Abstract
Purpose.
To determine whether choroidal imaging is feasible in preterm and term infants using an 840-nm portable spectral domain optical coherence tomography (SD-OCT) system without the use of enhanced-depth imaging techniques and to assess choroidal development by comparing choroidal thickness of preterm infants, term infants, and adults.
Methods.
SD-OCT images were obtained from 86 preterm infants, 59 term infants, and nine adults using a portable SD-OCT system plus nine adults using a tabletop system. An unprocessed image across the macula from one randomly selected eye of each participant was selected for determination of whether the choroidal-scleral junction (CSJ) could be visualized and for measurement of choroidal thickness.
Results.
Subfoveal CSJ was visualized in 96% of young-preterm infants (imaged from 30–36 weeks postmenstrual age [PMA]); 78% of term-aged preterm infants (imaged from 37–42 weeks PMA); 49% of term infants; and 39% of adult subjects. Racial pigmentation did not affect CSJ visibility in young-preterm infants (P = 0.57). Subfoveal choroidal thickness (SFCT) in young-preterm infants, term-aged preterm infants, term infants, and adults was 176 ± 53 μm, 289 ± 92 μm, 329 ± 66 μm, and 258 ± 66 μm, respectively, and these were all statistically significantly different from one another except term-aged preterms to adults.
Conclusions.
Infant choroid can be imaged with a portable SD-OCT system without enhanced depth imaging. Melanin in the RPE and choroid does not hinder outer choroidal imaging in young-preterm infants without advanced retinopathy of prematurity (ROP). In preterm infants, choroidal thickness increased with age but was thinner when compared to term infants suggesting delayed development due to ROP.
Keywords: choroid, retinopathy of prematurity, ocular development, optical coherence tomography
The choroid in preterm and term infants can be imaged with a portable 840-nm SD-OCT system. This finding may be related to less choroidal pigment at this early time of life. Choroidal imaging revealed delayed choroidal development in subjects born prematurely compared with term infants.
Introduction
The choroid is a highly vascular structure that supplies nourishment to RPE cells and to photoreceptors. Adult studies using optical coherence tomography (OCT) have implicated the choroid in the pathogenesis of a variety of ocular conditions such as glaucoma, diabetic retinopathy, and age-related macular degeneration.1–4
Choroidal vasculature begins to develop as early as the fourth week of gestation.5 At this time, the mesoderm surrounding the optic disk begins to differentiate into the early choriocapillaris.5 At approximately the sixth week of gestation, the primitive choriocapillaris completely encircles the optic cup.5 Connections with the posterior ciliary arteries and formation of vortex veins are completed by the second and third month of gestation and the choroid begins to mature by the fourth month of gestation.5 There are no reports of the choroidal vasculature in infants during the early period after preterm or term birth. Standard clinical OCT systems in ophthalmology with sources centered at ∼840 nm are commercially available for imaging the retina and inner choroid, but outer choroidal imaging is limited by backscattering at the RPE and inner choroid. OCT devices with 1050-nm sources provide deeper tissue penetration and less backscattering, making them ideal for choroidal imaging, but these systems are not commonly available in the ophthalmology clinic.6 Techniques such as enhanced-depth imaging (EDI) have been employed to provide better visualization of the choroid using standard clinical OCT machines.2,7–11
Portable spectral domain OCT (SD-OCT) imaging with an 840-nm to 100-nm bandwidth source (Bioptigen, Inc., Research Triangle Park, NC) has allowed clinicians to obtain SD-OCT images in subjects which were previously inaccessible. Using this device, clinicians have imaged preterm and term infants in the operating room, clinical exam visits, neonatal intensive care units, and newborn nursery, finding significant variation in retinal foveal morphology.12–22 However, SD-OCT has not been used to study the choroid during this critical part of ocular development.
Successful imaging of the choroid in preterm infants may provide meaningful information about the role of the choroid in foveal development and in retinopathy of prematurity (ROP). The purpose of this research was to determine the feasibility of visualizing the full thickness of the subfoveal choroid in preterm and term infants compared with adults through SD-OCT imaging at the bedside without the use of adjuvant anesthesia or sedation.7,23 Secondly, when imaging was feasible, we assessed choroidal development by comparing the choroidal appearance and thickness between preterm infants, term infants, and adults. Finally, we followed the choroidal development over time in a subgroup of the preterm infants.
Methods
This was part of a prospective institutional review board (IRB)–approved study of preterm infants who were enrolled at the Duke Neonatal Intensive Care Unit between January 20, 2009, and January 19, 2012, and healthy term infants who were enrolled at the Duke Birthing Center between August 19, 2010, and May 16, 2011. The prospective study designs for preterms in 2009 and for term infants in 2010 were to image the fundus, including choroid, with the SD-OCT; and the study design to analyze these OCT images for choroidal thickness was retrospective in 2011. Parents or legal guardians provided informed consent for each subject to participate. For comparison, adults aged under 50 years and without known history of ocular disease or premature birth were enrolled under a separate IRB-approved protocol and also provided informed consent for participation. This study adhered to the tenets of the Declaration of Helsinki. PubMed search included the terms “infant, pediatric, OCT, ROP, neonatal, preterm, choroid, and choroidal thickness.”
In total, 86 consecutive preterm infants, 59 healthy term infants, and 18 healthy adult subjects were included in the study to evaluate the feasibility of imaging the full thickness of the choroid at the fovea with SD-OCT. Preterm infants with severe systemic conditions and eye disease other than ROP (two preterm infants); infants not imaged within the window of 30 to 42 weeks postmenstrual age (PMA; five preterm infants); and infants in which the fovea was not captured on any eligible scan (one infant) were excluded, leaving 78 preterm infants in this study. A random number generator was used to select one eye visit per subject for analysis to assess choroid visibility. For each eye visit, the best captured central foveal frame was used for the analysis.
To assess choroidal development, we studied the previously selected foveal frame from all of the eye visits where the full thickness choroid was visible in the feasibility study. For all feasibility study eye visits in which the choroidal-scleral junction (CSJ) was not visible, we examined the fellow eye image at that visit; if the fellow eye CSJ was visible, the best foveal frame was included in the development study. For each eye visit, the scan with the best quality was used. This scan was used to measure from the foveal scans, choroid thickness was measured at a subfoveal site and two parafoveal sites 2000 microns away from the fovea. The 2000 microns parafoveal point was chosen because this was the farthest point away from the fovea where we believed we could obtain measurements in the majority of subjects. Because pediatric scans are not always centered, measurements farther away from 2000 microns would not have been possible in all subjects. This was selected before any measurements were performed or analyzed. For all pediatric scans, lateral measurement corrections were applied to compensate for the different estimated ocular axial lengths by age of each subject using the method described by Maldonado et al.14
Choroidal Imaging Feasibility Study Design
Because we were evaluating for change with development, we split the preterm infants into young-preterm (46 infants imaged from 30–36 weeks PMA) and term-aged preterm (32 infants imaged from 37–42 weeks PMA) infants. This cut point at 36 to 37 weeks was based on the definition of the World Health Organization on preterm birth as birth before 37 weeks of gestation. The term-aged preterm infants were compared with term infants, who were also aged 37 to 42 weeks PMA, to evaluate the choroid at comparable ages across the two groups. The percent success of imaging the full depth of the choroid was reported by age, race, ethnicity, and sex.
Choroidal Development Study Design
To determine the quantitative pattern of choroidal development by age, we included preterm, term infant, and adult eye imaging sessions in which there was full depth of the choroid at the fovea and no history of retinal disease or retinal detachment. We included only infants who never developed advanced ROP (maximum stage 2, no laser treatment, no plus disease, adequate follow-up) or retinal detachment. Seven of the preterm infants without sufficient follow-up and 27 with advanced ROP were excluded. Eight term infants were excluded due to subretinal fluid. Two preterm infants, 16 term infants, and 10 adult subjects were excluded because the full depth of the subfoveal choroid was not visible. Thus, 25 young-preterm infants, 17 term-aged preterm infants, 35 term infants, and eight adults were included in the choroidal development portion of the study, with one eye from one visit per subject (selected by a random number generator for eyes where the choroid was visible) analyzed.
To further evaluate choroidal development in individual subjects over time, we performed a secondary study of 13 preterm infants, each of whom had one eye imaging session in each of the two time periods (young-preterm and term-aged preterm) and of a subset of six preterm infants with five or more imaging sessions.
Clinical Examination and SD-OCT Imaging
Preterm infants underwent standard ROP screening. Term infants had dilated retinal examination without an eyelid speculum performed by a pediatric ophthalmologist within 48 hours of their birth as part of another imaging study to determine normal foveal morphology in healthy term infants. Examination data were recorded by the pediatric ophthalmologist in case report forms, and for the preterm infants, this included ROP data such as zone, stage, and vascular status (normal, pre-plus, plus).21 Note that eyes with pre-plus were not included in analysis of plus versus normal.
SD-OCT imaging was performed before or after ophthalmic examination on supine infants in a bed or incubator without sedation and without an eyelid speculum using a portable handheld SD-OCT (Bioptigen, Inc.) system and customized SD-OCT imaging parameters previously described.14 Healthy adults with no ocular conditions were imaged, nine using the portable SD-OCT, and nine using an equivalent table-top system by the same manufacturer with comparable settings. The tabletop axial measurements were modified with a correction factor (1.12 × tabletop thickness = handheld thickness) used to match the axial measurements of the handheld system. To calculate this factor, we used an eye model (Rowe Technical Design, Inc., Dana Point, CA) designed to mimic the human eye. Ten volumetric scans were taken in both the tabletop and handheld system, and two masked graders measured retinal thickness at the foveal center and at 1 mm laterally on each image. There was no significant difference among graders. There was significant difference between instruments with the tabletop measurements multiplied by 1.12 to achieve the handheld thicknesses. Scan lengths at the retina ranged from 6 to 10 mm with approximately 100 A-scans per millimeter at the retina across all subjects.
Image Processing, Measurements, Analysis
All SD-OCT images were converted into a digital imaging and communications in medicine (DICOM) format and qualitatively graded for pathology in medical imaging software (OsiriX; OsiriX Foundation, Geneva, Switzerland). The best-captured foveal frame from each imaging session was chosen for qualitative and quantitative analysis. To determine the central foveal frame, graders looked for the frame with the deepest foveal pit and thinnest inner retinal layers within the entire volumetric SD-OCT scan. The inner retinal surface; inner margin of the photoreceptor inner segment (IS) band; outer border of the RPE and CSJ; were delineated on the foveal scan using a custom program (Duke OCT Retinal Analysis Program, DOCTRAP, version 16.1), based in a commercial computing environment (MATLAB; MathWorks, Natick, MA).24 All segmentations were examined and manually corrected for deviations by a certified grader. A certified grader selected the foveal scan from a volume scan and identified the foveal center. A custom computing-based (MathWorks) program was used to extract and compute the choroidal thickness values from the semiautomatic DOCTRAP segmented images at 0.25-mm increments within 2 mm of foveal center.
Statistical Analysis
Outcome measures included choroidal thickness and subfoveal CSJ visibility (yes/no). Comparisons between three or more groups were made using a Kruskal-Wallis test and comparisons between two groups were made using a Wilcoxon rank sum test for equal medians. Matched pair analysis was done by Wilcoxon signed rank test for comparison between subfoveal, superior, inferior, nasal, and temporal choroidal thickness and for changes in choroidal thickness in the same infant. A Pearson correlation was used to assess the relationship between subfoveal choroidal thickness (SFCT) and age, gestational age, and birth weight. A Fisher's Exact Test was used to make comparisons between proportions. A P value of <0.05 was considered statistically significant. All statistical tests were run using statistical software (JMP; SAS Institute, Cary, NC).
Results
The results of the feasibility study in the four age groups are outlined in Table 1. There was a significant difference in visibility between the four age groups (P < 0.001) with the choroidal-scleral junction visible in 96% of young-preterm infants, in 78% of term-aged preterm infants, in 49% of term infants, and in 39% of the adult subjects. There was no difference in choroidal visibility by sex in any group and there was no difference in choroidal visibility by race in young-preterm infants (P = 0.57). But there was a significant difference of visibility by race in term-aged preterm infants (P = 0.01); term infants (P < 0.001); and adults (P = 0.023), with less choroidal visibility in African American and Hispanics compared with Caucasian subjects (Fig. 1).
Table 1.
Visibility of Full Thickness Choroid on SD-OCT Scan at the Fovea by Premature Birth, Age Group, Race, Ethnicity, and Sex
|
Young Preterm Infants, n
= 46 |
Term-Aged Preterm Infants, n
= 32 |
Term Infants, n
= 59 |
Adult Subjects, n
= 18, y |
|
| Demographics | ||||
| Age at imaging, median (SD), wk PMA | 34 (1.82) | 39.5 (1.87) | 39 (1.33) | 30 (8.05) |
| Gestational age, median (SD), wk PMA | 26 (2.60) | 26 (2.38) | 39 (1.33) | Term birth |
| Birth weight, median (SD), g | 800 (306) | 755 (204) | 3350 (471) | Unknown |
| Success of imaging CSJ number successfully imaged/ total number imaged (%) | ||||
| By race and ethnicity | ||||
| White | 21/21 (100%) | 18/19 (96.2%) | 19/20 (95.0%) | 5/7 (71.4%) |
| African American | 19/21 (90.4%) | 7/13 (53.9%) | 4/15 (26.7%) | 0/4 (0%) |
| Hispanic | 4/4 (100%) | 0/0 | 5/23 (21.7%) | 0/4 (0%) |
| Asian | 0/0 | 0/0 | 1/1 (100%) | 2/3 (66.7%) |
| By sex | ||||
| Female | 19/20 (95.0%) | 12/16 (75.0%) | 18/35 (51.4%) | 3/8 (37.5%) |
| Male | 25/26 (96.2%) | 13/16 (81.3%) | 11/24 (45.8%) | 4/10 (40.0%) |
| Total | 44/46 (95.7%) | 25/32 (78.1%) | 29/59 (49.2%) | 7/18 (38.9 %) |
Figure 1.
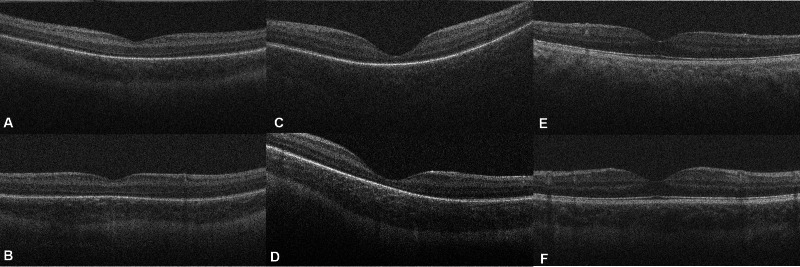
Single, unprocessed foveal B-scans from young premature infant eyes (A, B), term infant eyes (C, D), and adult eyes (E, F), acquired with a handheld SD-OCT system (Bioptigen). (A, C, E) Scans are from African American subjects while (B, D, F) are from Caucasian subjects. CSJ was visible in the African American premature infant (A) and Caucasian premature infant ([B], white arrow). CSJ was not visible in the African American term infant (C) and adult (E), but is noted in the Caucasian term infant (D) and adult (F).
Demographics including the age, sex, racial, and ethnic composition of the choroidal development study group are included in Table 2. The median ± SD of SFCT in young-preterm infants, term-aged preterm infants, term infants, and adults was 176 ± 53 μm, 289 ± 92 μm, 329 ± 66 μm, and 258 ± 66 μm, respectively (Fig. 2). Term-aged preterm infants had a significantly thicker choroid than young-preterm infants (P = 0.009) and a significantly thinner choroid than term infants (P = 0.03) at the fovea (Fig. 2), and this trend held across the retina (superior to inferior, Fig. 3). Adult SFCT was significantly thinner than that of term infants (P = 0.006) and significantly thicker than young-preterm infants (P = 0.03), but no different than term-aged preterm infants (P = 0.62; Fig. 2). In addition, an individual group analysis showed SFCT increased with age in preterm infants from 31 to 42 weeks PMA (R2 = 0.21, P = 0.002, Supplementary Fig. S1) and decreased with age in adults aged 20 to 37 years (R2 = 0.52, P = 0.043).
Table 2.
Demographics of Choroidal Development Study Cohort Without Advanced ROP and With Full Thickness Choroid Visible
|
Characteristics |
Young Preterm Infants, n
= 25 |
Term-Aged Preterm Infants, n
= 17 |
Term Infants, n
= 35 |
Adult Subjects, n
= 8 |
| Sex, n (%) | ||||
| Male | 10 (40) | 8 (53) | 11 (69) | 4 (50) |
| Female | 15 (60) | 9 (47) | 24 (31) | 4 (50) |
| Birth weight, g median (SD) | 800 (342) | 970 (227) | 3330 (500) | Unknown |
| Gestational age, wk PMA median (SD) | 26 (2.00) | 27 (1.67) | 39 (1.31) | Term birth |
| Age at imaging, wk PMA median (SD) | 33 (1.74) | 40 (1.84) | 39 (1.31) | 26 (5.8 y) |
| Race and ethnicity, n (%) | ||||
| African American | 14 (56) | 8 (47) | 8 (23) | 0 |
| White | 8 (32) | 9 (53) | 16 (46) | 5 (37.5) |
| Hispanic | 3 (9) | 0 | 10 (29)* | 0 |
| Asian | 0 | 0 | 1 (3) | 3 (62.5) |
The consent was translated into Spanish for part of the term infant study, thus increasing the number of Hispanic subjects in this group.
Figure 2.

Subfoveal choroidal thickness in all four groups. The median subfoveal choroidal thickness is greatest in term infants (329 ± 66 μm). The median subfoveal choroidal thicknesses of all age groups are significantly different from one another except for the comparison between adult subjects and term-aged preterm infants where there is no statistical difference.
Figure 3.
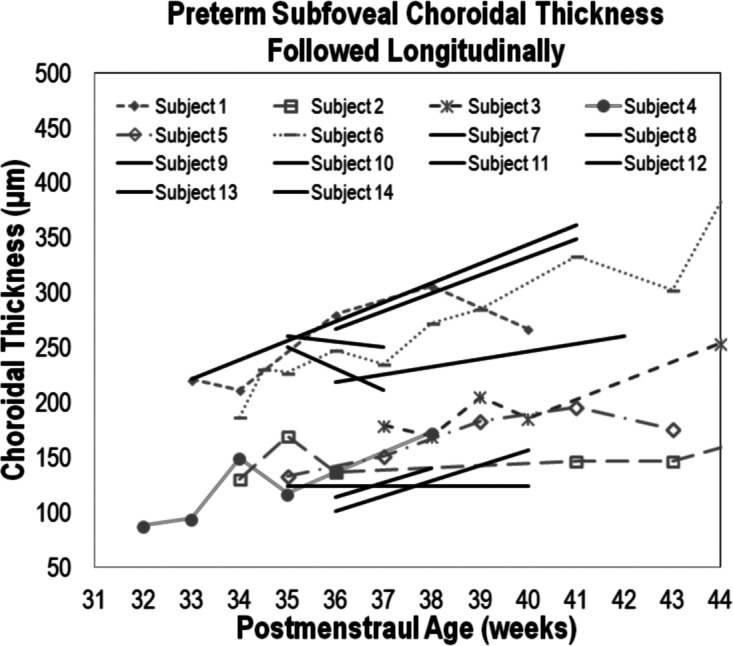
Preterm subfoveal choroidal thickness increased with age. Six subjects that had five or more visits (subjects 1–6) all showed the choroid thickness increasing with age. A Wilcoxon signed rank matched pair analysis including 13 subjects (P = 0.009) (subjects 1–14, excluding subject 3 since it did not have a visit in the young preterm age group) and a linear regression for all the preterm subjects in the study (P = 0.002), represented by the solid black line across the graph, also showed that choroid increased with age.
Choroidal thickening was evident with increasing age in preterm infants. A Wilcoxon signed rank matched pair analysis of 13 infants who had a visit in both the young-preterm and term-aged preterm infant groups showed that choroidal thickness increased in preterm infants with increasing age (P = 0.009, Fig. 3). In six of these preterm infants, SFCT was captured at multiple imaging sessions and also generally increased in thickness over time (Fig. 3).
In the young-preterm infants, the SFCT was weakly correlated to gestational age (R2 = 0.21, P = 0.021) but was not correlated with birth weight (R2 = 0.13, P = 0.07). In term-aged preterm infants, there was no significant correlation between SFCT and birth weight (R2 = 0.06, P = 0.34) or gestational age (R2 = 0.07, P = 0.32). In term infants, SFCT was not associated with birth weight (R2 = 0.02, P = 0.41) or postmenstrual age (R2 = 0.05, P = 0.18).
In both preterm groups, there was no significant difference in the central choroidal thickness by sex, race, or ethnicity (Table 3). In term infants, there was no significant difference in central choroidal thickness by sex, but there was a difference in choroidal thickness between Hispanics and Caucasians (P = 0.014, Table 3). It is critical to recognize, though, that 11 Hispanic eyes were not evaluated because of an inability to measure full choroidal thickness.
Table 3.
SFCT (Median ± SD) in Infants and Adults by Sex, Race, and Ethnicity
|
Young Preterm Infants |
Term-Aged Preterm Infants |
Term Infants |
Adult Subjects |
|
| Sex, median ± SD, μm | ||||
| Male | 174 ± 60 | 213 ± 90 | 331 ± 52 | 248 ± 72 |
| Female | 176 ± 49 | 324 ± 80 | 328 ± 73 | 263 ± 70 |
| Race and ethnicity, median ± SD, μm | ||||
| African American | 178 ± 36 | 222 ± 95 | 313 ± 63 | NA |
| White | 197 ± 64 | 324 ± 79 | 359 ± 63 | 258 ± 17 |
| Hispanic | 147 ± 97 | NA | 293 ± 60* | NA |
| Asian | NA | NA | 300 ± NA | 163 ± 112 |
| Total, median ± SD, μm | 176 ± 53 | 289 ± 92 | 329 ± 66 | 258 ± 66 |
Hispanic term infant subfoveal choroid is significantly thinner than Caucasian subfoveal choroid (P = 0.014).
In all infant groups, the subfoveal choroid was significantly thicker than the superior or inferior choroid at 2000 μm from the fovea, and in all groups except for the term-aged preterm infants, the inferior choroid was significantly thicker than the superior choroid (Table 4). In young-preterm infants, the subfoveal choroid was significantly thicker than the nasal choroid (P = 0.016, n = 7) and temporal choroid (P = 0.047, n = 7); and nasal and temporal choroidal thickness were not significantly different from one another (P = 0.22, n = 6). The overall choroid from superior to inferior appeared to vary less in thickness in young and term-aged preterm infants than term infants (Fig. 4). In term-aged preterm infants and term infants, an analysis of nasal and temporal measurements was not done due to the small number of horizontal scans in each group (only 3 subjects for term-aged preterm and 4 subjects for term infants).
Table 4.
Matched Paired Analysis of Subfoveal, Superior, and Inferior Choroidal Thicknesses at 2000 μm From the Fovea in Infants
|
Choroidal Thickness |
||||||
|
Subfoveal versus Superior |
Subfoveal versus Inferior |
Superior versus Inferior |
||||
|
Difference in Microns |
P |
Difference in Microns |
P |
Difference in Microns |
P |
|
| Term | 87 | <0.001 (n = 24) | 48 | <0.001 (n = 29) | 38 | 0.009 (n = 22) |
| Term-aged preterm | 64 | 0.008 (n = 8) | 38 | 0.003 (n = 14) | 32 | 0.11 (n = 8) |
| Young preterm | 34 | 0.007 (n = 12) | 23 | 0.043 (n = 14) | 21 | 0.023 (n = 9) |
Figure 4.
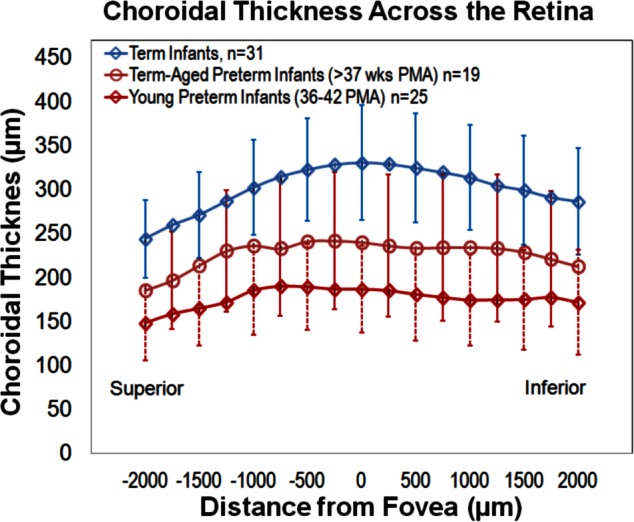
Choroidal thickness across vertical scans (superior to inferior) follow the same trend as central choroidal thickness with term infants having the greatest choroidal thickness followed by term-aged preterm infants then young preterm infants. Young preterm and term-aged preterm infants seem to have a slightly flatter overall choroid when compared to term infants.
Discussion
A structurally and functionally normal choroidal vasculature is essential for the function of the retina.25 Handheld SD-OCT imaging has allowed for noncontact in vivo high-resolution imaging of the retina without the use of anesthesia in term and preterm infants while still in infancy, but it has not previously been used to study the choroid in contrast to the adult. In this study, full subfoveal choroid was visible in 96% of preterm infants without the use of EDI techniques, but that visibility decreased with age, particularly in subjects of African American race and Hispanic ethnicity. We hypothesize that the ability to visualize the full depth of the choroid in preterm infants may be partially explained by the timing of development of pigmentation within RPE cells and choroidal melanocytes. Choroidal melanocytes derive from neural crest cell migration and RPE from optic cup, which is derived from the neuroectoderm, and they differ in developmental timing.26,27 RPE cell pigmentation occurs early in gestation, at approximately the second month. In contrast, choroidal pigmentation develops later, at approximately the eighth month.28 However, it is known that RPE cell density gradually increases in the macular area until up to 6 months of age.29 In this study, the preterm infants imaged before 37 weeks were imaged at a median age of 34 weeks PMA, near the proposed time of choroidal pigmentation development.28
Therefore, we propose that decreased choroidal melanin at this time after preterm birth (30–36 weeks PMA) is likely the reason for enhanced penetration of OCT signal through the choroid and subsequent reflectivity of outer choroidal structures and the CSJ. Unlike choroidal melanin, pigmentation of RPE cells does not vary by race, likely explaining the lack of variability in CSJ visibility by race in the young-preterm infants.30 In the term-aged preterm infants, CSJ visibility decreases as expected, coinciding with increased presence of choroidal melanin. However, the term-aged preterm infant subjects still had greater CSJ visibility than term infants of the same PMA, which suggests that prematurity may affect the timing of choroidal melanocyte development after birth.
The median SFCT measurements in young-preterm infants, term-aged preterm infants, and term infants increased from 176 ± 53 μm, 289 ± 92 μm, to 329 ± 66 μm, respectively, and these values were all statistically significantly different from one another. Although choroidal thicknesses increased with age in preterm infants, they remained significantly thinner than term infants, most likely representing delayed development. The median adult SFCT (258 ± 66 μm) was significantly less than that of term infants, which suggests a thinning of the choroid over time at some point after term age. On a previous study by Lin et al., the adult choroidal thickness was found to be comparable with choroidal thicknesses measured previously from the Cirrus (244 ± 56 μm) and Spectralis (223 ± 60 μm) systems.23 In addition, the adult choroid decreased in thickness with increasing age in our young adult population (aged 20–37 years), which agreed with the trend for data from other older adult studies—that choroidal thickness decreases with age.9,31 There was no significant difference in choroidal thickness in each group by sex or race and ethnicity, except for in term infants where there was a significant difference in choroidal thickness between Caucasian and Hispanic term infants.
This study demonstrates that choroidal development, both of pigmentation and thickness, continues after birth for preterm infants. Choroidal thickness in preterm infants increases with age; however, it lags behind that of term infants. The very few previous in vivo studies of the choroid in preterm infants in infancy have been either ultrasound based and focused on gross abnormalities such as choroidal hemorrhage, thickening, and calcification in eyes with retinal detachment from ROP,32–34 or based on fluorescein angiography with nonspecific choroidal filling abnormalities found in eyes with ROP.35 A recent OCT study of choroidal thickness in children aged between 4 and 10 years with a history of preterm or term birth showed that choroidal thickness at the fovea and seven of eight other locations in children born preterm was no different than that of children born full term at comparable ages,36 though this study was far after the period of early choroidal development.
It is known that the RPE is not fully developed at preterm birth, which may contribute to lack of completion of choroidal vascular development and thus a thinner choroid at term age.29 Although there have been limited studies of the molecular mechanism of choroidal vessel development, it is well accepted that a differentiated RPE plays a critical role.37–39 This has been demonstrated in animal models where an inactivation of VEGF expression in the RPE has led to the absence of choriocapillaris.40 For example, patients with colobomas have undeveloped choroidal vasculature.37 Furthermore, in vitro RPE cells are able to stimulate formation of choroidal cells.38,39 An alternative hypothesis for choroidal thickness in older preterm infants lagging below that of term infants could be the proposed mechanism of choroidal involution associated with ROP: that oxidative stress of the choroid causes damage to the vessels' endothelium and death as demonstrated in a rat model of ROP.41 However, the preterm infant lag in choroidal thickening could also be a manifestation of the known global developmental delay of preterm infants compared with normal term infants at the same age.42
These findings of thinner choroid in preterm infants and its delayed growth may play a role in future visual function in some infants. Photoreceptors are exclusively supplied with nutrients by the choroid and it is known that insufficient choroidal blood flow can result in photoreceptor death.25 Furthermore, during infancy, photoreceptors are still developing and have a high metabolic demand.43,44 In fact, Fulton et al. used multifocal electroretinography to show that infants with a history of mild ROP had a significantly decreased electroretinographic amplitude compared with normal controls.45 Park et al. found that children (aged 4–11 years) with a history of preterm birth had comparable choroidal thickness at the fovea and in most sites across the macula when compared with children with a history of term birth.36 The choroidal thickness at this later age did not correlate with visual acuity.36 Further studies are planned to evaluate the relationship between severity of ROP, systemic disease, birth age and weight, and choroidal thickness during infancy on visual acuity.
There are several limitations to this study. The ability to image the full thickness of the choroid at the fovea dropped off with age, particularly in African American and Hispanic subjects. Thus, although the CSJ was easily visible in most cases of preterm infants, without EDI, visualization of the choroid in the development analysis was limited in 16 of 51 (31%) term infants and 10/18 (56%) of adults. Although this demonstrates likely progressive onset of choroidal pigment with age, it also alerts one to potential imbalances in study populations when comparing choroidal thickness across racial and ethnic groups in the older subjects. For example, the subfoveal choroidal measurements in the term infants and adults in this pilot study are not fully representative of the population. We understand this potential limitation and are currently developing a method to incorporate EDI with handheld SD-OCT in a future study. This study is also limited by a relatively small sample size and by design as a cross-sectional study, with minimal longitudinal follow-up in few preterm infants. Finally, the preterm infants imaged at term age may represent a sicker population since they were still in the neonatal intensive care unit during this time, introducing potential bias to the analysis.
To our knowledge, this is the first in vivo description of human choroidal thickness and development in infancy (31–41 weeks PMA) of preterm and term infants using SD-OCT. It highlights the utility of handheld SD-OCT imaging in this population, the increase in thickness of the choroid, and decrease in choroidal signal likely due to increased pigmentation with age. Most importantly, this study demonstrates persisting thin choroid in preterm infants, which is likely to represent a delay in choroidal development. These findings highlight the importance of both choroidal and retinal vasculature in the developing infant eye and the potential to better understand the role of the choroid in retinopathy of prematurity through future clinical studies.
Supplementary Material
Acknowledgments
Supported by The Hartwell Foundation; Research to Prevent Blindness; The Andrew Family Foundation; Grant 1UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); and NIH Roadmap for Medical Research. The authors alone are responsible for the content and writing of the paper.
Disclosure: T.A. Moreno, None; R.V. O'Connell, None; S.J. Chiu, None; S. Farsiu, None; M.T. Cabrera, None; R.S. Maldonado, None; D. Tran-Viet, None; S.F. Freedman, None; D.K. Wallace, None; C.A. Toth, None
References
- 1. Yin ZQ, Vaegan , Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997; 6: 23–32 [PubMed] [Google Scholar]
- 2. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009; 29: 1469–1473 [DOI] [PubMed] [Google Scholar]
- 3. Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012; 32: 563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Switzer DW Jr, Mendonca LS, Saito M, Zweifel SA, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012; 32: 1265–1271 [DOI] [PubMed] [Google Scholar]
- 5. Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004; 48: 1045–1058 [DOI] [PubMed] [Google Scholar]
- 6. Wang RK, An L. Multifunctional imaging of human retina and choroid with 1050-nm spectral domain optical coherence tomography at 92-kHz line scan rate. J Biomed Opt. 2011; 16: 050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500 [DOI] [PubMed] [Google Scholar]
- 8. Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009; 148: 445–450 [DOI] [PubMed] [Google Scholar]
- 9. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147: 811–815 [DOI] [PubMed] [Google Scholar]
- 10. Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009; 147: 644–652 [DOI] [PubMed] [Google Scholar]
- 11. Imamura Y, Iida T, Maruko I, Zweifel SA, Spaide RF. Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. Am J Ophthalmol. 2011; 151: 297–302 [DOI] [PubMed] [Google Scholar]
- 12. Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009; 116: 2448–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott AW, Farsiu S, Enyedi LB, Wallace DK, Toth CA. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009; 147: 364–373 [DOI] [PubMed] [Google Scholar]
- 14. Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010; 51: 2678–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muni RH, Kohly RP, Charonis AC, Lee TC. Retinoschisis detected with handheld spectral-domain optical coherence tomography in neonates with advanced retinopathy of prematurity. Arch Ophthalmol. 2010; 128: 57–62 [DOI] [PubMed] [Google Scholar]
- 16. Muni RH, Kohly RP, Sohn EH, Lee TC. Hand-held spectral domain optical coherence tomography finding in shaken-baby syndrome. Retina. 2010; 30: S45–S50 [DOI] [PubMed] [Google Scholar]
- 17. Vinekar A, Sivakumar M, Shetty R, et al. A novel technique using spectral-domain optical coherence tomography (Spectralis, SD-OCT+HRA) to image supine non-anaesthetized infants: utility demonstrated in aggressive posterior retinopathy of prematurity. Eye (Lond). 2010; 24: 379–382 [DOI] [PubMed] [Google Scholar]
- 18. Maldonado RS, Freedman SF, Cotten CM, Ferranti JM, Toth CA. Reversible retinal edema in an infant with neonatal hemochromatosis and liver failure. J AAPOS. 2011; 15: 91–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maldonado RS, O'Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012; 130: 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maldonado RS, O'Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011; 118: 2315–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011; 31: 1470–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabrera MT, Maldonado RS, Toth CA, et al. Subfoveal fluid in healthy full-term newborns observed by handheld spectral-domain optical coherence tomography. Am J Ophthalmol. 2012; 153: 167–175.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin P, Mettu PS, Pomerleau DL, et al. Image inversion spectral-domain optical coherence tomography optimizes choroidal thickness and detail through improved contrast. Invest Ophthalmol Vis Sci. 2012; 53: 1874–1882 [DOI] [PubMed] [Google Scholar]
- 24. Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010; 18: 19413–19428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998; 116: 589–597 [DOI] [PubMed] [Google Scholar]
- 26. Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001; 128: 1059–1068 [DOI] [PubMed] [Google Scholar]
- 27. Bharti K, Miller SS, Arnheiter H. The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011; 24: 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mund ML, Rodrigues MM, Fine BS. Light and electron microscopic observations on the pigmented layers of the developing human eye. Am J Ophthalmol. 1972; 73: 167–182 [DOI] [PubMed] [Google Scholar]
- 29. Robb RM. Regional changes in retinal pigment epithelial cell density during ocular development. Invest Ophthalmol Vis Sci. 1985; 26: 614–620 [PubMed] [Google Scholar]
- 30. Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986; 27: 145–152 [PubMed] [Google Scholar]
- 31. Fujiwara A, Shiragami C, Shirakata Y, Manabe S, Izumibata S, Shiraga F. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol. 2012; 56: 230–235 [DOI] [PubMed] [Google Scholar]
- 32. de Juan E Jr, Shields S, Machemer R. The role of ultrasound in the management of retinopathy of prematurity. Ophthalmology. 1988; 95: 884–888 [DOI] [PubMed] [Google Scholar]
- 33. Pulido JS, Byrne SF, Clarkson JG, Di Bernardo CL, Howe CA. Evaluation of eyes with advanced stages of retinopathy of prematurity using standardized echography. Ophthalmology. 1991; 98: 1099–1104 [DOI] [PubMed] [Google Scholar]
- 34. Shapiro DR, Stone RD. Ultrasonic characteristics of retinopathy of prematurity presenting with leukokoria. Arch Ophthalmol. 1985; 103: 1690–1692 [DOI] [PubMed] [Google Scholar]
- 35. Lepore D, Molle F, Pagliara MM, et al. Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology. 2011; 118: 168–175 [DOI] [PubMed] [Google Scholar]
- 36. Park KA, Oh SY. Analysis of spectral-domain optical coherence tomography in preterm children: retinal layer thickness and choroidal thickness profiles. Invest Ophthalmol Vis Sci. 2012; 53: 7201–7207 [DOI] [PubMed] [Google Scholar]
- 37. Torczynski E. Choroid and suprachoroid. In: Jakobiec FA. ed Ocular Anatomy, Embryology and Teratology. Philadelphia: Harper & Row; 1982: 553–585 [Google Scholar]
- 38. Sakamoto T, Sakamoto H, Murphy TL, et al. Vessel formation by choroidal endothelial cells in vitro is modulated by retinal pigment epithelial cells. Arch Ophthalmol. 1995; 113: 512–520 [DOI] [PubMed] [Google Scholar]
- 39. Zhao S, Overbeek PA. Regulation of choroid development by the retinal pigment epithelium. Mol Vis. 2001; 7: 277–282 [PubMed] [Google Scholar]
- 40. Marneros AG, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005; 167: 1451–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shao Z, Dorfman AL, Seshadri S, et al. Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 6238–6248 [DOI] [PubMed] [Google Scholar]
- 42. Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002; 346: 149–157 [DOI] [PubMed] [Google Scholar]
- 43. Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986; 26: 847–855 [DOI] [PubMed] [Google Scholar]
- 44. Fulton AB, Hansen RM, Moskowitz A, Akula JD. The neurovascular retina in retinopathy of prematurity. Prog Retin Eye Res. 2009; 28: 452–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fulton AB, Hansen RM, Moskowitz A, Barnaby AM. Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc Ophthalmol. 2005; 111: 7–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


