same, as the raw scores presented in Figure 1. In Figure 6 the groupings are defined by relatively high performance (more than one standard deviation above the mean) and relatively low performance (more than one standard deviation below the mean). Thus, from the sample studied, there is a ‘high vocabulary’ group and a ‘low vocabulary’ group. The modeled trait scores are centered on age 10 years, with a 0 score at 10 years for the full group; younger children have negative scores, older children positive scores. Although the shape of change over time differs from other linguistic dimensions, it remains that children who perform at low levels follow the same growth trajectories as children who perform at high levels, confirmed by formal growth model computations. As with other measures, the low group does not catch up to the high group. Instead, they continue to follow a lower path that seems to level off at the upper age levels.
Figure 6.
Growth of latent trait receptive vocabulary for controls and children with specific language impairment (SLI)
Collectively, the figures illustrate that language acquisition in children with SLI shows weaknesses over time but also shows a somewhat surprisingly robust rate of change. The weakness is especially apparent at the beginning of a new phase of linguistic growth. This is not limited to a single linguistic dimension of weakness, but instead is apparent for vocabulary acquisition, lengthening utterance length, and finiteness marking. Across these dimensions, at different age levels, the SLI group, on average, performs equivalent to younger children without SLI, with an apparent gap of about 2 years or more. The weakness appears to be especially pronounced for finiteness marking, which is delayed more than 2 years at each phase of difficulty. The strengths are in the rate and trajectory of change, which parallels that of children without SLI. Clearly, children with SLI acquire language and they do so in the same elapsed amount of time as children without SLI, for those dimensions of language mastered early in childhood. The risk is that there will not be sufficient time from onset as they move through middle childhood to catch up to their age peers. This failure to catch up by early adolescence clarifies how parents of children with SLI are at higher risk for language impairments (Rice, Smith, & Gayán, 2009). They, too, probably did not ‘outgrow’ their earlier periods of SLI, leaving them with linguistic weaknesses as adults. The growth evidence suggests that although children with SLI can form grammatical simple clauses by middle childhood, with clear speech intelligibility, more complex grammatical constructions are vulnerable.
Questions raised by growth patterns
Much of the literature has focused on accounting for the language acquisition weaknesses of children with SLI, relative to their age peers. In contrast, the growth evidence reveals surprising strengths in language acquisition of children with SLI, especially during the early childhood period followed by an apparent leveling of acquisition in early adolescence. The findings pose a challenge for theoretical accounts of the etiology of SLI that focus on the weaknesses alone. Consider models that posit a weakness in a general learning mechanism (J. B. Tomblin, Mainela-Arnold, E., & Zhang, X., 2007). Such models could account for a generalized slow rate of language acquisition across all dimensions of language. Yet one wonders how children with SLI could accomplish such robust growth in language while encumbered with a limited general learning mechanism. Further, it is not clear how a general learning mechanism weakness would predict such different growth trajectories across the three dimensions of language acquisition. It seems more straightforward to assume the parallels in growth with younger children are accomplished by access to the same kinds of language learning mechanisms, which seem to vary across linguistic dimensions. Other models posit a breakdown or weaknesses in input processing mechanisms specific to speech or verbal memory limitations (Falcaro et al., 2008; Gathercole, 2006; L. B. Leonard, 1998; L.B. Leonard et al., 2007). Such limitations could account for particular problems with low salience surface forms, or perhaps limited memory for unfamiliar forms or clauses. On the other hand, it is not clear how input processing or memory limitations would account for how young children with SLI are able to overcome those limitations to acquire grammatical distinctions or new vocabulary in the same span of elapsed time as younger children without SLI, once the systems begin to show change, following the same growth patterns, across many different manifestations of surface form or familiarity of forms. Linguistic representation models such as the EOI (Rice & Wexler, 1996; Rice et al., 1995) have the advantage of positing acquisition differences across the dimensions of linguistic acquisition. A further advantage is the premise that young children have access to fundamental abstract grammatical properties which restrict a general trial and error approach to language acquisition. Such models, however, are silent about the mechanisms of growth that drive change over time, mechanisms that show strong parallels between children with and without SLI.
A general challenge for causal models is the apparent age dependency of the growth cycle, such that if children with SLI do not ‘catch up’ with their age peers by adolescence, it seems their language levels are ‘stuck’ at a level below age-referenced expectations. It appears that the increased acceleration needed for children to ‘catch up’ with their age peers is inconsistent with the general propensity to follow the same growth trajectories once the process begins. Further, if such increased acceleration is evident, there must be some mechanisms in place to re-adjust to the expected rates of change; if not, children would follow a faster acceleration than expected and rise to levels higher than their age peers, although this does not seem to be the case. Instead, the children with SLI seem predisposed to follow the same path when viewed over a broad age range.
All things considered, new perspectives are needed regarding the mechanisms that govern growth in language acquisition for children with SLI, and the nature of the weaknesses that lead to prolonged deficits in language acquisition that persist into adolescence and beyond. The growth curves point to three important distinctions: One is the onset of change, when a particular property of language acquisition begins to grow over time; the second is the trajectory of growth once activated; the third is an apparent deceleration or leveling of language levels that appears in early adolescence.
These properties have analogs in biological mechanisms, under gene regulation. The immune system provides an example of how onset, acceleration and deceleration can function at the cellular level. T cells are a type of white blood cell that destroys infected cells that are recognized as foreign. A recent discovery reveals that T cells require two signals to attack a target effectively: one that functions as an onset switch and another as an accelerator (Groopman, 2012; Sharma, Wagner, Wolchok, & Allison, 2011). In sequence, the two signals lead to activation of T cells, proliferation, and cell migration, all of which enhance the body’s immune response. The sequence includes a cumulative braking function, to ensure that the immune response does not over-react to perceived threats. This is accomplished by molecules produced in the sequence that eventually inhibit T cell activity. As summarized by Rice (2012), “This sequence of signals implies differentiated timing mechanisms, one signaling ‘turn on,’ followed by a second signal to ‘accelerate’ a function when needed, and a braking function built into the mechanism that leads to the restriction of T cell activity in order to minimize damage to normal tissues.” (p. x) Is there reason to expect such timing mechanisms to be implicated in SLI? Recent genetic studies provide some clues.
Candidate gene studies of reading and language abilities
There is growing evidence of likely genetic influences on SLI. Twin studies provide strong evidence of heritability of SLI in children, based on behavioural comparison of pairs of monozygotic and dizygotic twins (Dale et al., 1998; DeThorne, Petrill, Hayiou-Thomas, & Plomin, 2005; J. B. Tomblin & Buckwalter, 1998). More recently, candidate gene investigations with probands and their families yield discoveries at the molecular level that draw attention to possible gene effects. Behavioural phenotypes are the measured traits that result from gene expression as well as the influence of environmental factors and the interaction between the two. The relevant recent studies have used several kinds of phenotypes: Classification of children as SLI; performance levels on omnibus language tests or tests of vocabulary and grammar; age of first words; and reading levels. Approximately half of young children with SLI go on to develop reading impairments when they reach the age of reading instruction (Catts, 2004). Given this overlap of impairments, a reading phenotype could identify candidate genes for shared genetic influence on language and reading.
Several outcomes of interest are briefly summarized here. Detailed reviews of current discoveries can be found in recent papers (Paracchini, 2011; Smith, 2011). Relevant candidate gene association studies calculated the statistical probability that a behavioural phenotype co-occurred with a genetic variation measured at the molecular level of single nucleotide polymorphisms (SNPs, defined as variations in sequences of the basic components of DNA: Adenine(A), Cytosine(C), Guanine(G), and Thymine(T)). One gene of interest is KIAA0319, a candidate gene with two studies replicating associations of SNP variants located on this gene with language impairment phenotypes in different samples of children with SLI and their families (Newbury et al., 2011; M.L. Rice et al., 2009). Because this kind of investigation is relatively new, the demands for data collection are high, and few studies have been reported, the replication across two studies for a particular molecular location is important. Although the replication is encouraging, the outcomes are not definitive due to small sample sizes that need further replication. This gene, KIAA0319, has particularly interesting properties. The gene is reported to influence neuronal migration in embryonic rat brain, suggesting possibly similar effects in humans that remain to be established. In addition, KIAA0319 is thought to play a role in the etiology of reading disability. Recent investigation has identified mechanisms of gene expression of KIAA0319 that could regulate the effects of other genes, providing support for a possible regulatory role for this gene (Couto et al., 2010). A second gene of interest is CNTNAP2, a gene also known to be involved with early neuronal development (Poelmans, Buitelaar, Pauls, & Franke, 2011). Variants of this gene are significantly associated with performance of children with SLI on a non-word repetition task, which in turn is associated with performance on language assessments (Vernes et al., 2008). Further, variants of CNTNAP2 are significantly associated with early language development in the general population, measured by parent questionnaire for children at two years of age (Whitehouse, Bishop, Ang, Pennell, & Fisher, 2011). The same region of CNTNAP2 variants coincides with one associated with language delays, measured by age of first words, reported for children with autism (Alarcón et al., 2008). Another property of interest for CNTNAP2 is that it is a downstream target of FOXP2, a gene implicated in severe and rare forms of language impairment (Lai et al., 2000). Investigation of these three regulatory genes, KIAA0319, CNTNAP2, and FOXP2, collectively suggest potentially complex interactions among genes along the causal pathway, although definitive evidence is not available to establish regulatory gene effects as part of the etiology of SLI. The chain of evidence does, however, support the plausibility of such a claim (cf. Rice, 2012).
Gene regulation mechanisms as future directions
A possible link between growth trajectories of language in children with and without SLI and their genes requires closer consideration of molecular levels of gene expression. In many ways the functions of genes (or, more generally, DNA and RNA) are intrinsically timed, in well synchronized complex chemical interactions. Genes can be grouped into two types. One type is a list of ingredients for making a protein and the other type acts like a switch that lets a nearby gene be read (‘promoters’ and ‘enhancers’), or blocks it from being read (‘silencers’). This suggests the possibility that if promoters or enhancers are slow to activate a gene function that is necessary in the causal pathway, perhaps in the development of cortical structures needed for the beginnings of language acquisition, then language onset could be delayed. The example of the distinct signals in the immune system, in the functioning of T cells, indicates that such signaling systems can be differentiated for onset, acceleration, and deceleration, leading to delayed onset, spared acceleration once onset is established, but a limited time window before deceleration begins. Thus, there are parallels between the molecular mechanisms of gene expression and the observed patterns of language growth in children with SLI and unaffected children.
Another way in which timing mechanisms could enter into the causal pathways for SLI could be via epigenetic processes. Epigenetic models can be differentiated from genetic variant disease models (Feinberg, 2008). Epigenetics is defined as modifications of DNA or associated proteins, other than DNA sequence variations, that carry information content during cell division. Epigenetic mechanisms can be influenced by environmental factors such as nutrition, leading to possible interactions of genetic variants with such external factors (Bjornsson, Fallin, & Feinberg, 2004). Epigenetic change is thought to be at the heart of normal development (in the sense of cellular development), allowing for the likelihood that disruptions in epigenetic modifications can disturb normal developmental programs (Feinberg, 2008). A recent model of interest was developed in a program of investigation of decline in learning and memory associated with aging (Day & Sweatt, 2011). The model proposes an epigenetic code in the central nervous system that mediates synaptic plasticity, learning, and memory. Based on studies of humans and animal models, they propose that aberrant epigenetic modifications (involving a breakdown of timed signals for gene expression in signaling cascades) lead to declines in learning and memory as people age and in neurodevelopmental disorders of aging such as Alzheimer’s disease. It is conceivable that such breakdowns could be part of the pathways to SLI, in the cascades necessary to establish the neuronal infrastructure for the onset of language acquisition, the relative sparing of subsequent acceleration, and a time-limited period of growth followed by deceleration in early adolescence. Rice (2012) hypothesizes a growth signaling dysfunction (GSD), involving a breakdown in the synchronization of onset, growth, and deceleration of language acquisition and underlying neurocognitive circuits, as part of the biological basis of SLI.
Conclusions and clinical implications
The causes and etiological pathways of SLI remain unknown, but progress is evident on two fronts. One front is on the behavioural level, documenting how dimensions of language change over time for children with and without SLI, revealing crucial distinctions between the start of growth, rate of change, and leveling of change over time. The same growth trajectory does not fit across different dimensions of language. Even so, the same model of growth fits children with and without SLI, once adjusted for the group differences in level of performance at the first time of measurement. Children with SLI seem to have a delayed beginning for various dimensions of language relative to their age peers, a pattern that persists across childhood as new levels of language acquisition appear. Most striking, children with SLI, on average, increase their language levels at the same rate as children without SLI, indicating robust change over time, until they reach early adolescence when the rate of change seems to level off, leaving them with language levels below age expectations. On another front, candidate gene studies suggest a possible role for individual variations in genes known to be involved in neuronal development. Further consideration of mechanisms and processes involved in gene expression highlight the ways in which molecular level timing functions could influence higher cognitive processes of humans, such as a decline in memory with aging. Although there will be many challenges for the development of definitive evidence about the biological underpinnings of SLI, and ways in which environmental influences interact with these underpinnings, it is nevertheless clear that we have reason to adjust the way we think about language growth in children with SLI.
The notions of ‘delay’ or ‘impairment’ may suggest something consistently faulty in the language acquisition mechanisms available to children with SLI. Instead, children with SLI are different from and similar to children without SLI in dynamic ways, not static, involving cycles of change throughout childhood. Even when lower levels of performance on language assessments persist over time the children are nevertheless growing in their language abilities, which clearly can grow as rapidly as in children without SLI. At the same time, an apparent braking function that depresses language growth in early adolescence would leave adolescent children with a high risk of becoming adults with SLI. The genetics evidence suggests that the children of adults with a history of SLI could inherit gene variants or variants in gene regulatory mechanisms that set up a replication of the growth trajectories from one generation to another.
Although this scenario is consistent with available evidence, multiple caveats apply: The evidence is limited by relatively small samples of participants, and limited further by relatively small samples of the complex world of possible genetic mechanisms in causal pathways. Another limitation is that means of measurement for both the behavioural growth patterns and the genetic mechanisms are relatively new, with replication studies needed. Definitive findings await the outcomes of future investigations.
Even at these early stages, however, there is value in a new developmental perspective on language growth for the work of speech-language pathologists. A truly developmental perspective could impact essential components of intervention, such as the identification of goals for language intervention, determination of when to intervene for particular linguistic structures, expected rates of change, interactions with family members, and presumed causal mechanisms for SLI. Each of the components could benefit from an awareness of the need to consider a growth perspective. Intervention is likely to be most effective if the timing and sequence of goals and linguistic targets are attuned to a child’s progress on an expected growth curve for a given linguistic dimension. The challenge will be to align nurture with nature in ways that facilitate children’s language acquisition, to build on their strengths as well as remediate their weaknesses.
Acknowledgments
Preparation of this paper and the author’s research findings reported here were supported by the National Institutes of Health P30DC005803, R01DC001803, and R01DC005226 to the author, as well as by the University of Kansas Intellectual and Developmental Disabilities Research Center P30HD002528. Special appreciation is extended to the Speech Pathology Association of Australia and the organizers of the Speech Pathology Australia national conference, held in Hobart, Australia, 24–27 June, 2012.
References
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetics and genetic approach to common human disease. Trends in Genetics, 2004. 2004;20(8):350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Brown R. A first language: The early stages. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- Catts HW. Language impairments and reading disabilities. In: Kent RD, editor. THE MIT Encyclopedia of Communication Disorders. Cambridge, MA: MIT Press; 2004. pp. 329–331. [Google Scholar]
- Conti-Ramsden G, St Clair MC, Pickles A, Durkin K. Developmental trajectories of verbal and nonverbal skills in individuals with a history of Specific Language Impairment: From childhood to adolescence. Journal of Speech, Language, and Hearing Research. 2012;55:1716–1735. doi: 10.1044/1092-4388(2012/10-0182). [DOI] [PubMed] [Google Scholar]
- Couto JM, Livne-Bar I, Huang K, Xu Z, Cate-Carter T, Feng Y, et al. Association of reading disabilities with regions marked by acetylated H3 histones in KIAA0319. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2010;153B(2):447–462. doi: 10.1002/ajmg.b.30999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale PS, Simonoff E, Bishop DVM, Eley TC, Oliver B, Price TS, Plomin R. Genetic influence on language delay in two-year-old children. Nature Neuroscience. 1998;1:324–328. doi: 10.1038/1142. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeThorne LS, Petrill SA, Hayiou-Thomas ME, Plomin R. Low expressive vocabulary: High heritability as a function of more severe cases. Journal of Speech, Language, and Hearing Research. 2005;48:792–804. doi: 10.1044/1092-4388(2005/055). [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Falcaro M, Pickles A, Newbury DF, Addis L, Banfield E, Fisher SE, Conti-Ramsden G. Genetics and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes, Brain, and Behavior. 2008;7:393–402. doi: 10.1111/j.1601-183X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Epigenetics at the epicenter of modern medicine. Journal of the American Medical Association. 2008;299(11):1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Complexities and constraints in nonword repetititon and word learning. Applied Psycholinguistics. 2006;27:599–614. [Google Scholar]
- Groopman J. The New Yorker. 2012. Apr 23, The T-cell army; pp. 24–30. [Google Scholar]
- Hoffman L, Templin J, Rice ML. Linking outcomes from Peabody Picture Vocabulary Test forms using item response modeling. Journal of Speech, Language, and Hearing Research. 2012;55:754–763. doi: 10.1044/1092-4388(2011/10-0235). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Beitchman JH, Young A, Escobar M, Atkinson L, Wilson B, Wang M. Fourteen-year follow-up of children with and without speech/language impairments: Speech/language stability and outcomes. Journal of Speech, Language, and Hearing Research. 1999;42:744–760. doi: 10.1044/jslhr.4203.744. [DOI] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Levy ER, Hodgson S, Fox M, Monaco AP. The SPCH1 region on human 7q31: Genomic characterization of the critical interval and localization of translocations associated with speech and language disorder. American Journal of Human Genetics. 2000;67(2):357–368. doi: 10.1086/303011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard LB. Children with specific language impairments. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Leonard LB, Weismer SE, Miller CA, Francis DJ, Tomblin JB, Kail RV. Speed of processing, working memory, and language impairment in children. Journal of Speech, Language, and Hearing Research. 2007;50:408–428. doi: 10.1044/1092-4388(2007/029). [DOI] [PubMed] [Google Scholar]
- Newbury DF, Paracchini S, Scerri TS, Winchester L, Addis L, Richardson AJ. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behavioral Genetics. 2011;41:90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer PL, Hammill DD. Test of Language Development-Primary (TOLD-P:3) 3. Austin, TX: Pro-Ed; 1997. [Google Scholar]
- Paracchini S. Dissection of genetic associations with language-related traits in population-based cohorts. Journal of Neurodevelopmental Disorders. 2011;4(3):365–373. doi: 10.1007/s11689-011-9091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans G, Buitelaar JK, Pauls DL, Franke B. A theoretical molecular network for dyslexia: integrating available genetic findings. Molecular Psychiatry. 2011;16(4):365–382. doi: 10.1038/mp.2010.105. [DOI] [PubMed] [Google Scholar]
- Rice ML. A unified model of specific and general language delay: Grammatical tense as a clinical marker of unexpected variation. In: Levy Y, Schaeffer J, editors. Language competence across populations: Toward a definition of specific language impairment. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 63–94. [Google Scholar]
- Rice ML, Hoffman L, Wexler K. Judgments of omitted BE and DO in questions as extended finiteness clinical markers of specific language impairment (SLI) to 15 years: A study of growth and asymptote. Journal of Speech, Language, and Hearing Research. 2009;52:1417–1433. doi: 10.1044/1092-4388(2009/08-0171). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Redmond SM, Hoffman L. MLU in children with SLI and young control children shows concurrent validity, stable and parallel growth trajectories. Journal of Speech, Language, and Hearing Research. 2006;49:793–808. doi: 10.1044/1092-4388(2006/056). [DOI] [PubMed] [Google Scholar]
- Rice ML, Tomblin JB, Hoffman L, Richman WA, Marquis J. Grammatical tense deficits in children with SLI and nonspecific language impairment: Relationships with nonverbal IQ over time. Journal of Speech, Language, and Hearing Research. 2004;47:816–834. doi: 10.1044/1092-4388(2004/061). [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K. Toward tense as a clinical marker of specific language impairment in English-speaking children. Journal of Speech and Hearing Research. 1996;39:1239–1257. doi: 10.1044/jshr.3906.1239. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K. Rice/Wexler Test of Early Grammatical Impairment. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Rice ML, Wexler K, Cleave PL. Specific language impairment as a period of extended optional infinitive. Journal of Speech, Language, and Hearing Research. 1995;38:850–863. doi: 10.1044/jshr.3804.850. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Hershberger S. Tense over time: The longitudinal course of tense acquisition in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1998;41:1412–1431. doi: 10.1044/jslhr.4106.1412. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Marquis J, Hershberger S. Acquisition of irregular past tense by children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2000;43:1126–1145. doi: 10.1044/jslhr.4305.1126. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Redmond SM. Grammaticality judgments of an extended optional infinitive grammar: Evidence from English-speaking children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:943–961. doi: 10.1044/jslhr.4204.943. [DOI] [PubMed] [Google Scholar]
- Rice ML. Toward epigenetic and gene regulation models of specific language impairment: looking for links among growth, genes, and impairments. Journal of Neurodevelopmental Disorders. 2012;4(27) doi: 10.1186/1866-1955-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Blossom M. What do children with Specific Language Impairment do with multiple forms of DO? Journal of Speech, Language, and Hearing Research. 2013;56:222–235. doi: 10.1044/1092-4388(2012/11-0107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Smith SD, Gayán J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with Specific Language Impairment. Journal of Neurodevelopmental Disorders. 2009;1:264–282. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Smolik F, Perpich D, Thompson T, Rytting N, Blossom M. Mean length of utterance levels in 6-month intervals for children 3 to 9 years with and without language impairments. Journal of Speech, Language, and Hearing Research. 2010;53:1–17. doi: 10.1044/1092-4388(2009/08-0183). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Rice ML, Tager-Flusberg H. Tense marking in children with autism. Applied Psycholinguistics. 2004;25:429–448. [Google Scholar]
- Rowe ML, Raudenbush SW, Goldin-Meadow S. The pace of vocabulary growth helps predict later vocabulary skill. Child Development. 2012;83(2):508–525. doi: 10.1111/j.1467-8624.2011.01710x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nature Reviews: Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD. Approach to epigenetic analysis in language disorders. Journal of Neurodevelopmental Disorders. 2011;3(4):356–373. doi: 10.1007/s11689-011-9099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling AM, Rice ML, Warren SF. Finiteness marking in boys with Fragile X Syndrome. Journal of Speech, Language, and Hearing Research. 2012;55:1704–1715. doi: 10.1044/1092-4388(2012/10-0106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Buckwalter P. Heritability of poor language achievement among twins. Journal of Speech, Language and Hearing Research. 1998;41:188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. The prevalence of specific language impairment in kindergarten children. Journal of Speech and Hearing Research. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X. The dimensionality of language ability in school-aged children. Journal of Speech, Language, and Hearing Research. 2006;49:1193–1208. doi: 10.1044/1092-4388(2006/086). [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X, Buckwalter P, O’Brien M. The stability of primary language disorder: Four years after kindergarten diagnosis. Journal of Speech, Language, and Hearing Research. 2003;46:1283–1296. doi: 10.1044/1092-4388(2003/100). [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Mainela-Arnold E, Zhang X. Procedural learning in children with and without specific language impairment. Journal of Child Language Learning and Development. 2007;3:269–293. [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Fischer SE. A functional genetic link between distinct developmental language disorders. New England Journal of Medicine. 2008;359(22):2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler K. Optional infinitives, head movement and the economy of derivations. In: Lightfoot D, Hornstein N, editors. Verb movement. Cambridge, England: Cambridge University Press; 1994. pp. 305–350. [Google Scholar]
- Wexler K. Lenneberg’s dream: Learning, normal language development and specific language impairment. In: Levy Y, Schaeffer J, editors. Language competence across populations: Toward a definition of specific language impairment. Mahwah, NJ: Lawrence Erlbaum Assoc; 2003. pp. 11–62. [Google Scholar]
- Wexler K. Grammatical computation in the Optional Infinitive Stage. In: de Villiers J, Roeper T, editors. Handbook of generative approaches to language acquisition. New York: Springer Science+Business Media; 2011. pp. 53–118. [Google Scholar]
- Whitehouse AJO, Bishop DVM, Ang QW, Pennell CE, Fisher SE. CNTNAP2 variants affect early language development in the general population. Genes, Brain and Behavior. 2011;10(4):451–456. doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]