Abstract
Background
Fast, noninvasive identification of ischemic territories at rest (prior to tissue-specific changes) and assessment of functional status can be valuable in the management of severe coronary artery disease. This study investigated the utility of cardiac phase-resolved Blood-Oxygen-Level-Dependent (CP-BOLD) CMR in detecting myocardial ischemia at rest secondary to severe coronary artery stenosis.
Methods and Results
CP-BOLD, standard-cine, and T2-weighted images were acquired in canines (n=11) at baseline and within 20 minutes of ischemia induction (severe LAD stenosis) at rest. Following 3-hours of ischemia, LAD stenosis was removed and T2-weighted and late-gadolinium-enhancement (LGE) images were acquired. From standard-cine and CP-BOLD images, End-Systolic (ES) and End-Diastolic (ED) myocardium were segmented. Affected and remote sections of the myocardium were identified from post-reperfusion LGE images. S/D, quotient of mean ES and ED signal intensities (on CP-BOLD and standard-cine), was computed for affected and remote segments at baseline and ischemia. Ejection fraction (EF) and segmental wall-thickening (sWT) were derived from CP-BOLD images at baseline and ischemia. On CP-BOLD images: S/D was greater than 1 (remote and affected territories) at baseline; S/D was diminished only in affected territories during ischemia and the findings were statistically significant (ANOVA, post-hoc p<0.01). The dependence of S/D on ischemia was not observed in standard-cine images. Computer simulations confirmed the experimental findings. ROC analysis showed that S/D identifies affected regions with similar performance (AUC:0.87) as EF (AUC:0.89) and sWT (AUC:0.75).
Conclusions
Preclinical studies and computer simulations showed that CP-BOLD CMR could be useful in detecting myocardial ischemia at rest. Patient studies are needed for clinical translation.
Keywords: coronary artery disease, acute coronary syndrome, ischemia, BOLD, MRI
Noninvasive imaging approaches that can rapidly assess an ongoing ischemia can be of great value in managing patients with clinically significant coronary artery disease. Although a number of imaging approaches exist for the identification of myocardial territories supplied by stenotic coronary arteries, generally all available imaging methods require provocative stress and/or exogenous contrast media. The most desirable imaging approach is one that can non-invasively and rapidly identify ischemic territories prior to the onset of tissue specific changes (development of edema or necrosis) and can permit the assessment of functional/volumetric status while minimizing patient discomfort.
Previous studies have shown that ongoing ischemia may be detected with CMR (Cardiac Magnetic Resonance) on the basis of stress perfusion and changes in functional indices. More recently, it has been shown that myocardial edema may be utilized as a marker of ongoing ischemia using animal models 1 and patients 2. While the edema approach eliminates the need for provocative stress, both approaches require separate acquisitions for accurate assessment of functional indices. In this work, we propose and test a new CMR approach for a rapid assessment of myocardial ischemia. The proposed method is based on cardiac phase-resolved steady-state free precession (SSFP) magnetic resonance (MR) signal changes originating primarily from alterations in oxygenation (%HbO2) and secondarily from changes in regional myocardial blood volume (MBV) in the myocardial territory supplied by a stenotic artery. Since the proposed approach can generate functional and tissue specific indices in one acquisition, the proposed approach can provide opportunities to rapidly determine the presence and territory of myocardial ischemia.
It is known that (a) MBV is a function of cardiac phase, increasing during diastole and decreasing during systole 3,4; and (b) MBV directly determines the oxygen extraction by cardiomyocytes 5. Thus, MBV and %HbO2 are expected to be different between systole and diastole. Hence, under normal (healthy) conditions, one expects the MBV and oxygen extraction to be maximal during diastole, and minimal in systole. In addition to these effects, as MBV increases, each unit volume of ventricle, i.e., each voxel in a myocardial image, contains a slightly higher proportion of blood, and a correspondingly smaller proportion of myocardial tissue 6. Thus, even at a stable level of %HbO2, the number of deoxygenated hemoglobin molecules within a voxel increases as MBV increases. Moreover, a number of studies have also shown that with increasing grade of coronary stenosis, MBV in the myocardial territory supplied by a stenotic artery increases in systole 7–9. Thus based on these studies, the relative MBV and %HbO2 changes between systole and diastole are expected to be different between myocardial territories supplied by healthy and stenotic coronary arteries.
Cardiac phase-resolved BOLD SSFP CMR might provide a unique opportunity to capture these physiological changes and hence assess ongoing ischemia. It is known that T1 of myocardium is dependent on blood volume 10 and that T2 is dependent on blood oxygenation saturation 11. Since BOLD SSFP signals are approximately T2/T1 weighted 12,13, it may be possible to capture the changes in MBV 4 (via T1) and %HbO2 14 (via T2) in one acquisition. In particular, since coronary artery stenosis leads to an increased systolic MBV that is expected to be accompanied by decreases in %HbO2 (due to increased oxygen extraction), we hypothesized that the BOLD SSFP method can be used to detect the presence of coronary stenosis even at rest (i.e. without pharmacological stress). Under conditions of coronary stenosis, the physiological changes in basal MBV and %HbO2 are expected to work synergistically to enhance the SSFP-based detection capacity of myocardial territories supplied by stenotic arteries. In this work, we test these hypotheses using a canine animal model of severe coronary artery stenosis and computer/numerical simulations. In particular, we examine whether the systolic to diastolic myocardial SSFP signal intensity ratio (S/D) is greater than 1 in health and is diminished during ischemia. In addition, we investigate the effects of acute coronary occlusion on ejection fraction, wall thickening, and myocardial edema.
Methods
Numerical Computer Simulations
To establish the theoretical foundation and to lend additional support to our hypothesis that MBV and %HbO2 synergistically contribute to cardiac phase-dependent myocardial BOLD SSFP signal changes, numerical simulations were performed using a two-pool exchange model 13,15. T1, T2, and SSFP signal changes were computed assuming that the relative MBV is 9% (systole) and 15% (diastole) 16 and myocardial %HbO2 is 30% (systole) and 80% (diastole) 17. Systolic and diastolic T1 changes were computed from the simulations of the dual flip angle technique18 with flip angles = 3° and 15°. The cardiac phase-dependent changes in T2 were computed from simulations of the T2-preparation method 11 with T2-preparation durations of 24 ms and 48 ms. SSFP signals were computed assuming TR = 6.2 ms and flip angle of 70°. To evaluate phasic changes in T1, T2, and BOLD SSFP signal intensities, relative changes in T1, T2, and SSFP signal intensities were computed between systole and diastole, and used to define Systolic to Diastolic Ratios (S/D). Similar computations were performed to determine the expected S/D values on the basis of standard cine SSFP images assuming TR = 3.0 ms and flip angle=70°. To evaluate whether ischemia-induced cardiac phase-resolved changes in MBV and %HbO2 can mediate changes in S/D, we performed additional simulations. Simulations assumed that during ischemia, MBV was 15% (systole and diastole) and %HbO2 = 10% during systole and was unchanged in diastole. All other parameters were held to the values as before in simulating the signal behavior of standard cine SSFP and BOLD SSFP.
Animal Protocol
Mongrel dogs (n=14, 5 female, 20–25 kg) were studied under the protocols approved by the Institutional Animal Care and Usage Committee. Animals were acclimated for seven days and were pre-medicated with Buprenex (0.01–0.02mg/kg IM or SQ), anesthetized with Propofol (3.5–7.0 mg/kg IV), intubated and placed on gas anesthesia with Isoflurane (2–5%) and Oxygen (1 L/min) prior to surgery. Subsequently, dogs were ventilated with a Drager Narkomed 2A Anesthesia machine (Drager, Lubeck, Germany a left lateral thoracotomy was performed. Catheters were inserted into the descending aorta and the right and left atria and were routed so that they exit the body via the chest cavity. A MR-compatible hydraulic occluder was placed around the LAD artery. An intercoastal block was performed with Bupivicaine (0.5%, 3mg/kg/SQ) and Buprenex (0.01–0.02mg/kg IM). The ribs, muscle, and subcutaneous layers were closed with sutures and were allowed to recover for 7 days prior to CMR studies. On the day of the CMR studies, dogs were fasted, sedated using Innovar (Fentanyl 0.4mg/ml and Droperidol 20 mg/ml, IM) and anesthetized using Propofol (3.5–7.0mg/kg, IV). Animals were intubated, placed on CMR scanner table and ventilated with a Narkomed Anesthesia machine, Isoflurane (2–3%) and Oxygen (1 L/min at a rate of 12/15 breaths/minute). ECG, SPO2, ETCO2, invasive BP, and coronary Doppler flow monitoring were established. Severe LAD stenosis was created at the time of CMR studies by inflating the hydraulic occluder to reduce Doppler signals by 90–95% of baseline values and the constancy of the Doppler signals was evaluated every 30 minutes. This stenosis was maintained for 3 hrs followed by reperfusion. Following the final CMR studies, animals were euthanized using Euthasol (1cc/5 kg IV), hearts were excised and myocardial slices were stained with TTC19.
Imaging Protocol
All imaging studies were performed on a clinical 1.5T MRI system (MAGNETOM Espree®, Siemens Healthcare, Germany). Animals were positioned in feet-first right-anterior oblique position and a surface coil was placed over the chest for signal reception. The study protocol is shown in Figure 1. Following scout scans to localize the axes of the heart, whole-heart shimming 20 was performed and the shim values were held constant throughout the study. No imaging acceleration schemes were utilized and built-in coil normalizations 21 were performed to ensure signal homogeneity within the myocardium. In addition, breath-holds (mechanical suspension of the ventilator) were limited to no more than 25 seconds to avoid spontaneous breathing of the animals during the acquisition.
Figure 1.
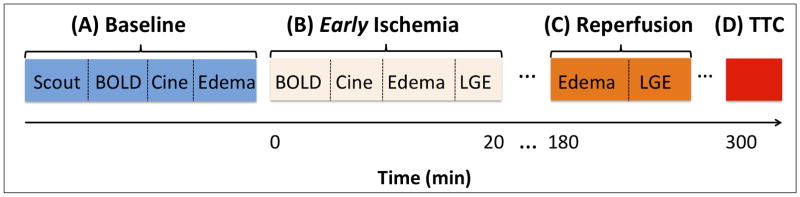
Study Protocol. Baseline scans (A) were acquired prior to scans during ischemia (B), lasting for 20 minutes, followed by scans during reperfusion (C), 3-hours after induction of ischemia followed by reperfusion, and histological confirmation of infarction (D).
CP-BOLD SSFP imaging
Following scout scans, a breath-held, retrospectively gated, flow and motion-compensated 2D short-axis cine BOLD SSFP sequence 22 was prescribed over the mid-ventricle at rest (without and with severe LAD stenosis). Scan parameters were: field-of-view = 240×145 mm2; spatial resolution = 1.2×1.2×8mm3; readout bandwidth = 930 Hz/pixel; flip-angle = 70°; TR/TE = 6.2/3.1 ms; and temporal resolution = 37.2 ms.
Standard Cine SSFP imaging
Animals were also scanned under the same physiological conditions at rest with and without stenosis using a standard retrospectively gated, 2D cine SSFP sequence, immediately before or after BOLD imaging. Scan parameters were: field-of-view = 240×145 mm2; spatial resolution = 1.2×1.2×8mm3; readout bandwidth = 930 Hz/pixel; flip-angle = 70°; TR/TE = 2.5/1.3 ms; and temporal resolution = 37.5 ms. No parallel imaging was utilized and coil normalization and shimming were carried out as above.
Edema Imaging
T2-STIR images1 (matched to the BOLD imaging slices) were acquired at baseline, under LAD stenosis (< 20 minutes of ischemia induction), and subsequent to reperfusion after 3-hours of ischemia with the following imaging parameters: TE = 64 ms, TR = 2 R-R intervals (synchronized with every other heartbeat), TI = 170 ms, Echo train length = 15; Echo spacing = 7.1 ms, readout bandwidth = 235 Hz/pixel, spatial resolution = 1.2×1.2×8.0 mm3. Edema images were acquired immediately after the cine acquisitions (12±2 min post stenosis) and following 3 hours of ischemia.
Late Gadolinium Enhancement (LGE) imaging
LGE scans (matched to the BOLD imaging slice) were performed within 20 mins of inducing ischemia and 3 hours after the infliction of stenosis to determine the site of myocardial injury. Imaging protocol for LGE MRI: PSIR reconstruction with TurboFLASH readout 23; spatial resolution = 1.3×1.3×8mm3; TE/TR = 3.9/8.2 ms; TI = 200–220 ms; flip angle = 25°; readout bandwidth = 140 Hz/pixel.
Image Analysis
All SSFP studies were analyzed with custom Matlab (The Mathworks, Inc, USA) software developed in our laboratory. Endocardial and epicardial borders were manually traced for an image in systole and the RV groove was manually identified. The borders were propagated (tracked) automatically in all images of the cardiac cycle using a myocardial border tracking method 24. Subsequently, the myocardium was further segmented automatically into 6 segments per image according to standard practice 25. Based on the automatically estimated blood volume in the ventricular cavity over the full cine stack, end-systole (ES) and end-diastole (ED) were automatically identified as those having the minimum and maximum blood volume, respectively. Using post-reperfusion LGE images, a remote (non-infarcted territory) was identified on the edema-weighted images and was used to determine edematous territories using a 2 standard deviation threshold criterion as before 26.
Systolic to Diastolic Ratios
On the basis of presence of tissue damage on the LGE images acquired 3 hours post reperfusion, two regions of myocardial segments were identified on the BOLD SSFP and cine SSFP images: “affected” as those affected by the LAD stenosis and “remote” typically in the LCX territory. The same regions (myocardial segments) were used to evaluate BOLD and cine SSFP signal changes under baseline or stenosis conditions. The average pixel intensity of a region was measured for each cardiac phase and recorded as a time series, which was subsequently, smoothed using a moving average filter of length 3. On the basis of the smoothed time series, the S/D of a region was defined as:
where ES and ED are as defined above. This process was repeated for the remote and affected regions under baseline and stenosis conditions, on BOLD and standard cine image stacks.
Cardiac Function
Ejection Fraction (EF) was estimated from BOLD SSFP images using the endocardial delineations used in the analysis described above. Myocardial wall thickening (WT) was estimated again from BOLD SSFP images using the myocardial delineations from the analysis described above, by excluding papillary muscles, and measuring the transmural length of the myocardium using 360 equally spaced radial chords in ES (WTS) and ED (WTD). For each of the six myocardial regions, the segmental Wall Thickening (sWT), defined as the per-segment average of 100% × [WTs − WTD]/WTD was computed.
Statistical Analysis
Data are reported as mean ± SEM. One-tailed t-tests were used to test the null hypothesis that S/D ≤ 1 for any region independent of condition. A two-way repeated measurement ANOVA was used to test the within subject effects of region (two categories: remote and affected) and condition (two categories: baseline and ischemia) and their interaction on S/D derived either based on BOLD or standard cine studies. The same test was used to assess the effects of region and condition on sWT measurements derived based on BOLD studies. The identity of the canine was used as a fixed effect. Bonferroni post-hoc tests were used when appropriate. A paired t-test was used to test the null hypothesis that EF at baseline and ischemia are equal. Receiver Operating Characteristic (ROC) analyses were performed to determine the diagnostic power of the proposed biomarker (S/D) in relation to EF and sWT using groupings of positive and negative diagnoses, shown in the Table. Differences in area-under-the-curve (AUC) between ROC curves, were compared using the critical z-ratio27. Normality of study data was tested by the Shapiro-Wilks and Kolmogorov-Smirnov tests to indicate the appropriateness of parametric testing. The significance level for all tests was set at p<0.05. All analyses were performed using OriginPro (OriginLab Corporation, ver. 8, Northampton, MA).
Table.
Grouping of positive and negative diagnoses for the different ROC analysis.
| ROC comparison | Imaging Biomarkers | Positive (+) | Negative (−) |
|---|---|---|---|
| A | Ejection Fraction (EF) | Ischemia | Baseline |
| B | Systolic to Diastolic Ratios (S/D), segmental Wall Thickening (sWT) | Affected region during ischemia | Remote region during ischemia |
Results
Out of the 14 animals, insufficient occluder fidelity (variable Doppler flow throughout the ischemia period) was evident in 3 animals and results from these animals were excluded from further analysis. Overall, 11 animals were studied and 22 BOLD and cine (baseline and ischemia), 33 edema-weighted (baseline, ischemia, reperfusion) and 22 LGE (during ischemia and post reperfusion) images were available for analysis. LGE studies (during ischemia) showed that no animal experienced infarction, while all LGE studies post reperfusion showed myocardial injury. All study data were found to follow normal distribution and are reported as mean ± SE. For post-hoc tests, least square means and standard errors of the comparison are shown when appropriate.
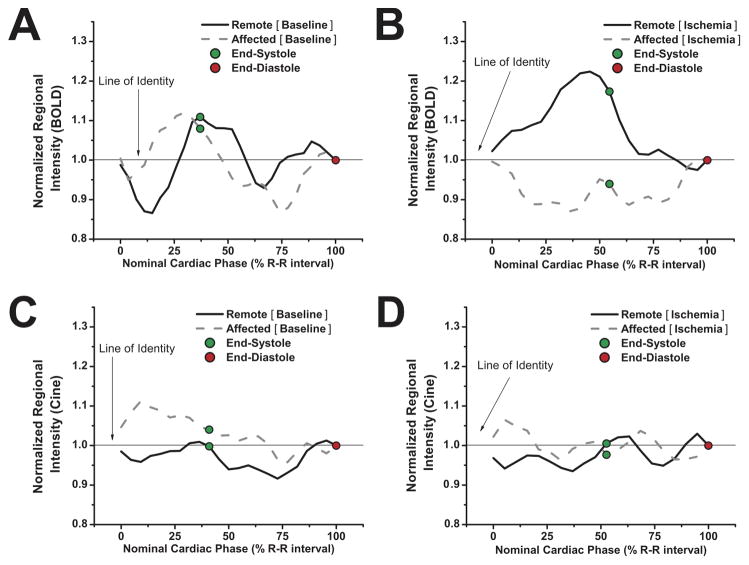
Figure 2 shows representative, regional mean BOLD signal intensities from remote and affected regions under baseline (Figure 2A) and ischemia (Figure 2B), normalized to ED values, obtained from a canine study. During baseline conditions (Figure 2A), both regions have S/Ds that are greater than 1 (1.1 and 1.07, for remote and affected respectively). Under ischemia (Figure 2B), however, in this animal, the remote territory exhibited a S/D greater than 1 (at 1.17). Conversely, the S/D for the affected (LAD) region was below 1 (at 0.93), in line with the hypothesis that S/D values are diminished during ischemia. For the same animal (and corresponding myocardial territories), Figures 2C and 2D show that the normalized regional mean signal intensities from a standard cine SSFP acquisition (which has minimal or no sensitivity to the BOLD effect), do not exhibit the same variation in S/D, as observed in Figures 2A and 2B.
Figure 2.
Normalized regional mean SSFP intensities obtained with CP-BOLD and standard cine acquisitions. Regional mean SSFP intensities obtained with CP-BOLD acquisitions from affected and remote regions under baseline (A) and ischemia (severe LAD stenosis, B), normalized by the regional mean intensities at end-diastole (shown with red circles) as a function of nominal percentage of cardiac cycle. Similar signal profile from standard cine, under baseline (C) and ischemia (severe LAD stenosis, D) are also shown. In all panels, green circles indicate end-systole. A line of identity (S/D =1) is also shown for reference. S/D: Systolic to Diastolic Ratio.
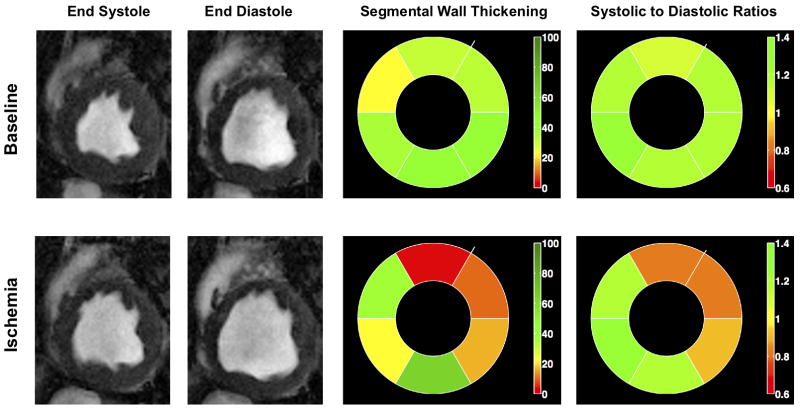
Figure 3 shows ES and ED BOLD images and bulls-eye plots of S/D ratios derived from the ES and ED BOLD images, as well as the sWT of the myocardium under baseline and ischemia from the same animal in Figure 2. Observe that under baseline conditions, all regions of the myocardium exhibit high S/D (green color). However, under ischemia the affected regions corresponding to the LAD territory exhibit lower S/D (red color), while all other regions remain green (S/D > 1). Also note that the baseline sWT varies among regions, and in the presence of ischemia, sWT is reduced. However, this reduction is not confined to the affected region, and in fact, remote regions also exhibit large deviations compared to their baseline values.
Figure 3.
BOLD end systolic and end diastolic images, circumferential polar plots (bulls eye) of systolic to diastolic ratios (S/D) and segmental Wall Thickening (sWT) from a representative animal under baseline and ischemia (severe LAD stenosis) are shown.
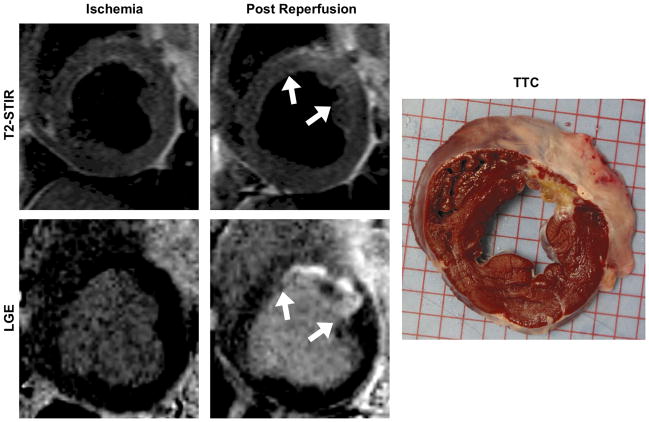
In Figure 4, edema-weighted and LGE images, as well as a gross histology (TTC) image acquired from the same animal in Figures 2 and 3, are shown. Note that edema appears to be absent at baseline and during early ischemia but is clearly present following reperfusion after a 3-hr ischemic period. Similarly, LGE images show that no enhancement is present in the early ischemic phase of the study, but is clearly present following the 3-hr ischemic period followed by reperfusion, and is confirmed by the gross histological, TTC, staining.
Figure 4.
Edema-weighted (T2-STIR) and late gadolinium enhancement (LGE) images from the same animal as in Figure 3, obtained within 20 minutes of ischemia induction and 3-hours post reperfusion, and TTC image confirming the infarct territory are shown. No edema (absence of elevation in T2-weighted signal) or infarction was observed within the 20 minutes of ischemia induction (left); post reperfusion (preceded by 3-hr ischemia), both edema and infarction were readily apparent in regions where Systolic to Diastolic ratio (S/D) changes were observed during the early ischemic phase (Figure 3).
Our statistical analysis showed that EF was markedly reduced during ischemia compared to baseline (0.25±0.03 (ischemia) vs. 0.45±0.03 (baseline); p<0.001; Figure 5A). sWT measurements (Figure 5B) showed region to have a statistically significant effect (remote vs. affected: 0.58 (remote) vs. 0.43 (affected); p<0.009), but not condition (0.58 (baseline) vs. 0.42 (ischemia); p=0.07). The interaction term and all post-hoc statistical comparisons were not significant (baseline: 0.66±0.08 (remote) vs. 0.51±0.08 (affected); ischemia: 0.51±0.08 (remote) vs. 0.34±0.08 (affected); remote: 0.66±0.08 (baseline) vs. 0.51±0.08 (ischemia); affected: 0.51±0.08 (baseline) vs. 0.34±0.08 (ischemia); all P=NS); indicating that wall thickness may vary across regions, and that regional ischemia may affect sWT measurements in a global fashion. Subject effects were not statistically significant (p~1).
Figure 5.
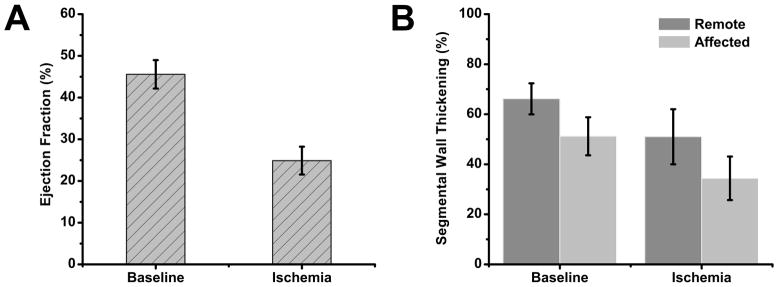
Ejection Fraction (EF) and Segmental Wall Thickening (sWT). Experimental data are reported as mean ± SEM.
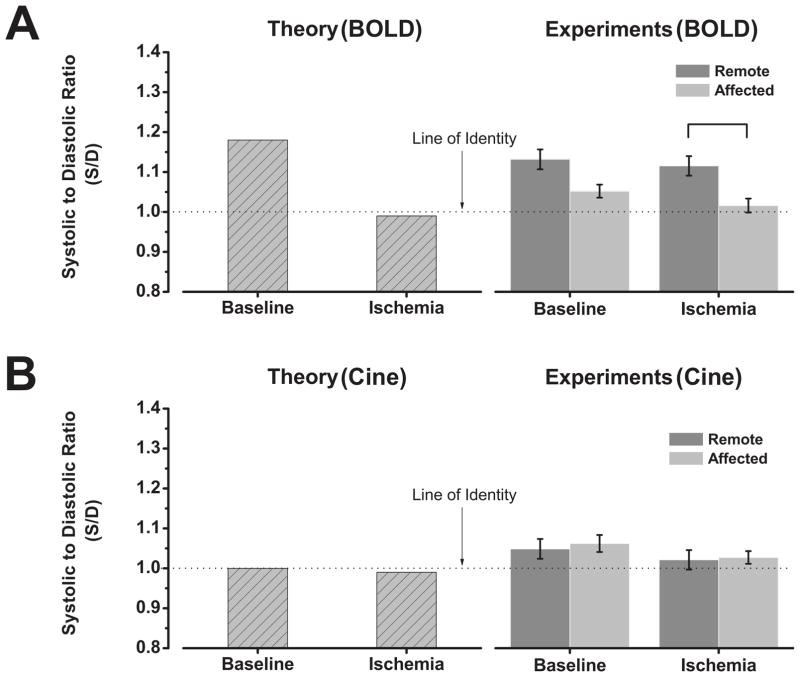
Figure 6 shows both simulated and experimental SSFP signal variations between ES and ED under baseline and ischemia, under BOLD (Figure 6A) and standard cine acquisitions (Figure 6B). Simulations showed that during baseline, S/D > 1; and in the presence of ischemia S/D < 1 (Figure 6A, Theory). Statistical analysis (Figure 6A, Experiments), showed that mean S/D values obtained from remote (1.13±0.025) and affected regions (1.05±0.016) under baseline conditions or from remote regions during ischemia (1.11±0.02) was likely greater than 1 (p < 0.05, t-test). However, the same test showed that this did not hold for S/D values obtained from affected regions during ischemia (1.01±0.017). Furthermore, controlling for repeated measures, the ANOVA test found region to be a significant factor (1.12 (remote) vs. 1.03 (affected); p=0.001), while condition (1.09 (baseline) vs. 1.06 (ischemia)) and interaction were not significant. Post-hoc tests found statistically significant differences in S/D between remote and affected (LAD) regions under ischemia (1.11±0.026 (remote) vs. 1.01±0.026 (affected), P=0.03), but not for other comparisons (baseline: 1.13±0.026 (remote) vs. 1.05±0.026 (affected), P=0.13; remote: 1.13±0.03 (baseline) vs. 1.11±0.03 (ischemia), P=1; affected: 1.05±0.03 (baseline) vs 1.01±0.03 (ischemia), P=1). Subject effects were not statistically significant (p~1).
Figure 6.
Bar plots showing agreement between theoretical and experimental findings on S/D based on a BOLD sensitive acquisition (A) and a standard cine acquisition (B). Experimental data are reported as mean ± SEM. Bar indicates p<0.05.
If a standard cine acquisition is used (Figure 6B, Theory), where the BOLD effect is expected to be minimal, simulations showed that ES and ED SSFP signal intensities are approximately equal, at baseline and ischemia (i.e., S/D ~ 1). The ANOVA test (Figure 6B, Experiments), did not find any statistically significant effect for region (1.04 (remote) vs. 1.03 (affected); p=0.61) and condition (1.05 (baseline) vs. 1.02 (ischemia); p=0.09) and their interaction (p=0.87). Subject effects were not statistically significant (p~1).
EF may be capable of discriminating between baseline and ischemia at rest (Figure 7A). The proposed method based on S/D, achieves comparable performance with EF (AUC=0.87 vs. 0.89) and is able to identify the affected territory solely using BOLD images acquired during ischemia (Figure 7B). sWT (AUC=0.75) appears to underperform S/D; however, this difference was not statistically significant (z-ratio=0.96, p=0.33).
Figure 7.
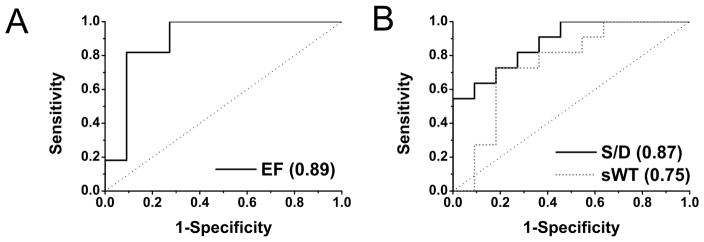
Receiver operating characteristic (ROC) curves corresponding to the biomarker in relation to the Table: A EF baseline vs. ischemia; B S/D and sWT only during ischemia, remote vs. affected regions. In the legends of each plot, area-under-the-curve (AUC) values are provided. The cut-off point that maximizes sensitivity and specificity for S/D is 1.07. Abbreviations are the same as in Figures 2 and 6 or the Table.
Discussion
Recently, the use of CMR for evaluating myocardial ischemia has received significant attention 28,29. A number of non-invasive imaging approaches have been proposed for the identification of ischemic territories, although these methods typically require provocative (exercise or pharmacological) stress 30,31. Although the edema approach is appealing given it is performed at rest and does not require contrast injections, it requires the appearance of edema within the ischemic period, which may be related to the time of onset of ischemia, and may be limited by the lack of specificity of an ongoing ischemia or a recent reversible injury. In this work, we demonstrated a contrast-free CMR approach for rapidly assessing myocardial ischemia without provocative stress, prior to the evolution of edema. The proposed method relies on the differences between systolic and diastolic BOLD SSFP signal intensities, attributable to changes in MBV and %HbO2, to determine the ischemic territory. In particular, we demonstrated that with ischemia, regional S/D values are significantly reduced, which may allow for the detection of ischemic territories at rest. We also showed that the proposed approach could complement standard volumetric indices, ejection fraction and segmental wall thickening, for localizing ischemic territories. Hence the deployment of cine BOLD MRI in place of standard cine MRI provides incremental improvement for the assessment of myocardial ischemia without resorting to provocative stress or appearance of edema.
Our simulations and experimental findings demonstrate the capability of the proposed method to identify ischemic territories on the basis of cardiac phase-dependent BOLD signal changes under rest. These findings confirm our hypothesis that systolic and diastolic BOLD SSFP signal intensities should be different in the absence of ischemia and that such differences are marginalized in the presence of ischemia. In addition, consistent with the theoretical prediction, we also found that when the BOLD effect is minimized (minimal TR, standard cine SSFP), the S/D ratio of SSFP signal intensities to be indifferent in the presence or absence of coronary artery stenosis. Since T1 effects are equally present in standard SSFP and BOLD SSFP acquisitions, it appears that the T2 effect (which is sensitive to changes in TR and is affected by changes in %HbO2)15 is the dominant source of the observed effect.
More recently, it was shown that myocardial edema could be useful in identifying ischemic territories prior to the appearance of myocardial necrosis 30,32. Using canines, Abdel-Aty and colleagues showed that the appearance of edema may be observed as early as 30 minutes following ischemic insult to the myocardium 32. In this study, we showed that S/D changes in the affected territories are apparent even before the appearance of edema. This suggests that the alterations in MBV and %HbO2, in response to coronary obstruction, precede intracellular edema, making the proposed approach more attractive as a very early marker of myocardial ischemia.
While we observed the S/D ratio to be significantly reduced in the setting of ischemia, it appears that the baseline S/D values vary throughout the myocardium and may not be uniform, as observed in Figure 6. These variations may be explained on the basis of phasic differences in coronary blood flow 33. Additional studies are likely necessary to determine/confirm the source of these regional differences.
In addition, the derivation of S/D depends on the accurate identification of ES and ED. Our determination of ES and ED based on measuring ventricular blood volume is considered to be the most accurate quantitative method for determining these key cardiac phases. Furthermore, Monte Carlo sensitivity analysis (not shown here) perturbing the locations of ES and ED showed that, small perturbations (± 1 cardiac phase with the current temporal resolution of 37 ms) have a minimal and statistically insignificant effect on S/D.
It is known that wall motion abnormalities and ejection fraction changes accompany significant ischemia. Although there are cut-offs for healthy EF, accepted cutoffs for sWT are not available. Since changes in EF are not unique to acute myocardial ischemia, additional indicators are necessary to confirm ongoing ischemia. The lack of accepted cutoffs for sWT in the literature may be justified in part due to the segmental wall thickening (sWT) varying among territories even under baseline, as we observed. In addition, since regional ischemia induces regional and global alteration in cardiac contraction, sWT appears to have limited ability to identify regional ischemic territories solely on the basis of wall motion. This is consistent with the findings of others that sWT measurements between remote and affected territories are in fact correlated and cannot precisely identify ischemic territories 34.
Our analysis found that sWT had a large AUC (Area-Under-the-Curve) for identifying affected from remote territories in the presence of ischemia. Similarly, the ANOVA test also showed that S/D could also reliably identify the affected territory when all the data and repeated measures are taken into account. Although EF and sWT are markers that aim to quantify the anatomical changes induced by ischemia, their exact values may vary among individuals and myocardial territories, respectively. On the other hand, S/D is a marker of MBV and %HbO2 that is altered due to ischemia, which is independent of anatomy but on tissue specific changes. Further investigations are necessary to evaluate whether the anatomic information (EF, sWT) and tissue-specific information (S/D), derived from a single MR study, can be optimally combined to further increase accuracy 35.
Since the differences in S/D ratios between non-ischemic and ischemic segments from this study was approximately 15%, additional improvements in sensitivity are expected to be necessary to extend this approach to investigate stable but significant coronary artery disease. Since 3T BOLD SSFP is expected to yield approximately a 3-fold increase in sensitivity 36 to changes in myocardial %HbO2, we anticipate further studies at 3T to be of great value. In addition, it is expected that more advanced image registration and myocardial-tracking methods that operate at the pixel-level would further increase sensitivity and specificity.
The current study only examined the cardiac phase-resolved BOLD SSFP signal changes under severe (flow limiting) acute stenosis. It would be useful to examine whether appreciable S/D signal differences can also be detected in the setting of clinically significant but stable (non-flow limiting) coronary artery stenosis. We anticipate that S/D ratios to be directly related to stenosis extent and hence with increased sensitivity (for instance by imaging at 3T), it may be possible to detect critically significant coronary artery disease, even when LV function is within normal limits, without resorting to provocative stress. Should such changes be detectable, it could provide a truly noninvasive imaging paradigm for diagnosing ischemic heart disease.
Limitations
This is a pilot study investigating S/D changes using an oxygen sensitive imaging sequence in the presence of coronary artery stenosis under rest in 11 animals. The proposed method remains to be validated in patients. Image processing methods that can rapidly and automatically segment and analyze cardiac phase-dependent myocardial BOLD images are also expected to be useful for effective translation of the proposed approach into the clinical arena.
Since this study used a 2D flow-compensated CP-BOLD SSFP sequence 22, it is possible that through-plane motion of the heart may have an effect on the results. However, to limit the effect of through-plane motion on phase-dependent signal and to accurately segment the LV myocardial wall, we only considered the mid-ventricular section of the myocardium, where it is expected that such motion is significantly lower than the basal slices. In fact, standard cine imaging of the same animal under the same physiological conditions, typically acquired within 5 minutes of the CP-BOLD acquisition did not show any statistically significant effects on systolic to diastolic differences between regions, lending further support that the contribution to signal changes from through-plane motion effects are insignificant. It is expected that with further pulse sequence development to extend the image acquisitions to 3D, along with flow- and motion-compensation, potential through-plane effects can be minimized and would facilitate a smooth clinical translation of the proposed approach.
The two-compartment model is expected to provide a reasonable approximation for the myocardial system as demonstrated previously 36. Nevertheless, there may be some limitation to this model since it assumes that the spin exchange between the vascular and extra-vascular space is fast, which may not be fully accurate. Bauer et al 37 have suggested that the spin exchange in the myocardium may conform to the intermediate exchange regime, which can be a source that underestimates the T2 values and ultimately underestimate the S/D contrast between ischemic and non-ischemic territories. In addition, the simulations also do not utilize the actual MBV or myocardial %HbO2. In this work, simulations were performed to lend a mechanistic insight into the direction of signal changes in conventional and BOLD cine acquisitions due to modulations in systolic and diastolic MBV and myocardial %HbO2. A more accurate model would require one to take into account the random motion of spins (via Monte Carlo simulation) along with measured values for MBV and myocardial %HbO2. However, such details on the physical parameters that elucidate the cardiac phased-resolved changes in MBV and %HbO2 in disease and health are currently unavailable due to the lack of direct measurement tools.
In addition, this study did not evaluate the changes in S/D values in chronic infarcts. Based on the biophysical mechanisms and on the basis of our simulation results, we anticipate that in the chronic phase, S/D to be approximately 1 and to vary minimally across the cardiac cycle since the infarcted tissue would be replaced by fibrotic tissue. To the extent that the fibrotic tissue receives blood from residual (spared from ischemia) or neovascular capillary beds, there would be some variation between systolic and diastolic signal intensities; however, such variations would be expected to be significantly lower than that in healthy myocardium. Hence, it is possible that S/D measurements alone cannot identify acute ischemic territories and that in addition to estimating S/D ratios, it may be important to rule out previous myocardial infarctions as well. Further studies are necessary to investigate the difference in S/D values between acute and chronic infarction.
Conclusion
Using a controlled canine model, we have provided the first evidence that it is possible to identify ischemic territories secondary to severe coronary artery stenosis on the basis of cardiac phase-resolved myocardial BOLD CMR without exogenous contrast agents or provocative stress, prior to the evolution of myocardial edema. The proposed method may be valuable in determining the presence of ongoing ischemia in patients.
CLINICAL PERSPECTIVE.
Diagnosis of myocardial ischemia secondary to clinically significant coronary artery stenosis is typically performed with the induction of provocative (exercise or pharmacological) stress. However, provocative stress generally imparts discomfort to the patient, is associated with small but significant number of adverse events and is contraindicated in people with lung disorders and atrioventricular blocks. Hence, approaches that can provide diagnostic information without additional stress to the vulnerable patient are of great interest. Recent studies have shown that provocative stress may be obviated in cases of acute ischemia, since the ensuing myocardial edema can be used as a marker of ischemia prior to tissue necrosis. In this study, using state-of-the art cardiac phase-resolved blood-oxygen-level-dependent (BOLD) CMR and a controlled animal model, we demonstrate that it may be possible to detect an on-going ischemia at rest even before the appearance of tissue specific changes (eg. edema, necrosis, etc), which typically occur downstream of the ischemic cascade. The proposed CMR method relies on the pathological alterations in the phasic changes of myocardial oxygenation and blood volume within the myocardial segments subtended by the stenotic coronary artery. Since our approach is capable of generating functional and tissue specific indices in one acquisition without requiring exogenous contrast media, we anticipate cardiac phase-resolved BOLD CMR can provide opportunities to rapidly and non-invasively assess myocardial ischemia at rest. Detecting the ischemic cascade at a very early stage can be of critical importance as it can facilitate successful patient management prior to the appearance of myocardial tissue damage.
Acknowledgments
Sources of Funding
This work was supported by grants from NIH/NHLBI (HL091989) and the American Heart Association (SDG 0735099N).
LIST OF ABBREVIATIONS
- AUC
Area Under the Curve
- BOLD
Blood-Oxygen-Level-Dependent
- CMR
Cardiovascular Magnetic Resonance
- ETCO2
End Tidal CO2
- LAD
Left Anterior Descending coronary artery
- LCX
Left-CircumfleX coronary artery
- LGE
Late Gadolinium Enhancement
- MBV
Myocardial Blood Volume
- S/D
Systolic to Diastolic ratio of mean segmental intensities from SSFP images
- SSFP
Steady-State Free Precession
- STIR
Short Tau Inversion Recovery
- sWT
Segmental Wall Thickening
- TTC
Triphenyltetrazolium Chloride (Staining)
- WT
Wall Thickening
- WTS
Wall Thickening at end systole
- WTD
Wall Thickening at end diastole
Footnotes
Disclosures
Drs. Rohan Dharmakumar and Debiao Li hold research grants from the National Institutes of Health/the National Heart, Lung and Blood Institute and receive research support from Siemens Healthcare. Dr. Dharmakumar also holds a research grant from the American Heart Association.
References
- 1.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 2.Raman SV, Simonetti OP, Winner MW, 3rd, Dickerson JA, He X, Mazzaferri EL, Jr, Ambrosio G. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55:2480–8. doi: 10.1016/j.jacc.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu EX, Tang H, Wong KK, Wang J. Mapping cyclic change of regional myocardial blood volume using steady-state susceptibility effect of iron oxide nanoparticles. J Magn Reson Imaging. 2004;19:50–8. doi: 10.1002/jmri.10426. [DOI] [PubMed] [Google Scholar]
- 4.Wansapura J, Gottliebson W, Crotty E, Fleck R. Cyclic variation of T1 in the myocardium at 3 T. Magn Reson Imaging. 2006;24:889–93. doi: 10.1016/j.mri.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Bai XJ, Iwamoto T, Williams AG, Jr, Fan WL, Downey HF. Coronary pressure-flow autoregulation protects myocardium from pressure-induced changes in oxygen consumption. Am J Physiol. 1994;266:H2359–68. doi: 10.1152/ajpheart.1994.266.6.H2359. [DOI] [PubMed] [Google Scholar]
- 6.Klocke FJ, Li D. Testing coronary flow reserve without a provocative stress. A “BOLD” approach. J Am Coll Cardiol. 2003;41:841–2. doi: 10.1016/s0735-1097(02)02930-3. [DOI] [PubMed] [Google Scholar]
- 7.Wei K, Tong KL, Belcik T, Rafter P, Ragosta M, Wang XQ, Kaul S. Detection of coronary stenoses at rest with myocardial contrast echocardiography. Circulation. 2005;112:1154–60. doi: 10.1161/CIRCULATIONAHA.104.513887. [DOI] [PubMed] [Google Scholar]
- 8.Wei K, Le E, Jayaweera AR, Bin JP, Goodman NC, Kaul S. Detection of noncritical coronary stenosis at rest without recourse to exercise or pharmacological stress. Circulation. 2002;105:218–23. doi: 10.1161/hc0202.101986. [DOI] [PubMed] [Google Scholar]
- 9.Lindner JR, Skyba DM, Goodman NC, Jayaweera AR, Kaul S. Changes in myocardial blood volume with graded coronary stenosis. Am J Physiol. 1997;272:H567–75. doi: 10.1152/ajpheart.1997.272.1.H567. [DOI] [PubMed] [Google Scholar]
- 10.Donahue KM, Weisskoff RM, Chesler DA, Kwong KK, Bogdanov AA, Jr, Mandeville JB, Rosen BR. Improving MR quantification of regional blood volume with intravascular T1 contrast agents: accuracy, precision, and water exchange. Magn Reson Med. 1996;36:858–67. doi: 10.1002/mrm.1910360608. [DOI] [PubMed] [Google Scholar]
- 11.Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imaging. 1991;1:275–83. doi: 10.1002/jmri.1880010303. [DOI] [PubMed] [Google Scholar]
- 12.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic resonance imaging: physical principles and sequence design. New York: Wiley; 1999. [Google Scholar]
- 13.Dharmakumar R, Wright G. Understanding Steady-State Free Precession: A Geometric Perspective. Concepts in Magnetic Resonance. 2005;26A:1–10. [Google Scholar]
- 14.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265–70. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 15.Dharmakumar R, Qi X, Hong J, Wright GA. Detecting microcirculatory changes in blood oxygen state with steady-state free precession imaging. Magn Reson Med. 2006;55:1372–80. doi: 10.1002/mrm.20911. [DOI] [PubMed] [Google Scholar]
- 16.Judd RM, Levy BI. Effects of barium-induced cardiac contraction on large- and small-vessel intramyocardial blood volume. Circ Res. 1991;68:217–25. doi: 10.1161/01.res.68.1.217. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Oellerich WF, Gropler RJ. Magnetic resonance assessment of myocardial oxygenation. In: Manning WPD, editor. Cardiovascular Magnetic Resonance. Philadelphia, PA: Churchill Livingstone; 2001. pp. 447–454. [Google Scholar]
- 18.Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003;49:515–26. doi: 10.1002/mrm.10407. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 20.Shah S, Kellman P, Greiser A, Weale P, Zuehlsdorff S, Jerecic R. Rapid Fieldmap Estimation for Cardiac Shimming. Proceedings 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine; Honolulu. 2009. p. 565. [Google Scholar]
- 21.Jellus V, Horger W, Kiefer B. Image Quality Improvement of Composed MR Images by Applying a Modified Homomorphic Filter. MAGNETOM Flash. 2009:180–184. doi: 10.1007/s00330-008-1011-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Tsaftaris SA, Liu Y, Tang R, Klein R, Zuehlsdorff S, Li D, Dharmakumar R. Artifact-reduced two-dimensional cine steady state free precession for myocardial blood-oxygen-level-dependent imaging. J Magn Reson Imaging. 2010;31:863–71. doi: 10.1002/jmri.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–83. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsaftaris SA, Andermatt V, Schlegel A, Katsaggelos AK, Li D, Dharmakumar R. A dynamic programming solution to tracking and elastically matching left ventricular walls in cardiac cine MRI. 15th IEEE International Conference on Image Processing (ICIP); 2008; 2008. pp. 2980–2983. [Google Scholar]
- 25.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–7. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro MD, Guarraia DL, Moloo J, Cury RC. Evaluation of acute coronary syndromes by cardiac magnetic resonance imaging. Top Magn Reson Imaging. 2008;19:25–32. doi: 10.1097/RMR.0b013e31816fd81d. [DOI] [PubMed] [Google Scholar]
- 29.Walls MC, Verhaert D, Min JK, Raman SV. Myocardial edema imaging in acute coronary syndromes. J Magn Reson Imaging. 2011;34:1243–50. doi: 10.1002/jmri.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–44. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 31.Kwong RY, Arai AE. Detecting patients with acute coronary syndrome in the chest pain center of the emergency department with cardiac magnetic resonance imaging. Crit Pathw Cardiol. 2004;3:25–31. doi: 10.1097/01.hpc.0000116584.57152.06. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 33.Ootaki Y, Ootaki C, Kamohara K, Akiyama M, Zahr F, Kopcak MW, Jr, Dessoffy R, Fukamachi K. Phasic coronary blood flow patterns in dogs vs. pigs: an acute ischemic heart study. Med Sci Monit. 2008;14:BR193–7. [PubMed] [Google Scholar]
- 34.Guth BD, White FC, Gallagher KP, Bloor CM. Decreased systolic wall thickening in myocardium adjacent to ischemic zones in conscious swine during brief coronary artery occlusion. Am Heart J. 1984;107:458–64. doi: 10.1016/0002-8703(84)90086-3. [DOI] [PubMed] [Google Scholar]
- 35.Pepe MS, Thompson ML. Combining diagnostic test results to increase accuracy. Biostatistics. 2000;1:123–40. doi: 10.1093/biostatistics/1.2.123. [DOI] [PubMed] [Google Scholar]
- 36.Dharmakumar R, Arumana J, Tang R, Harris K, Zhang Z, Li D. Assessment of regional myocardial oxygenation changes in the presence of coronary artery stenosis with balanced SSFP imaging at 3.0T: Theory and experimental evaluation in canines. J Magn Reson Imaging. 2008;27:1037–1045. doi: 10.1002/jmri.21345. [DOI] [PubMed] [Google Scholar]
- 37.Bauer WR, Nadler W, Bock M, Schad LR, Wacker C, Hartlep A, Ertl G. The relationship between the BOLD-induced T(2) and T(2)(*): a theoretical approach for the vasculature of myocardium. Magn Reson Med. 1999;42:1004–10. doi: 10.1002/(sici)1522-2594(199912)42:6<1004::aid-mrm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]