Abstract
Liver fibrosis is the result of chronic liver injury, and it represents a widespread medical problem. The aim of this study is to investigate the antifibrotic activity of isoquinoline alkaloid berberine in carbon tetrachloride (CCl4)-induced damage in mice. Hepatic fibrosis was induced by intraperitoneal (i.p.) administration of CCl4 (2 mL/kg, 20% v/v in olive oil) twice a week for 8 weeks. Berberine at the doses of 3 and 9 mg/kg and silymarin at the dose of 50 mg/kg were given i.p. once daily for the next 2 weeks. CCl4 intoxication increased the levels of serum transaminases and induced oxidative stress in the liver. Hepatic fibrosis was evidenced by a massive deposition of collagen, which coincided with increased expression of tumor necrosis factor (TNF)–α and transforming growth factor (TGF)–β1 and the activation of hepatic stellate cells. The high-dose berberine (9 mg/kg) ameliorated oxidative stress, decreased TNF-α and TGF-β1 expression, increased the levels of matrix metalloproteinase (MMP)–2, and stimulated the elimination of fibrous deposits. Berberine at the dose of 9 mg/kg exhibited stronger therapeutic activity against hepatic fibrosis than silymarin at the dose of 50 mg/kg. In vitro analyses show an important scavenging activity of berberine against oxygen and nitrogen reactive species. The results of this study suggest that berberine could ameliorate liver fibrosis through the suppression of hepatic oxidative stress and fibrogenic potential, concomitantly stimulating the degradation of collagen deposits by MMP-2.
Key Words: α–smooth muscle actin, berberine, liver fibrosis, matrix metalloproteinases, transforming growth factor-β1, tumor necrosis factor-α
Introduction
Liver fibrosis is a frequent event that follows chronic insult to the liver parenchyma. It is characterized by an excessive accumulation of extracellular matrix (ECM) proteins, including different collagen types. Cytokines and growth factors, such as tumor necrosis factor (TNF)–α and transforming growth factor (TGF)–β1, released by activated Kupffer cells, mediate the process of hepatic fibrogenesis.1 The profibrotic role of TGF-β1 is closely connected with the activation of hepatic stellate cells (HSCs),2 which are transformed into myofibroblast-like cells that are responsible for the overproduction of ECM proteins. The key mediators of ECM degradation and elimination of fibrous deposits are enzymes belonging to the family of matrix metalloproteinases (MMPs) and their inhibitors.3
Berberine is a naturally occurring isoquinoline alkaloid that can be found in the root, rhizome, and stem bark of many plant species from Berberidaceae and other families4 which are traditionally known for their beneficial effects on the digestive tract and liver. Numerous studies have shown that berberine possesses a wide range of pharmacological activities, including antioxidant5 and anti-inflammatory activity.6 These effects are most likely responsible for the hepatoprotective activity observed in acute toxic liver damage.7–12 Zhang et al.13 showed that berberine could be effective in protecting against the development of liver fibrosis induced by multiple hepatotoxic factors.
In the present study, we use a murine model of subchronic carbon tetrachloride (CCl4) intoxication to determine the therapeutic potential of berberine in well-established liver fibrosis and the mechanism of its antifibrotic activity. Silymarin, a mixture of three flavonolignans—silybin, silydianin, and silychristin—is considered an important hepatoprotective agent.14 We use silymarin as the reference drug.
Materials And Methods
Chemicals
Berberine was purchased from Polyphenols Laboratories AS (Sandnes, Norway), CCl4 from Kemika (Zagreb, Croatia), and acetic acid and methanol from T.T.T. (Sveta Nedjelja, Croatia). Silymarin, olive oil, 1,1,3,3,-tetramethoxypropane (TMP), trichloroacetic acid (TCA), sodium dodecylsulphate (SDS), bovine superoxide dismutase (SOD), xanthine, xanthine oxidase, cytochrome c, ethylenediaminetetraacetic acid (EDTA), 2,2-diphenyl-1-picryl-hydrazyl (DPPH•), hydrogen peroxide (H2O2), sodium nitroprusside dihydrate, naphthylethylenediamine dihydrochloride, sulfanilamide, and gelatin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ethanol and sodium phosphate were purchased from Kemika (Zagreb, Croatia). Diagnostic kits for the determination of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were obtained from Herbos Diagnostics (Sisak, Croatia), and those for alkaline phosphatase (ALP) were obtained from DiaSys Diagnostic Systems (Holzheim, Germany). Mouse monoclonal antibodies against TGF-β1 (ab92486), α–smooth muscle actin (α-SMA; ab18460), TNF-α (ab1793), and MMP-2 (ab7032) were purchased from Abcam (Cambridge, England, United Kingdom). The EnVision+System was purchased from DAKO Corporation (Carpinteria, CA, USA). Horseradish peroxidase (HRP)–conjugated anti–rabbit IgG was from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and HRP-conjugated anti–mouse IgG was from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). All other chemicals were of the highest grade commercially available.
Animals
Male Balb/c mice from a breeding colony of the School of Medicine, University of Rijeka, 2–3 months old, were divided into six groups with five animals per group. The mice were fed a standard rodent diet (pellet, type 4RF21 GLP; Mucedola, Milan, Italy). The animals were maintained in a 12 h light/dark cycle, at constant temperature (20°C±1°C) and humidity (50%±5%). All experimental procedures were performed in compliance with the appropriate laws and institutional guidelines and were approved by the ethics committee of the School of Medicine, University of Rijeka.
Experimental design
We administered CCl4 dissolved in olive oil (20%, v/v, 2 mL/kg) intraperitoneally (i.p.) twice a week for 8 weeks (groups II–VI), except for the control group (group I) that received olive oil only. Seventy-two hours after the last CCl4 injection, the CCl4-only group (group II) was sacrificed. The CCl4 control group (group III) was observed for spontaneous resolution of fibrosis for an additional 2 weeks. Berberine dissolved in dimethyl sulfoxide and diluted with sterile saline solution (final DMSO 5% v/v) was administered i.p. at the dose of 3 and 9 mg/kg daily for 2 weeks (groups IV and V, respectively), whereas mice from the control (group I) and CCl4 control (group III) groups received the vehicle instead. Silymarin (50 mg/kg) dissolved in the same solvent as berberine was administered to group VI. The doses were selected on the basis of our previous study.15 Animals were sacrificed 24 h after the last dose of berberine, silymarin, or saline, and heparinized blood was taken by cardiac puncture. Plasma was separated after centrifugation of blood samples at 5000 g for 10 min. The abdomen was opened and the livers were perfused with saline, excised, blotted dry, weighed, and divided into samples. If not used the same day, the samples were stored at −80°C.
Evaluation of in vitro antioxidant activity
DPPH• radical-scavenging assay
The DPPH• scavenging activity of the tested samples was measured according to the method of Blois.16 Briefly, 0.1 mM solution of DPPH• in ethanol was prepared, and this solution (0.5 mL) was added to the sample solution in ethanol (1.5 mL) at different concentrations (0.20–1600 μg/mL). The mixture was shaken vigorously, left to stand for 30 min in the dark, and then the absorbance was measured at 517 nm. The capability to scavenge the DPPH• radical is calculated using the following equation: (%)=[(A0 – A1)/A0]×100%, where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the sample, corrected for the absorbance of the sample itself. Trolox was used for comparison. All determinations were done in triplicate.
Nitric oxide radical-scavenging assay
The nitric oxide (NO•) scavenging activity of the tested samples was conducted according to the method described by Rai et al.17 with a slight modification. NO• generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen to produce nitrite ions, which were measured by the Griess reaction. An aliquot of the sample (1.0 mL) at various concentrations (3.13–800 μg/mL) was mixed with 10 mM sodium nitroprusside (1.0 mL) in phosphate-buffered saline (pH 7.4) and incubated at 25°C for 150 min. After incubation, 1.0 mL of the reaction mixture was mixed with 1% sulfanilamide (0.5 mL). After 5 min, 0.1% naphthylethylenediamine dihydrochloride (0.5 mL) was added, the solution was mixed, and the absorbance of a pink-colored chromophore was measured at 540 nm against the corresponding blank solution. Trolox was used as a standard. All experiments were performed in triplicate. NO•-scavenging activity is expressed as the percentage of inhibition according to the following equation: (%)=[(A0−A1)/A0]×100%, where A0 is the absorbance of the control without a sample and A1 is the absorbance in the presence of the sample.
H2O2 scavenging activity
The H2O2 scavenging ability of the tested samples was determined according to the method of Ruch et al.18 with minor modifications. A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). An aliquot of 3.4 mL of sample dissolved in 50% ethanol in phosphate buffer (0.1 mM, pH 7.4) with various concentrations (12.5–800 μg/mL) was mixed with 600 μL of the H2O2 solution. Trolox was used as the reference compound. The concentration of H2O2 was measured by reading the absorbance values at 230 nm of the reaction mixtures at 10 min against a blank solution containing the reaction mixture without H2O2. The percent of scavenging of H2O2 by berberine, silymarin, and the reference compound is calculated according to the formula: (%)=[(A0−A1)/A0]×100%, where A0 is the absorbance of the control and A1 is the absorbance in the presence of the tested samples. All determinations were done in triplicate.
Serum markers of liver damage
Plasma levels of AST, ALT, and ALP were measured using a Bio-Tek EL808 Ultra Microplate Reader (BioTek Instruments, Winooski, VT, USA), according to the manufacturer's instructions.
Determination of oxidative stress
The liver samples were homogenized in 50 mM phosphate buffer saline, pH 7.4 and centrifuged at 12,000 g for 15 min at 4°C. The homogenates were used for the measurement of lipid peroxidation, and the supernatants were used for the determination of Cu/Zn SOD activity and protein content.19
The Cu/Zn SOD activity was measured as the decrease in cytochrome c reduction by superoxide radicals generated with xanthine/xanthine oxidase system, as previously described.15 Briefly, the reaction mixture containing 0.05 mM cytochrome c and 1 mM xanthine was added to the supernatant. The reaction was started by the addition of 0.5 units of xanthine oxidase, and the increase in the absorbance was spectrophotometrically measured at 550 nm (Cary 100; Varian, Mulgrave, Australia). The calibration curve was constructed with known concentrations of Cu/Zn SOD.
Lipid peroxidation was assessed by the thiobarbituric acid-reactive substances (TBARS) assay as previously described.15 In brief, samples were mixed with 8% SDS, incubated at room temperature, and 20% acetic acid solution was added. The mixture was centrifuged at 10,000 g for 15 min. The 0.8% aqueous solution of TCA was added to the supernatant and heated in a boiling water bath. After cooling in cold water, the resulting chromogen was extracted with a 15:1 1-butanol/pyridine solution, the organic phase was separated by centrifugation at 3000 g, and the amount of TBARS was spectrophotometrically determined at 532 nm. TBARS levels were determined using the standard curve prepared from TMP.
Determination of hepatic hydroxyproline
The hydroxyproline content in the liver was determined after digestion of hepatic tissue in 6 M HCl at 110°C, as previously described.15 Briefly, samples were neutralized by 10 M NaOH and 3 M HCl. Chloramine T in citrate-acetate buffer was added, followed by the addition of Ehrlich reagent, and the samples were incubated in a water bath at 60°C. The absorbance of each sample was spectrophotometrically measured at 550 nm (Cary 100; Varian).
Histopathology
Liver specimens were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm thick sections. Liver sections were stained with Mallory trichrome stain. Hepatic fibrosis was assessed by use of the Ishak scoring system.20 The stage of fibrosis was grading as minimal fibrosis (Ishak score=1), mild fibrosis (Ishak score=2), moderate fibrosis (Ishak score=3 or 4), severe fibrosis and incomplete cirrhosis (Ishak score=5), and cirrhosis (Ishak score=6).
Immunohistochemistry
Immunohistochemical studies were performed on paraffin-embedded liver tissues using antibodies against TNF-α diluted 1:100, TGF-β1 diluted 1:50, α-SMA diluted 1:100, and MMP-2 diluted 1:200 employing the DAKO EnVision+System as previously described.15 Briefly, slides were incubated with peroxidase block, washed, and primary antibodies were added and incubated overnight at 4°C. Later, samples were incubated with a peroxidase-labeled polymer conjugated to secondary antibodies. The immunoreaction was visualized with diaminobenzidine solution. Tissues were counterstained with hematoxylin, dehydrated in a gradient of alcohol, cleared in xylol, and mounted with mounting medium. Stained slides were analyzed by light microscopy, and high-power field pictures (×400 magnification) of the livers were taken of each mouse (Olympus BX51, Tokyo, Japan). The average gray level of immunoreactivity was measured by the Cell F v3.1 software, Olympus Soft Imaging Solutions (Münster, Germany).
MMP zymography
The gelatinolytic activity in tissue homogenates was measured by the gelatin zymography as previously described.15 Briefly, after tissue homogenization in radioimmunoprecipitation assay buffer with added inhibitors of proteases, 10 μg of liver tissue lysates were separated by a 10% SDS-PAGE gel containing 0.1% gelatin. Gels were washed in 2.5% Triton X-100; briefly washed in the reaction buffer containing 50 mM Tris-HCl, pH 7.5, 5 mM CaCl2, 1 μM ZnCl2, and 0.02% NaN3; and incubated at 37°C for 42 h. The MMP gelatinolytic activity was visualized by staining the gels with 0.1% Coomassie blue R-350, and gels were later destained with methanol–acetic acid–water (30:10:60 v/v). The intensity of the bands was measured using image-analysis software (NIH Image J software, available at http://rsb.info.nih.gov/ij).
Statistical analysis
Data were analyzed by nonparametric Kruskal–Wallis test, and post hoc comparisons were carried out with Dunn's multiple comparison test. The data were analyzed by StatSoft STATISTICA version 10.0 software. For in vitro analyses, differences were estimated by Student's t-test (Microsoft Excel 2000 software). The concentration of the sample that provides 50% inhibition (IC50) was obtained by interpolation from linear regression analysis. Values in the text are means±standard deviation. Differences with P<.05 were considered statistically significant.
Results
In vitro antioxidant activity
The ability of berberine and silymarin to scavenge reactive species was assessed on the basis of their IC50 values. As shown in Table 1, berberine was less reactive toward NO• radical than Trolox but showed a significantly higher NO•-scavenging activity (P<.05) than silymarin. In contrast, berberine proved to be a more effective H2O2 scavenger than Trolox, although it was less potent than silymarin. However, DPPH•-scavenging activity of berberine was pronouncedly lower compared with either silymarin or Trolox, showing only marginal activity.
Table 1.
In Vitro Reactive Oxygen Species Scavenging Activity of the Tested Compounds
| |
IC50 (μg/mL) |
||
|---|---|---|---|
| Sample | DPPH•scavenging activity | NO•scavenging activity | H2O2 scavenging activity |
| Berberine | 1156.2±23.6a | 103.33±7.59a | 37.07±0.74a |
| Silymarin | 15.91±0.89b | 128.45±2.39b | 30.09±0.84b |
| Trolox | 1.54±0.15c | 51.00±2.74c | 152.56±0.74c |
Each value is expressed as mean±standard deviation (n=3).
Means within rows sharing the same letter are not significantly different from each other (P>.05).
IC50 value, the effective concentration at which 2,2-diphenyl-1-picryl-hydrazyl (DPPH•), nitric oxide (NO•), and hydrogen peroxide (H2O2) were scavenged by 50%.
Serum activities of AST, ALT, and ALP
The activities of ALT, AST, and ALP in serum significantly increased (P<.05) after 8 weeks of CCl4 intoxication (Table 2). The activity of transaminases and ALP spontaneously decreased in the CCl4 control group when compared with the CCl4-only group. The administration of berberine caused an additional decrease in the enzyme activities. Groups receiving berberine at the dose of 9 mg/kg and silymarin at the dose of 50 mg/kg had AST, ALT, and ALP values that were similar to controls.
Table 2.
Plasma Markers of Liver Damage, Body and Liver Weight, and Hydroxyproline Content
| Body weight gain*(g) | Liver weight†(g/100 g b.w.) | Hyp (μg/g liver) | AST (U/L) | ALT (U/L) | ALP (U/L) | |
|---|---|---|---|---|---|---|
| Control | 4.98±0.46a | 5.64±0.05a | 180±14a | 37.4±2.9a | 24.8±1.9a | 66.2±5.5a |
| CCl4 | 1.12±0.18b | 8.65±0.09b | 455±51b | 105.7±7.8b | 77.5±3.7b | 118.9±7.1b |
| CCl4+vehicle | 1.83±0.23c | 6.71±0.30c | 622±97c | 51.4±6.5c | 35.1±2.5c | 79.4±6.2c |
| CCl4+berberine 3 mg/kg | 2.57±0.26d | 6.98±0.59c | 479±61b | 45.3±4.1c | 30.4±2.2d | 74.8±5.4c |
| CCl4+berberine 9 mg/kg | 4.21±0.44a | 6.92±0.39c | 246±30d | 39.7±3.3cd | 27.3±0.8e | 69.5±3.8d |
| CCl4+silymarin 50 mg/kg | 3.36±0.38e | 6.87±0.38c | 230±28d | 41.1±2.9cd | 26.7±1.1e | 67.4±4.3d |
Each value represents the mean±SD for five mice.
Body weight gain represents a difference between the initial and final body weight.
Relative liver weight is expressed as g of liver weight/100 g of body weight (b.w.).
abcde: Means within rows sharing the same letter are not significantly different from each other (P>.05).
Hyp, hydroxyproline; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; CCl4, carbon tetrachloride; SD, standard deviation.
Liver weight and hydroxyproline content
The relative liver weight of CCl4-intoxicated mice markedly increased when compared with the control group, which was accompanied by a significant increase in hydroxyproline content (P<.05; Table 2). In mice that were observed for a spontaneous reversion of fibrosis, the relative liver weight decreased when compared with the CCl4-only group, while the hydroxyproline content increased. The treatment with berberine or silymarin did not have a notable impact on the relative weight of livers, whereas the hydroxyproline content significantly decreased in mice receiving berberine at the dose of 9 mg/kg and silymarin at the dose of 50 mg/kg (P<.05). Body weight gain during the experiment was the most prominent in control mice and in mice treated with a high dose of berberine. In contrast, mice from the CCl4 and the CCl4 control groups showed a severe body weight gain limitation.
Measurement of oxidative stress
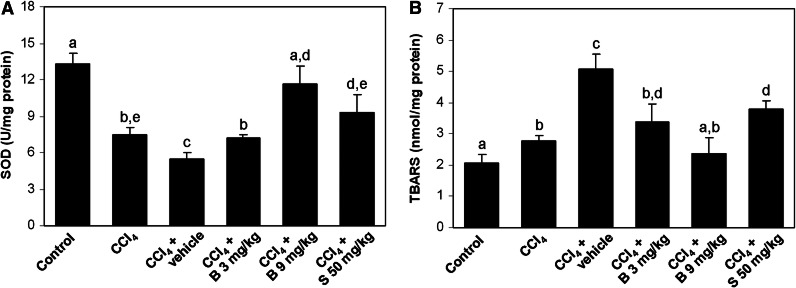
The significant increase in the TBARS level and the decrease in Cu/Zn SOD activity (P<.05 compared with controls) in the livers of CCl4-intoxicated mice suggested induction of oxidative stress (Fig. 1). Oxidative stress was further enhanced in the CCl4 control group. Berberine administration markedly attenuated the increase in the TBARS levels and the decrease in the Cu/Zn SOD activity in a dose-dependent manner, returning their levels to the normal values by the high dose of berberine. Silymarin at the dose of 50 mg/kg was less effective in the amelioration of oxidative stress when compared with the high-dose berberine.
FIG. 1.
The hepatic Cu/Zn SOD activity (A) and TBARS level (B) in experimental groups. Results are shown as the mean±SD; n=5 mice per group. abcdeMeans sharing the same letter are not significantly different from each other (P>.05). SOD, superoxide dismutase; TBARS, thiobarbituric acid-reactive substances; B, berberine; S, silymarin.
Liver histopathology
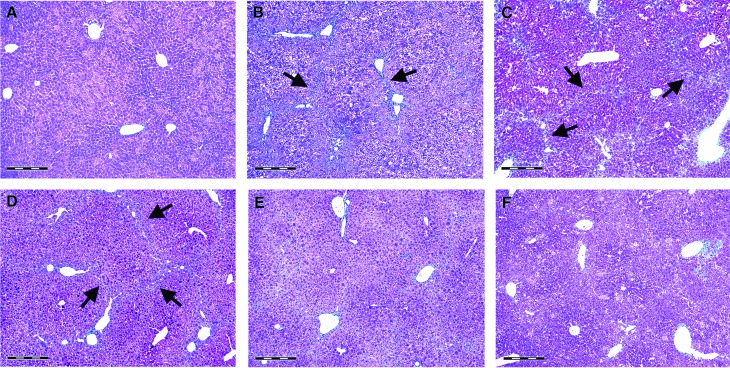
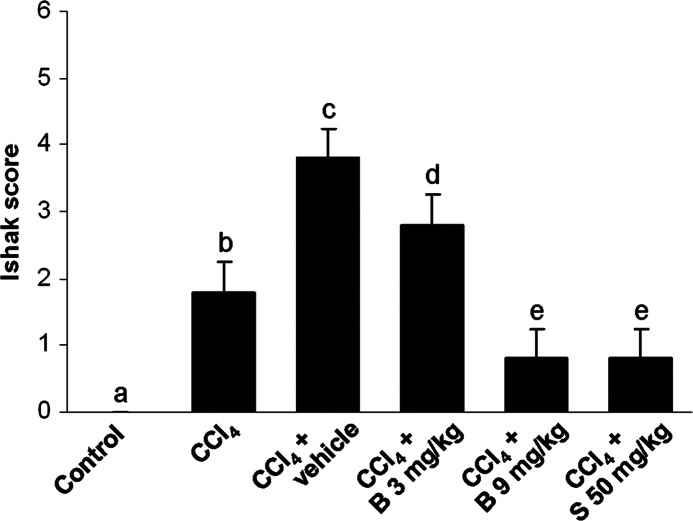
Figure 2 shows liver sections stained for collagen with Mallory trichrome stain. The livers from control mice exhibited normal hepatic structure, with only a few traces of collagen found in the blood vessel walls (Fig. 2A). Liver sections from mice receiving CCl4 for 8 weeks showed disorganization of hepatic cords, degenerative hepatocytes, and fibrous expansion of most portal areas, with occasional portal-to-portal bridging (Fig. 2B). In the CCl4 control group, the increase in collagen deposits and fibrous expansion of portal areas were found, with marked bridging that progressed into pseudolobules (Fig. 2C). In the livers of mice receiving 3 mg/kg of berberine, bridging fibrotic septa and collagen deposits were partially reduced in extent when compared with the CCl4 control group (Fig. 2D). In contrast, the livers of mice receiving 9 mg/kg berberine showed recovered histoarchitecture, with sporadic fibrosis in periportal areas (Fig. 2E), comparable with silymarin at the dose of 50 mg/kg (Fig. 2F). The degree of hepatic fibrosis was determined using Ishak scoring system (Fig. 3).
FIG. 2.
Mallory trichrome staining for collagen detection in control mice (A), mice receiving CCl4 for 8 weeks (B), CCl4 control group (C), and mice receiving berberine at the doses of 3 mg/kg (D) and 9 mg/kg (E) and silymarin at the dose of 50 mg/kg (F) for 2 weeks. Arrows show collagen fibrils. Scale bars=500 μm. CCl4, carbon tetrachloride. Color images available online at www.liebertpub.com/jmf
FIG. 3.
Scoring on hepatic fibrosis. Results are shown as the mean±SD, n=5 mice per group. abcdeMeans sharing the same letter are not significantly different from each other (P>.05).
Hepatic inflammatory response
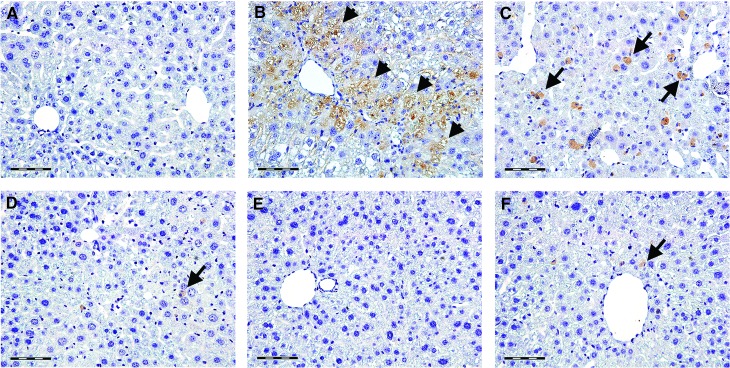
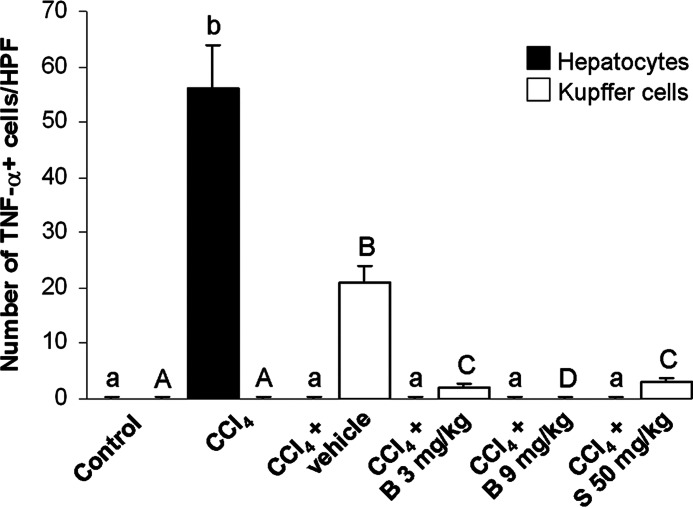
The expression of TNF-α and its hepatic distribution is shown in Figures 4 and 5. The livers of control mice were negative to TNF-α (Fig. 4A). In the CCl4-only group, TNF-α immunopositivity was diffusely found in hepatocytes across necrotic areas (Fig. 4B). In contrast, the livers of CCl4 control mice showed TNF-α positive staining in Kupffer cells only (Fig. 4C). Only sporadic TNF-α immunopositivity was found in the livers of mice treated with low-dose berberine (Fig. 4D) and silymarin (Fig. 4F). In mice treated with a high dose of berberine, hepatic TNF-α staining was negative (Fig. 4E). The intensity of TNF-α immunostaining is shown in Figure 5.
FIG. 4.
The expression and tissue distribution of TNF-α in control mice (A), mice receiving CCl4 for 8 weeks (B), CCl4-treated mice 2 weeks after CCl4 cessation (C), and mice receiving berberine at the doses of 3 mg/kg (D) and 9 mg/kg (E) and silymarin at the dose of 50 mg/kg (F) for 2 weeks. Arrows show TNF-α immunopositive Kupffer cells, arrowheads show TNF-α positive staining in hepatocytes. Scale bars=50 μm. A representative immunohistochemical stain for TNF-α from five mice. TNF, tumor necrosis factor. Color images available online at www.liebertpub.com/jmf
FIG. 5.
The measurement of the TNF-α immunostaining intensity (n=5). Means sharing the same letter (abchepatocytes; ABCKupffer cells) are not significantly different from each other (P>.05).
Liver fibrosis
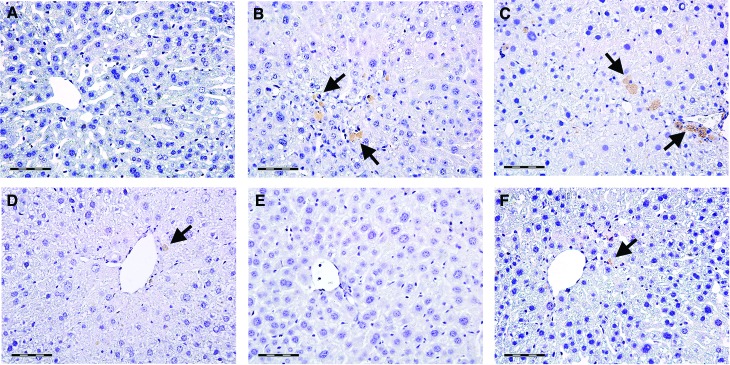
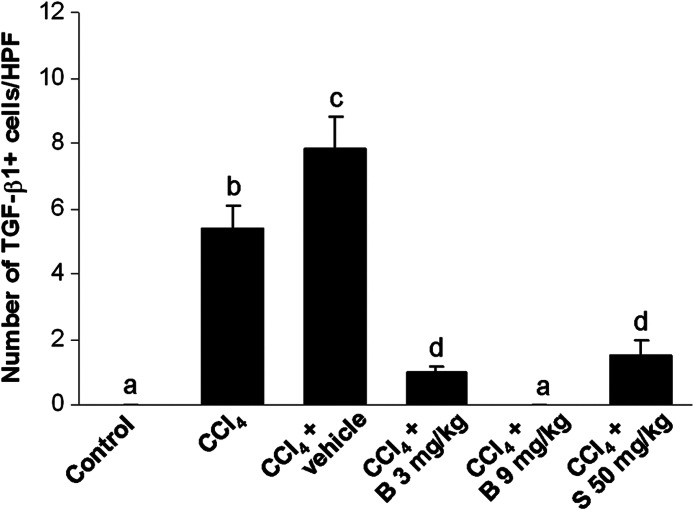
Figure 6 shows the immunohistochemical detection of TGF-β1 in the livers. All control liver tissues were TGF-β1 negative (Fig. 6A). In the livers of mice from the CCl4-only group (Fig. 6B), and particularly the CCl4 control group (Fig. 6C), TGF-β1 immunopositive Kupffer cells were detected. The treatment with berberine at the dose of 3 mg/kg (Fig. 6D) and silymarin at the dose of 50 mg/kg (Fig. 6F) markedly reduced hepatic TGF-β1 expression. In the livers of mice treated with high-dose berberine, TGF-β1 staining was negative (Fig. 6E). Figure 7 shows the number of TGF-β1 immunopositive macrophages in the livers.
FIG. 6.
The expression and tissue distribution of TGF-β1 in control mice (A), mice receiving CCl4 for 8 weeks (B), CCl4-treated mice 2 weeks after CCl4 cessation (C), and mice receiving berberine at the doses of 3 mg/kg (D) and 9 mg/kg (E) and silymarin at the dose of 50 mg/kg (F) for 2 weeks. Arrows show TGF-β1 immunopositive Kupffer cells. Scale bars=50 μm. A representative immunohistochemical stain for TGF-β1 from five mice. TGF, transforming growth factor. Color images available online at www.liebertpub.com/jmf
FIG. 7.
The count of TGF-β1 immunopositive cells per at least 10 high power fields. abcdMeans sharing the same letter are not significantly different from each other (P>.05).
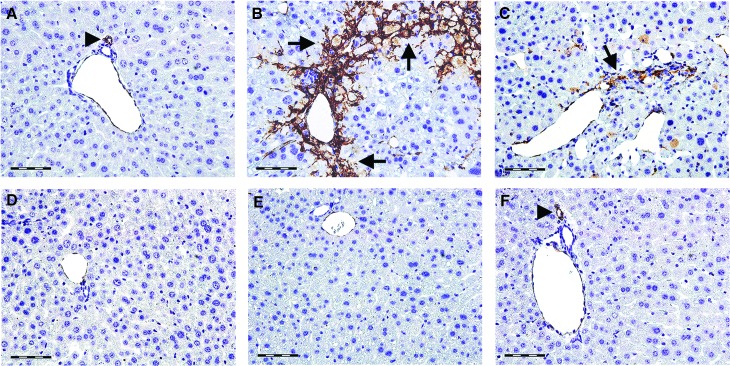
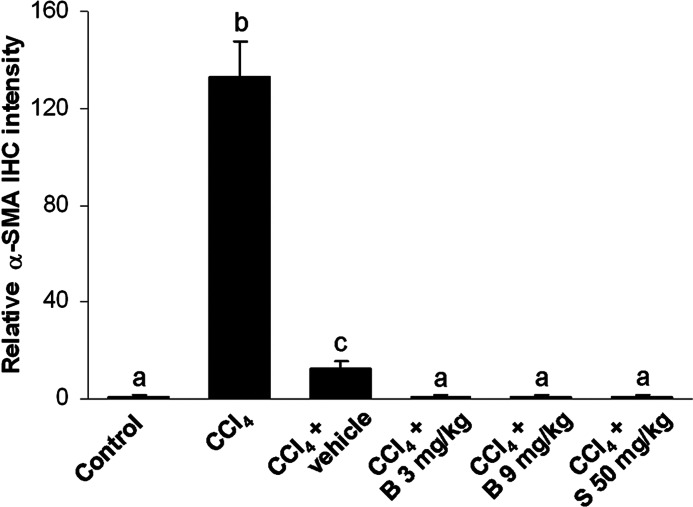
Hepatic α-SMA immunoreactivity, which detects activated HSCs, is shown in Figure 8. In control livers, α-SMA immunopositivity was found in the vascular walls, whereas other cells remained negative (Fig. 8A). The livers of CCl4-intoxicated mice showed strong α-SMA immunoreactivity in perisinusoidal areas (Fig. 8B). Two weeks after CCl4 cessation, weak α-SMA immunopositivity was still present in the CCl4 control group but it was substantially reduced (Fig. 8C). The livers of mice treated with berberine at the dose of 3 mg/kg (Fig. 8D) and silymarin at the dose of 50 mg/kg (Fig. 8F) showed sporadic α-SMA-positivity; whereas in mice receiving berberine at the dose of 9 mg/kg (Fig. 8E), α-SMA immunopositivity was expressed similar to the control group. Figure 9 shows the intensity of α-SMA immunostaining.
FIG. 8.
The expression and tissue distribution of α-SMA in control mice (A), mice receiving CCl4 for 8 weeks (B), CCl4-treated mice 2 weeks after CCl4 cessation (C), and mice receiving berberine at the doses of 3 mg/kg (D) and 9 mg/kg (E) and silymarin at the dose of 50 mg/kg (F) for 2 weeks. Arrowheads show α-SMA positive blood vessels, arrows show α-SMA immunopositivity in activated hepatic stellate cells. Scale bars=50 μm. A representative immunohistochemical stain for α-SMA from five mice. SMA, smooth muscle actin. Color images available online at www.liebertpub.com/jmf
FIG. 9.
The measurement of the α-SMA immunostaining intensity (n=5). abcMeans sharing the same letter are not significantly different from each other (P>.05).
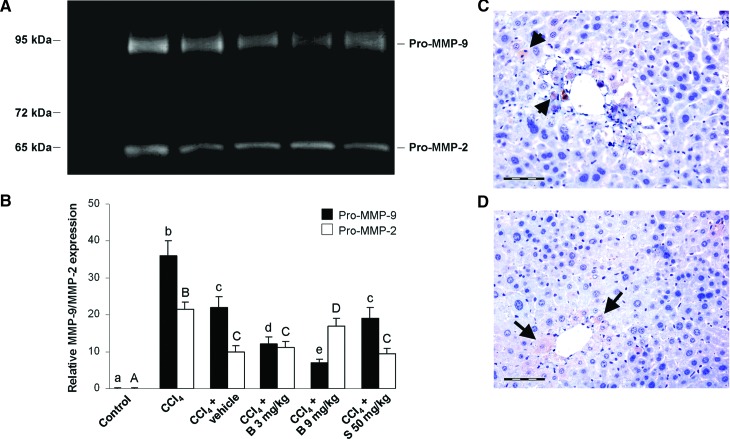
To investigate whether pro-MMP-2 and -9 were activated during the liver fibrosis and regeneration, crude protein extracts of liver tissues were subjected to zymographic analysis. A representative result of three zymography experiments is shown in Figure 10. Liver extracts in equal protein quantities (10 mg/kg) of hepatic homogenates contained mainly the latent forms of MMP-2 and MMP-9 at 65 and 92 kDa (Fig. 10A). In CCl4-intoxicated mice, the MMP-2 and MMP-9 proteins were clearly detected in comparison with controls (Fig. 10B). The treatment with berberine resulted in the downregulation of MMP-9 in a dose-dependent manner; however, MMP-2 expression increased in mice treated with high-dose berberine when compared with those treated with a lower dose. Silymarin had no significant effect on either the MMP-2 or MMP-9 activity 2 weeks after the beginning of the treatment. Figure 10C shows MMP-2 immunopositive nonparenchimal liver cells in the CCl4 control group, whereas Figure 10D reveals MMP-2 immunopositivity in the hepatocytes of mice treated with berberine at the dose of 9 mg/kg.
FIG. 10.
(A) A representative SDS-PAGE gelatin zymogram. MMP-9 and MMP-2 were identified by molecular weights relative to marker. (B) Expression of latent forms of 92 kDa MMP-9 and 65 kDa MMP-2 in experimental groups. The densitometric analysis of pro-MMP expression detected in gelatin zymograms. The bar graph shows the expression of latent forms of MMP-9 and MMP-2 (mean±SD, n=5). (C) MMP-2 immunopositivity in the CCl4 control group. (D) MMP-2 immunopositive hepatocytes in mice treated with berberine 9 mg/kg (arrows). Scale bars=50 μm. abcde,ABCDMeans sharing the same letter are not significantly different from each other (P>.05). MMP, matrix metalloproteinase. Color images available online at www.liebertpub.com/jmf
Discussion
Chronic liver injury initiates a cascade of events that result in an excessive production of ECM and hepatic fibrosis.21 In this study, we used a murine model of subchronic CCl4 administration for the induction of hepatic fibrogenesis. CCl4 intoxication and subsequent oxidative stress resulted in body weight loss, hepatocellular damage, and the release of hepatic aminotransferases and ALP into the blood. The increase in the plasma enzyme activity and body weight loss were dose-dependently ameliorated by berberine. Hepatic oxidative stress was markedly attenuated by high-dose berberine, as indicated by the normalization of the Cu/Zn SOD activity and the TBARS levels. The same results, however, could not be achieved by silymarin.
Previous in vitro studies showed relatively weak activity of berberine against free radicals, such as DPPH•, when compared with Trolox or vitamin C, which was attributed to the lack of the group bearing abstractable hydrogen.22,23 Nevertheless, in vivo studies revealed that berberine exerts a significant antioxidant and free radical-scavenging effects,5,24 which agrees with the results of the current study. We also show that berberine is a potent scavenger of NO• and H2O2, a physiologically relevant reactive species. Due to the prominent role of reactive oxygen and nitrogen species in the activation of HSCs and fibrogenesis,25 the inhibition of their formation by berberine could be considered the main mechanism of its antifibrotic and hepatoprotective activity. In addition, berberine is quickly metabolized in vivo into compounds with at least one phenolic group, such as jatrorrhizine,26 which exert even stronger free radical-scavenging activity than berberine.27
In the pathogenesis of liver fibrosis, several multifunctional cytokines, including TGF-β and TNF-α, have the most important role. Derived primarily from activated Kupffer cells, the TGF-β family of proteins has been identified as a profibrogenic master cytokine. A member of this family, TGF-β1, induces apoptosis of hepatocytes, activates HSCs, and promotes their epithelial-mesenchymal transition and ECM synthesis.28 In contrast to TGF-β1, the role of TNF-α, produced mainly by activated monocytes and macrophages during fibrogenesis, is still vague. In vitro studies demonstrated that TNF-α inhibits collagen synthesis,29 whereas it stimulates MMP-2/9 expression and activation.30,31 However, in several in vivo models of chronic liver injury, TNF-α has been found to stimulate both inflammation and fibrosis.32,33 The results of the current study are in agreement with the later findings, showing active production of TNF-α by macrophages in fibrotic livers. The treatment with berberine reduced TNF-α as well as TGF-β1 expression in the livers, even more effectively than silymarin. These results contribute to the hypothesis that the inhibition of TNF-α and TGF-β1 could be a part of therapeutic strategy for the treatment of liver inflammation and fibrosis.32 Our results showed increased perisinusoidal α-SMA immunopositivity in CCl4-intoxicated mice, suggesting the activation of HSCs and their transformation into myofibroblast-like, collagen-producing cells. HSCs were gradually entering the quiescent stage 2 weeks after CCl4 cessation. Meanwhile, they were still producing collagen, thus increasing the hepatic hydroxyproline content. The deactivation of HSCs is considered a key prerequisite for the resolution of fibrosis,34 the process in which MMPs and their specific inhibitors play a crucial role.35 In earlier stages of fibrogenesis, MMP-9 activates latent TGF-β,36 thus potentiating the initial phase of HSC activation and the subsequent collagen production. The downregulation of MMP-9 in the later stages of fibrogenesis appears to be mediated primarily by TGF-β, through a negative feedback mechanism.37 The results of our study confirm this hypothesis, as the decrease in MMP-9 expression in the CCl4 control group was accompanied by increased TGF-β1 expression.
Previous findings suggested that MMPs could be selectively involved in the fibrolytic process.38 The gelatinolytic activity of MMPs may be modulated by a specific drug treatment regime and could be heavily dependent on the type of injury.3 Thus, particular drugs exerting antifibrotic activity may differentially activate MMPs and initialize ECM degradation. In this study, silymarin did not significantly modulate either MMP-2 or MMP-9 expression. However, the treatment with berberine progressively decreased the MMP-9 expression in the livers, whereas the MMP-2 activity was increased by the high-dose berberine. Interestingly, Chen et al.39 showed that the resolution of thioacetamide-induced liver fibrosis by silymarin in rats was mediated through downregulation of MMP-2. Although MMP-2 is considered to be increased during fibrogenesis,1,40 this enzyme could also play an important role in collagen degradation.41 Activated HSCs serve as the main source of MMP-2 in fibrotic liver.35 Nevertheless, our results demonstrated that increased MMP-2 production in berberine-treated mice coincided with the deactivation of HSCs during the resolution of fibrosis. This suggests that other types of cells that are distinct from HSCs may serve as the source of MMP-2. Our findings demonstrated that hepatocytes could represent an alternative source of MMP-2, which agrees with similar findings.38
In conclusion, the results of this investigation show that treatment with berberine markedly ameliorates hepatic inflammation and fibrosis in mice. Antifibrotic activity of berberine is mediated, at least in part, through the suppression of hepatic oxidative stress and the activation of MMP-2 in the livers. Notably, berberine exerts a strong antifibrotic activity at a lower dose than the reference drug silymarin. The presented data indicate that berberine should be considered a therapeutic agent in the treatment of hepatic fibrosis, although further pharmacokinetic and clinical studies are required to confirm this hypothesis.
Acknowledgment
This research was supported by grants from the Ministry of Science, Education, and Sport, Republic of Croatia (Project 062-0000000-3554 and 006-0061117-1238).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Parola M. Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Knittel T. Mehde M. Grundmann A. Saile B. Scharf JG. Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 4.Birdsall TC. Kelly GS. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;2:94–103. [Google Scholar]
- 5.Yokozawa T. Ishida A. Kashiwada Y. Cho EJ. Kim HY. Ikeshiro Y. Coptidis Rhizoma: protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J Pharm Pharmacol. 2004;56:547–556. doi: 10.1211/0022357023024. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CL. Chi CW. Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Janbaz KH. Gilani AH. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71:25–33. doi: 10.1016/s0367-326x(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JM. Wang CJ. Chou FP. Tseng TH. Hsieh YS. Lin WL. Chu CY. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch Toxicol. 2002;76:664–670. doi: 10.1007/s00204-002-0351-9. [DOI] [PubMed] [Google Scholar]
- 9.Ye X. Feng Y. Tong Y. Ng KM. Tsao S. Lau GK. Sze C. Zhang Y. Tang J. Shen J. Kobayashi S. Hepatoprotective effects of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced acute liver hepatotoxicity in rats. J Ethnopharmacol. 2009;124:130–136. doi: 10.1016/j.jep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y. Siu KY. Ye X. Wang N. Yuen MF. Leung CH. Tong Y. Kobayashi S. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin Med. 2010;5:33. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domitrović R. Jakovac H. Blagojević G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology. 2011;280:33–43. doi: 10.1016/j.tox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X. Zhang J. Tong N. Chen Y. Luo Y. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol Pharm Bull. 2012;35:796–800. doi: 10.1248/bpb.35.796. [DOI] [PubMed] [Google Scholar]
- 13.Zhang BJ. Xu D. Guo Y. Ping J. Chen LB. Wang H. Protection by and anti-oxidant mechanism of berberine against rat liver fibrosis induced by multiple hepatotoxic factors. Clin Exp Pharmacol Physiol. 2008;35:303–309. doi: 10.1111/j.1440-1681.2007.04819.x. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan SC. Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 15.Domitrović R. Jakovac H. Antifibrotic activity of anthocyanidin delphinidin in carbon tetrachloride-induced hepatotoxicity in mice. Toxicology. 2010;272:1–10. doi: 10.1016/j.tox.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 17.Rai S. Wahile A. Mukherjee K. Saha BP. Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol. 2006;104:322–327. doi: 10.1016/j.jep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Ruch RJ. Cheng SJ. Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Ishak K. Baptista A. Bianchi L. Callea F. De Groote J. Gudat F. Denk H. Desmet V. Korb G. MacSween RN. Phillips MJ. Portmann BG. Poulsen H. Scheuer PJ. Schmid M. Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto H. Rippe R. Niemela O. Lin M. Roles of oxidative stress in activation of Kupffer and Ito cells in liver fibrogenesis. J Gastroenterol Hepatol. 1995;10:S50–S53. doi: 10.1111/j.1440-1746.1995.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 22.Luo A. Fan Y. Antioxidant activities of berberine hydrochloride. J Med Plants Res. 2011;5:3702–3707. [Google Scholar]
- 23.Račkova L. Majekova M. Koštálová D. Štefek M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg Med Chem. 2004;12:4709–4715. doi: 10.1016/j.bmc.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Zhou JY. Zhou SW. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia. 2011;82:184–189. doi: 10.1016/j.fitote.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo F. Nakamura N. Akao T. Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos. 2006;34:2064–2072. doi: 10.1124/dmd.106.011361. [DOI] [PubMed] [Google Scholar]
- 27.Keawpradub N. Dej-adisai S. Yuenyongsawad S. Antioxidant and cytotoxic activities of Thai medicinal plants named Khaminkhruea: Arcangelisia flava, Coscinium blumeanum and Fibraurea tinctoria. Songklanakarin J Sci Technol. 2005;27:455–467. [Google Scholar]
- 28.Bissell DM. Roulot D. George J. Transforming growth factor β and the liver. Hepatology. 2001;34:859–867. doi: 10.1053/jhep.2001.28457. [DOI] [PubMed] [Google Scholar]
- 29.Hernández-Muñoz I. de la Torre P. Sánchez-Alcázar JA. García I. Santiago E. Muñoz-Yagüe MT. Solís-Herruzo JA. Tumor necrosis factor alpha inhibits collagen alpha 1(I) gene expression in rat hepatic stellate cells through a G protein. Gastroenterology. 1997;113:625–640. doi: 10.1053/gast.1997.v113.pm9247485. [DOI] [PubMed] [Google Scholar]
- 30.Han YP. Tuan TL. Wu H. Hughes M. Garner WL. TNF-α stimulates activation of pro-MMP2 in human skin through NF-κB mediated induction of MT1-MMP. J Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CW. Lin CC. Lin WN. Liang KC. Luo SF. Wu CB. Wang SW. Yang CM. TNF-α induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L799–L812. doi: 10.1152/ajplung.00311.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kimura K. Ando K. Ohnishi H. Ishikawa T. Kakumu S. Takemura M. Muto Y. Moriwaki H. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol. 1999;11:1491–1500. doi: 10.1093/intimm/11.9.1491. [DOI] [PubMed] [Google Scholar]
- 33.Simeonova PP. Gallucci RM. Hulderman T. Wilson R. Kommineni C. Rao M. Luster MI. The role of tumor necrosis factor-α in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol. 2001;177:112–120. doi: 10.1006/taap.2001.9304. [DOI] [PubMed] [Google Scholar]
- 34.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 35.Hemmann S. Graf J. Roderfeld M. Roeb E. Expression of MMPs and TIMPs in liver fibrosis—a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955–975. doi: 10.1016/j.jhep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Yu Q. Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 37.Shek FW. Benyon RC. Walker FM. McCrudden PR. Pender SL. Williams EJ. Johnson PA. Johnson CD. Bateman AC. Fine DR. Iredale JP. Expression of transforming growth factor-β 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe T. Niioka M. Ishikawa A. Hozawa S. Arai M. Maruyama K. Okada A. Okazaki I. Dynamic change of cells expressing MMP-2 mRNA and MT1-MMP mRNA in the recovery from liver fibrosis in the rat. J Hepatol. 2001;35:465–473. doi: 10.1016/s0168-8278(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 39.Chen IS. Chen YC. Chou CH. Chuang RF. Sheen LY. Chiu CH. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J Sci Food Agric. 2012;92:1441–1447. doi: 10.1002/jsfa.4723. [DOI] [PubMed] [Google Scholar]
- 40.Overall CM. Wrana JL. Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-β1 in human fibroblasts. J Biol Chem. 1991;266:14064–14071. [PubMed] [Google Scholar]
- 41.Zhou X. Hovell CJ. Pawley S. Hutchings MI. Arthur MJ. Iredale JP. Benyon RC. Expression of matrix metalloproteinase-2 and −14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492–501. doi: 10.1111/j.1478-3231.2004.0946.x. [DOI] [PubMed] [Google Scholar]