Abstract
Studies in adults have demonstrated a relationship between lowered heart rate variability (HRV) and poor health. However, less is known about the role of autonomic arousal in children’s well-being. The aim of the current study was to examine resting HRV in children with chronic pain compared to healthy control children and, further, to examine children’s HRV following a series of acute experimental pain tasks in both groups. Participants included 104 healthy control children and 48 children with chronic pain aged 8–17 years. The laboratory session involved a 5-minute baseline electrocardiogram followed by four pain induction tasks: evoked pressure, cold pressor, focal pressure, and a conditioned pain modulation task. After the tasks were complete, a 5-minute post-task electrocardiogram recording was taken. Spectral analysis was used to capture high-frequency normalized power and the ratio of low-to-high frequency band power, signifying cardiac vagal tone and sympathetic balance, respectively. Results revealed that children with chronic pain had significantly lower resting HRV (signified by low high-frequency normalized power and high ratio of low-to-high frequency band power) compared to healthy children; moreover, a significant interaction between groups and time revealed that children with chronic pain displayed a static HRV response to the pain session compared to healthy children, whose HRV was reduced concomitant with the pain session. These findings suggest that children with chronic pain may have a sustained stress response with minimal variability in response to new acute pain stressors.
Keywords: laboratory pain, pediatric pain, cold pressor, experimental pain, childhood pain, stress task
Introduction
Heart rate variability (HRV), or the beat-to-beat alteration in heart rate, offers a noninvasive indicator of autonomic nervous system activity.1 Low HRV – indicative of reduced parasympathetic cardiac control – has been associated with disorders ranging from diabetes mellitus2 to sleep problems, as well as difficulty regulating emotions.3 There is also evidence for a link between decreased HRV and functional pain conditions such as irritable bowel syndrome.4 HRV may therefore represent a potent biomarker of general stress and health.5
Although the relationship between low HRV and compromised health has been extensively studied in adults, there has been less focus on children. Low HRV has been linked with anxiety and emotional disorders in children,6,7 but little research has been conducted in pediatric chronic pain. One study in children with recurrent abdominal pain indicated lower resting HRV relative to healthy controls,8 but others found no group differences.9,10 However, these studies included small sample sizes,8,9 or examined a narrow age range of relatively young children.10
As observed in the laboratory, HRV is sensitive to acute stress. Non-clinical children have shown HRV reductions following the tilt table test or standing tests,11 psychological stress,12 and playing violent video games.13 Two studies compared physiological reactivity in children with recurrent abdominal pain to healthy controls in response to a social stress task14 and a cold pressor task.15 Children with recurrent abdominal pain had elevated arousal, evidenced by increased heart rates, in response to these stressors; however, HRV was not examined. Recently, children with recurrent abdominal pain evidenced increased parasympathetic and sympathetic HRV in response to a cognitive task compared to controls.16 Coactivation of these arousal and resting responses is somewhat unexpected, and may denote an unbalanced autonomic system in children with recurrent abdominal pain. As yet, HRV response to pain-related stressors has not been examined in children with chronic pain.
HRV may also be an important biomarker of chronic allo-static stress load. Children with chronic pain typically experience considerable stress in trying to cope with their pain, and the total stress load, including anxiety experienced by these children, can further exacerbate their pain and contribute to pain-related disability. Studies on adults indicate that HRV biofeedback, which guides users to breathe at the optimal respiratory frequency to maximally increase their HRV, leads to improvements in perceived stress,17 anxiety,18 as well as autonomic regulation in response to a laboratory stress task.19 These results suggest that treatments targeting HRV may be useful in reducing stress and anxiety. A recent study in adolescents with chronic fatigue syndrome, a condition that is characterized by severe impairment and multiple symptoms including pain, found that hypersensitivity to sensory stimuli was associated with both disability and with low HRV in relation to a tilt table test.20 These findings similarly suggest that interventions addressing autonomic dysfunction, as indexed by low HRV, may have utility for children with multiple symptoms including pain. HRV may be an important biomarker to assess when evaluating interventions for pediatric chronic pain.
The first aim of this study was to examine resting HRV in children with chronic pain versus healthy control children; the second aim was to compare the groups’ HRV response to laboratory pain tasks. We used spectral analysis of HRV, a widely employed and recommended method,21 to capture high-frequency normalized power, refecting the parasympathetic system and vulnerability to stress,22 and the ratio of low-to-high frequency band powers, assessing sympathetic balance.23 We hypothesized that compared to healthy controls, children with chronic pain would display lower resting HRV. Based on studies using other autonomic measures,14,15 laboratory stressors should result in greater arousal and HRV declines in the pain group relative to healthy children. Therefore, we hypothesized that the pain group would evidence lowered HRV in response to the laboratory pain tasks compared to controls. Anxiety has also been implicated in altered acute pain sensitivity including enhancing descending pain facilitation pathways.24 In order to explore whether potential group differences could be explained by pain-related anxiety, we examined anxiety levels across the laboratory session.
Materials and methods
Participants
Participants included 152 children aged 8–17 (see Table 1 for demographic information): 104 healthy controls and 48 with chronic pain. Controls were recruited through advertisements, community events, and referrals from previous participants. The chronic pain sample was primarily recruited through a multidisciplinary, tertiary clinic specializing in pediatric chronic pain. Inclusion criteria for the chronic pain group followed the commonly accepted definition of a pain problem persisting for 3 months or longer.25
Table 1.
Demographic data by group and for the total sample
| Control group (n = 104) | Pain group (n = 48) | Total sample (n = 152) | |
|---|---|---|---|
| Gender [female – n (%)] | 56 (53.8%) | 30 (62.5%) | 86 (56.6%) |
| Mean age in years (SD) | 13.4 (2.8) | 14.2 (2.6) | 13.7 (2.8) |
| Ethnicity [n (%)] | |||
| Hispanic/Latino | 28 (26.9%) | 12 (25.0%) | 40 (26.3%) |
| Non-Hispanic/non-Latino | 76 (73.1%) | 36 (75.0%) | 112 (73.7%) |
| Race [n (%)] | |||
| White | 51 (50.5%) | 31 (64.6%) | 82 (55.0%) |
| African-American | 20 (19.8%) | 6 (12.5%) | 26 (17.4%) |
| Asian | 2 (2.0%) | 2 (4.2%) | 4 (2.7%) |
| Multi-racial | 28 (27.7%) | 9 (18.8%) | 37 (24.8%) |
| Pubertal status [n (%)] | |||
| Early puberty | 30 (28.8%) | 15 (31.3%) | 45 (29.6%) |
| Late puberty | 74 (71.2%) | 33 (68.8%) | 107 (70.4%) |
| Mean BMI (SD) | 21.0 (4.9) | 21.1 (5.7) | 21.1 (5.2) |
Notes: Racial data were unavailable for three control participants. Early puberty = Tanner stages I–II, Late Puberty = Tanner stages III–IV.
Abbreviations: BsMI, body mass index; SD, standard deviation.
Presenting pain diagnoses were: 58.3% headaches (migraines, and myofascial, vascular, tension, stress-related or other type of headache), 47.9% functional neurovisceral pain disorder (functional bowel, uterine, or bladder disorder), 8.3% type 1 or type 2 complex regional pain syndrome, 35.4% myofascial pain (of any part of the body excluding headaches), 22.9% fbromyalgia, and 6.3% joint pain (please note that percentages sum to more than 100% due to multiple pain diagnoses). Multiple pain diagnoses were present in 58.3% (n = 28) of the sample.
Eligibility was confirmed by telephone. Parents were asked whether their child met any of the following exclusionary criteria: acute illness/injury that may impact laboratory performance (eg, fever) or that affects sensitivity of the extremities (eg, Raynaud’s disease); daily use of opioids; developmental delay; autism; or significant anatomic impairment that could preclude participation in pain induction. Written informed consent was obtained from parents, and children provided written assent. The study was approved by the University of California Los Angeles Institutional Review Board. Each participating family member received $50 cash for their participation.
Procedures
Participants were escorted to the laboratory where their height and weight were recorded, and leads for physiological recording were attached. Participants were asked to sit quietly for a 5-minute baseline physiology recording, during which they watched a neutral nature DVD with no sound. The nature video was included to reduce excessive movement by participants, which would lead to artifacts in the data. Detailed procedures have been described in previous studies.26,27
Participants then completed the laboratory pain tasks: an evoked pressure task;28 a cold pressor task; a focal pressure task; and a conditioned pain modulation task.27 The evoked pressure task involved a series of 5-second trials of pressure applied to the left thumbnail; stimuli were first presented in a predictable, ascending manner up to participants’ rating of moderate pain (6 on a 0–10 numeric rating scale where 0 = no pain; 10 = worst/most pain). Pressure stimuli were then presented in random order to verify the pressure level corresponding to participants’ ratings of moderate pain. The cold pressor task consisted of a single immersion of the right hand in 5°C water; the trial had an uninformed 3-minute ceiling. The focal pressure task consisted of a single trial of pressure delivered to the second dorsal phalanx of the middle finger of the right hand via a dull lucite point; the trial had an uninformed 3-minute ceiling. The conditioned pain modulation task involved phasic pressure stimuli delivered to the left thumbnail, and simultaneous immersion of the right hand in 5°C water.27
Total time for the laboratory session was approximately 30–40 minutes. After the completion of the final task, another 5-minute post-session recording of HRV was taken during which subjects sat quietly. Participants were then unhooked from the physiological monitoring equipment.
The current investigation is presented as a separate report due to its focus on the physiological data recorded before and after pain induction; prior analyses have focused on self-reported psychological26 and clinical data,29 and behavioral aspects of pain responsivity.27,30
Measures
Pubertal status
Pubertal status was assessed with a child self-report instrument31 consisting of schematic drawings, including written descriptions of five stages of secondary sexual characteristics in two separate dimensions.32,33 A single score, ranging from I – prepubertal to V – adult, was computed by averaging the two ratings.34 Tanner III–V indicated late puberty and Tanner I and II indicated early puberty.
Numeric rating scale – anxiety
A 0–10 numeric rating scale assessed pain-related anxiety. After each task, participants were asked to rate how nervous, afraid, or worried they felt, at its worst, during the task (0 = none, 10 = worst/most).
Body mass index (BMI)
BMI was calculated from participants’ height and weight using the common BMI formula [(weight in pounds/height in inches)2 * 703].
HRV analysis
HRV was recorded using a two-lead electrocardiogram attached to the upper chest. The signal was sampled at 1000 Hz and timing of each R-wave performed using a BIOPAC recording system and AcqKnowledge software (BIOPAC Systems Inc, Santa Barbara, CA, USA). All data were also visually checked for quality control. Periods with excessive noise, usually due to movement, were eliminated from the analysis. Heart rate was recorded for a 5-minute period at the beginning and at the end of the laboratory session. HRV recordings were not obtained during the laboratory tasks due to the insufficient time period within each task to capture necessary data, and also due to the nature of the tasks, which involved excessive participant movement to capture reliable data.
Heart period data were imported into the Kubios HRV software35 (University of Kuopio, Kuopio, Finland), and HRV measures were determined for two 2- minute epochs from the baseline and post-session periods. For each assessment period, the HRV measures for the two epochs were averaged. If only one epoch was available because of artifact, this was used (10.8% of pre-task baseline files; 19.3% of post-task files). Heart rate data for four subjects’ pre-task baseline periods (four healthy, zero pain) and seven post-task periods (six healthy, one pain) did not contain sufficient clean data to score HRV. Mean heart rate for each recording period was also calculated by Kubios using the same heart period data.
Spectral analysis was used to capture high-frequency normalized power and the ratio of low-to-high frequency band powers using the Kubios algorithms for fast Fourier transformation.35
A frequency domain analysis method was selected over a time domain approach because frequency-based analyses provide a more discrete parsing of the frequency components of heart rate variability and therefore a clearer interpretation of parasympathetic and sympathetic influences.1,36 Although there are several frequency-based approaches, spectral analysis is widely used in experimental studies of HRV and was chosen because it has been shown to provide a robust measure of vagal tone.37 Specifically, the spectral analysis partitions the total variance in HRV into low (0.04–0.15 Hz) and high (0.15–0.40 Hz) frequency bands. Through assessing autonomic nervous system influences on cardiovascular activity, the spectral analysis provides a representation of sympathetic and parasympathetic interaction.
Respiration
Respiration was recorded using the BIOPAC recording system and software and a respiratory effort transducer (TSD201, BIOPAC Systems Inc) fastened around the waist. The signal was sampled at 250 Hz, and a rate detector algorithm was used to calculate respiration rate throughout each recording period. Respiration rate was averaged across the time period and included for files with ≥2 consecutive minutes of clean data. Respiration rate was primarily monitored to check for the presence of significant Group X Time interactions in respiration patterns, which might have influenced potential HRV results.37
Statistical analysis
Using a conservative cutoff of >3 standard deviations above the mean to identify outliers within each time period resulted in the exclusion of seven baseline periods (six healthy, one pain) and six post-task periods (two healthy, four pain). In total, 141 baseline periods (94 healthy, 47 pain) and 139 post-task periods (96 healthy, 43 pain) were included in analyses. Independent sample t-tests for continuous data and Chi-square tests for categorical data examined mean differences between the pain group and controls on sex, age, puberty status, and race/ethnicity. Separate Group (pain; control) × Time (baseline; post-task) repeated measure ANOVAs were conducted for each of the HRV variables (high-frequency normalized power and the ratio of low-to-high frequency band power). Significant interactions were examined using post hoc tests of the estimated marginal means to examine simple effects. Significance for all tests was set at P < 0.05.
Results
Demographic and baseline data
Descriptive statistics for the sample are provided in Table 1. There were no significant differences between the pain and control groups on sex, race, ethnicity, age, BMI, or pubertal status.
Heart rate variability
The raw means for baseline and post-task HRV for the pain and control groups are shown in Table 2. The ANOVAs examining group differences in HRV indicated significant Group × Time interactions for both high-frequency normalized power (F(1, 126) = 5.47, P = 0.021) and the ratio of low-to-high frequency band power (F(1, 126) = 4.59, P = 0.034). There was also a main effect of Time for high-frequency normalized power (F(1, 126) = 10.06, P = 0.002) and the ratio of low-to-high frequency band power (F(1, 126) = 11.56, P = 0.001). The results, including the estimated marginal means, are presented in Table 3.
Table 2.
Heart rate variability measures by group (mean [SD])
| Control group | Pain group | Total sample | |
|---|---|---|---|
| High-frequency normalized power | |||
| Baseline | 59.58 (15.7) n = 94 |
51.59 (17.8) n = 47 |
56.92 (16.8) n = 141 |
| Post-task | 51.03 (17.9) n = 96 |
52.39 (18.2) n = 43 |
51.45 (17.9) n = 139 |
| Ratio of low-to-high frequency band power | |||
| Baseline | 0.847 (0.57) n = 94 |
1.262 (0.88) n = 47 |
0.985 (0.71) n = 141 |
| Post-task | 1.379 (1.05) n = 96 |
1.272 (0.91) n = 43 |
1.345 (1.00) n = 139 |
Abbreviation: SD, standard deviation.
Table 3.
Results of repeated measure ANOVAs on the marginal means for HRV
| Dependent variables | Predictors | Marginal mean (SE) | F (DF) | P-value | |
|---|---|---|---|---|---|
| High-frequency normalized power | Time | Baseline | 56.89 (1.51) | 10.06 (1) | 0.002** |
| Post-task | 52.49 (1.63) | ||||
| Group | Control group | 56.36 (1.60) | 1.39 (1) | 0.240 | |
| Pain group | 53.03 (2.32) | ||||
| Time × group | Baseline control group | 60.18 (1.71) | 5.47 (1) | 0.021* | |
| Baseline pain group | 53.60 (2.48) | ||||
| Post-task control group | 52.53 (1.85) | ||||
| Post-task pain group | 52.45 (2.69) | ||||
| Ratio of low-to-high frequency band power | Time | Baseline | 0.992 (0.063) | 11.56 (1) | 0.001** |
| Post-task | 1.255 (0.083) | ||||
| Group | Control group | 1.036 (0.071) | 1.97 (1) | 0.163 | |
| Pain group | 1.211 (0.103) | ||||
| Time × group | Baseline control group | 0.821 (0.071) | 4.59 (1) | 0.034* | |
| Baseline pain group | 1.162 (0.103) | ||||
| Post-task control group | 1.250 (0.094) | ||||
| Post-task pain group | 1.260 (0.137) |
Notes:
P < 0.05;
P < 0.01.
Abbreviations: HRV, heart rate variability; SE, standard error; F(DF), F statistic (degrees of freedom).
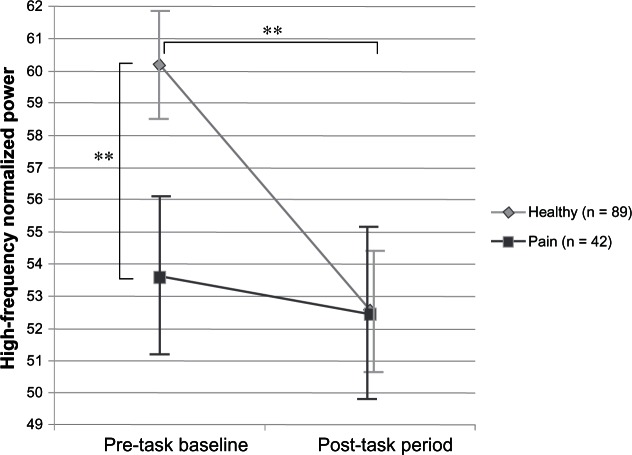
Figure 1 depicts the Group × Time interaction for high-frequency normalized power. At baseline, controls had higher resting HRV than children with chronic pain, although after the laboratory session, children’s HRV was similar regardless of group. The interaction for the ratio of low-to-high frequency band power was similar but inverse, consistent with the inverse relationship between high-frequency normalized power and the ratio of low-to-high frequency band power.
Figure 1.
Estimated marginal means of high-frequency normalized power for pre-task baseline and post-task periods in the chronic pain and control groups.
Note: **P ≥ 0.01.
The post hoc pairwise tests of the marginal means showed a significant baseline difference between the groups for high-frequency normalized power (F(1, 126) = 4.77, P = 0.03) and the ratio of low-to-high frequency band power (F(1, 126) = 7.40, P = 0.01); there were no post-task differences between the groups on either HRV measure. The tests of simple effects also revealed an effect of time for the HRV measures in the control group, but not in the pain group, a finding indicating a significant reduction in high-frequency normalized power (F(1, 126) = 23.68, P = 0.00) and increase in the ratio of low-to-high frequency band power (F(1, 126) = 23.94, P = 0.00) from baseline to post-task in controls only. When the analyses were repeated controlling for child age, BMI, and average respiration, the significant interactions held for both high-frequency normalized power (F(1, 122) = 4.69, P = 0.035) and the ratio of low-to-high frequency band power (F(1, 122) = 3.93, P = 0.049).
Heart rate data are presented inTable 2. Heart rate was significantly higher overall in the pain group (F(1, 129) = 12.09, P = 0.00) but did not significantly change from baseline to post-task for either group (F(1, 129) = 1.05, P = 0.307).
Anxiety ratings across the session
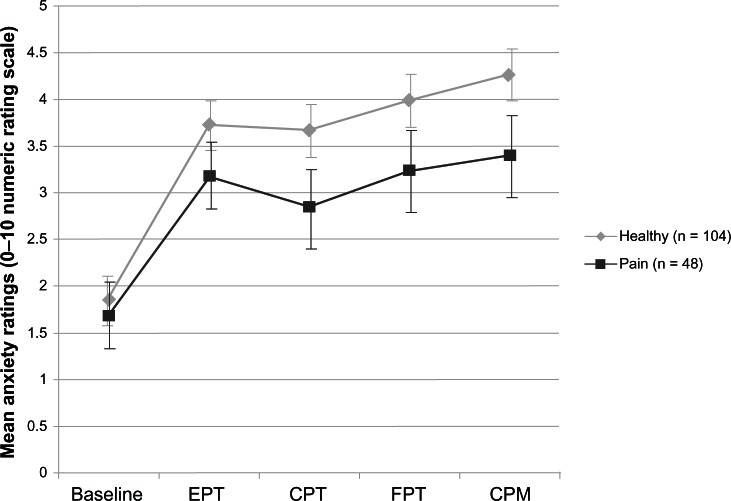
To explore the possibility that differences in pain-related anxiety may have explained the group disparities in HRV from baseline to post-session, a Group × Time repeated-measures ANOVA on anxiety ratings for each laboratory task was performed. Figure 2 shows mean anxiety ratings for each group. There were no significant Group main effects or interactions (Group main effect: F = 3.44(1), P = 0.07; Group × Time interaction: F = 1.05(4), P = 0.38), indicating similar levels of anxiety across groups. The average anxiety rating for the pain group did not exceed 3.5, while the average for controls did not exceed 4.5, suggesting that the session was only moderately anxiety-provoking for both groups.
Figure 2.
Mean anxiety ratings (0–10 Numeric Rating Scale) across the laboratory session for the pain and control groups.
Abbreviations: EPT, evoked pressure task; CPT, cold pressor task; FPT, focal pressure task; CPM, conditioned pain modulation task.
Discussion
To our knowledge, this is the first study to examine changes in HRV in response to laboratory pain tasks in children with chronic pain and healthy children. Supporting hypothesis 1, children with chronic pain demonstrated lower HRV (ie, lower high-frequency normalized power and a higher ratio of low-to-high frequency band power) at baseline compared to healthy children. Reduced high frequency-normalized power and elevated ratio of low-to-high frequency band power reflect a dominant sympathetic balance, in which the “fight or flight” response is hyperactive while the parasympathetic “brakes” on the autonomic nervous system are under-utilized.38 Contrary to hypothesis 2, children with chronic pain had static HRV in response to pain induction, whereas healthy children’s HRV was significantly reduced, indicating an appropriate autonomic nervous system stress response to laboratory pain. These differences could not be explained by different subjective emotional experiences because both groups rated the laboratory pain tasks as similarly anxiety-provoking. These findings suggest that an underlying physiological difference may explain the results. Heart rate was higher in the chronic pain group compared to controls at both baseline and post-task but did not change over the laboratory session for either group, suggesting some underlying increased arousal in the pain group, which nevertheless did not account for the differential response of the two groups.
The autonomic imbalance seen in this sample of children with chronic pain is consistent with adult studies showing reduced HRV in patients with irritable bowel syndrome,4 chronic back pain,39 fibromyalgia,40 and general chronic pain.41 Although there is limited previous research examining HRV in pediatric chronic pain, the balance of evidence appears to support autonomic dysregulation in children with chronic pain,8,14,15 rather than the converse.9,10 Inconsistent findings may be related to small sample sizes, examination of a limited developmental period, and varied markers of autonomic functioning. The bulk of the pediatric and adult literature, added to our findings, supports a cross- developmental view of low HRV as a biomarker of disease and pain states.38 Although our findings agree with prior research showing lower baseline HRV in children with recurrent abdominal pain,8 two other studies found no differences in HRV between children with recurrent abdominal pain and healthy controls.9,10 It should be noted that in contrast to these earlier investigations, only approximately 48% of the children with chronic pain in our sample presented with abdominal pain. Moreover, the age ranges of the children examined in the two studies reporting null findings were younger than the current sample. The mean ages in these two studies were 9.3 years and 10.7 years, whereas the mean age of our chronic pain sample was 14.2 years. Thus, these prior reports examined a largely prepubertal sample whereas we tested a postpubertal sample.
Our findings are consistent with existing studies indicating that healthy children show a marked HRV response to laboratory tests (eg, standing/tilt table tests;11 social stress tasks12). However, the characteristics of the healthy samples in these prior studies were somewhat different to those of the current study. The investigation using social stress tasks, for example, included only females and the age range encompassed young adulthood (aged 12–23 years). The report on tilt table tests studied a younger, prepubertal sample (mean age = 10.3 years), whereas we examined an older, postpubertal sample (mean age = 13.4 years). Thus, our healthy control sample was not strictly comparable with those tested in these earlier studies. Nevertheless, our results are also consistent with prior work indicating that girls with high levels of somatic complaints tended to show more limited HRV in response to a social stress task than girls with few somatic complaints.42 In another study, children with recurrent abdominal pain were more likely than healthy children to demonstrate increased sympathetic-and parasympathetic-mediated HRV following success on a cognitive task, but no such group differences on a task resulting in failure.16 These findings suggest a role for subjective meaning of the laboratory experience in determining HRV responsivity among children with chronic pain. Our pain tasks used uninformed ceilings, and children were unable to judge whether they had been “successful.” Future research should explore the possible impact of cognitions on the autonomic nervous system response to stressors.
It was not possible to determine from our data whether a common biological mechanism underlies both altered HRV and development of chronic pain, or whether the experience of pain over time lowers HRV. De-conditioning has been shown to lead to lowered HRV,43 and reduced exercise is commonly reported in pediatric chronic pain patients.44 A genetic explanation is also viable; low HRV may be an endophenotype for a broad range of dysfunctions involving physiological, affective, and cognitive regulation.45 It may be that the pain group did not show HRV responsivity due to habituation as a result of experiencing pain frequently. However, this explanation was unlikely given that the pain and healthy groups reported similar levels of anxiety across the session. More likely, the findings speak to physiological differences in autonomic nervous system functioning. Children with chronic pain may be characterized by persistent, lowered HRV and associated activation of systems that produce low HRV. When this state is prolonged, it produces excess stress on the system components consistent with allostatic load.45 Similar to the blunting response of cortisol in chronic pain patients,46 it is possible that chronic activation and exhaustion of the autonomic nervous system lead not only to a lowered resting HRV response, but also to a lack of autonomic flexibility following the pain tasks.
Autonomic flexibility in response to changing environmental demands plays a key role within recent conceptualizations of stability and instability in biological systems.47 Autonomic imbalance has been associated with a lack of dynamic flexibility and health. Extended activation of the sympathetic branch carries high energy demands, which over time, can lead to an exhausted system, premature aging and poor health.38 Given the low resting variability and sympathetic dominance in children with chronic pain, it is not surprising that we found continued low variability, rather than a dynamic response to the changing demands of the laboratory session. In contrast, the healthy control children’s cardiovascular system responded appropriately and flexibly to the session.
Limitations to our findings should be noted. First, the chronic pain group included heterogenous pain conditions, and we were not able to examine differences between healthy children and children with specific pain conditions. Given the broad array of health issues associated with lowered HRV, however, it is likely that the findings reflect chronic non-disease-specific pain conditions. Second, although none of the sample used opioids, we did not exclude medication use in the chronic pain sample and it is possible that other medications influenced the results. However, a pediatrician determined that no patient-reported medications would have been likely to have influenced cardiovascular function. The patient-reported medications consisted of allergy/asthma medications, antibiotics, antidepressants, over-the-counter pain medications, and anticonvulsants. Future studies should ask parents to bring in all medications taken by children on the day of the study. Finally, we were unable to determine the causal relationships in the link between chronic pain and HRV, given the cross sectional design of the study.
Conclusion
Epidemiological studies have shown that reduced HRV is a risk factor for all-cause mortality and morbidity.48 Our findings suggest that the relationship between low HRV and compromised health appears relatively early in life. Children with chronic pain demonstrated not only lowered resting HRV relative to their healthy counterparts, but their autonomic nervous system response was also blunted following laboratory pain induction. This autonomic imbalance, if not addressed, may lead to further stress on the body and a cascade of protracted health problems. Longitudinal studies of children with low HRV and chronic pain are necessary to understand the nature of the relationship between autonomic functioning and well-being.
Acknowledgments
This study was supported by Grant R01DE012754, awarded by the National Institute of Dental and Craniofacial Research (Principal Investigator: Lonnie K Zeltzer); by University of California, Los Angeles Clinical and Translational Research Center, Clinical and Translational Science Institute Grant UL1RR033176 (Principal Investigator: Lonnie K Zeltzer); and by Grant 1K01AT005093, awarded by the National Center for Complementary and Alternative Medicine (PI: Subhadra Evans).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler D, Laude D, Akila F, Elghozi JL. Time- and frequency-domain estimation of early diabetic cardiovascular autonomic neuropathy. Clin Auton Res. 2001;11(6):369–376. doi: 10.1007/BF02292769. [DOI] [PubMed] [Google Scholar]
- 3.Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10:229–240. [Google Scholar]
- 4.Mazurak N, Seredyuk N, Sauer H, Teufel M, Enck P. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neurogastroenterol Motil. 2012;24(3):206–216. doi: 10.1111/j.1365-2982.2011.01866.x. [DOI] [PubMed] [Google Scholar]
- 5.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Monk C, Kovelenko P, Ellman LM, et al. Enhanced stress reactivity in paediatric anxiety disorders: implications for future cardiovascular health. Int J Neuropsychopharmacol. 2001;4(2):199–206. doi: 10.1017/S146114570100236X. [DOI] [PubMed] [Google Scholar]
- 7.Yeragani VK, Rao KA, Pohl R, Jampala VC, Balon R. Heart rate and QT variability in children with anxiety disorders: a preliminary report. Depress Anxiety. 2001;13(2):72–77. doi: 10.1002/da.1019. [DOI] [PubMed] [Google Scholar]
- 8.Sowder E, Gevirtz R, Shapiro W, Ebert C. Restoration of vagal tone: a possible mechanism for functional abdominal pain. Appl Psychophysiol Biofeedback. 2010;35(3):199–206. doi: 10.1007/s10484-010-9128-8. [DOI] [PubMed] [Google Scholar]
- 9.Olafsdottir E, Ellertsen B, Berstad A, Fluge G. Personality profiles and heart rate variability (vagal tone) in children with recurrent abdominal pain. Acta Paediatr. 2001;90(6):632–637. [PubMed] [Google Scholar]
- 10.Jarrett M, Heitkemper M, Czyzewski D, Zeltzer L, Shulman RJ. Autonomic nervous system function in young children with functional abdominal pain or irritable bowel syndrome. J Pain. 2012;13(5):477–484. doi: 10.1016/j.jpain.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longin E, Dimitriadis C, Shazi S, Gerstner T, Lenz T, Konig S. Autonomic nervous system function in infants and adolescents: impact of autonomic tests on heart rate variability. Pediatr Cardiol. 2009;30(3):311–324. doi: 10.1007/s00246-008-9327-8. [DOI] [PubMed] [Google Scholar]
- 12.Hollenstein T, McNeely A, Eastabrook J, Mackey A, Flynn J. Sympathetic and parasympathetic responses to social stress across adolescence. Dev Psychobiol. 2012;54(2):207–214. doi: 10.1002/dev.20582. [DOI] [PubMed] [Google Scholar]
- 13.Ivarsson M, Anderson M, Akerstedt T, Lindblad F. Playing a violent television game affects heart rate variability. Acta Paediatr. 2009;98(1):166–172. doi: 10.1111/j.1651-2227.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- 14.Dorn LD, Campo JC, Thato S, et al. Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(1):66–75. doi: 10.1097/00004583-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Dufton L, MJ D, LS S, BE C. Self-reported and laboratory-based responses to stress in children with recurrent pain and anxiety. J Pediatr Psychol. 2011;36(1):95–105. doi: 10.1093/jpepsy/jsq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puzanovova M, Arbogast PG, Smith CA, Anderson J, Diedrich A, Walker LS. Autonomic activity and somatic symptoms in response to success vs failure on a cognitive task: a comparison of chronic abdominal pain patients and well children. J Psychosom Res. 2009;67(3):235–243. doi: 10.1016/j.jpsychores.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolan RP, Kamath MV, Floras JS, et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J. 2005;149(6):1137. doi: 10.1016/j.ahj.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Reiner R. Integrating a portable biofeedback device into clinical practice for patients with anxiety disorders: results of a pilot study. Appl Psychophysiol Biofeedback. 2008;33(1):55–61. doi: 10.1007/s10484-007-9046-6. [DOI] [PubMed] [Google Scholar]
- 19.Prinsloo GE, Derman WE, Lambert MI, Laurie Rauch HG. The effect of a single session of short duration biofeedback-induced deep breathing on measures of heart rate variability during laboratory-induced cognitive stress: a pilot study. Appl Psychophysiol Biofeedback. 2013 Feb 24; doi: 10.1007/s10484-013-9210-0. Epub. [DOI] [PubMed] [Google Scholar]
- 20.Wyller VB, Helland IB. Relationship between autonomic cardiovascular control, case definition, clinical symptoms, and functional disability in adolescent chronic fatigue syndrome: an exploratory study. Biopsychosoc Med. 2013;7(1):5. doi: 10.1186/1751-0759-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallen EL, Kamath MV, Ghista DN. Power spectrum of heart rate variability: a non-invasive test of integrated neurocardiac function. Clin Invest Med. 1988;11(5):331–340. [PubMed] [Google Scholar]
- 22.Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995;19(2):225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 23.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 24.Rhudy JL. The importance of emotional processes in the modulation of pain. Pain. 2009;146(3):233–234. doi: 10.1016/j.pain.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 25.McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Payne LB, Seidman LC, Lung K, Zeltzer LK, Tsao JCI. Relationship of neuroticism and laboratory pain in healthy children: does anxiety sensitivity play a role? Pain. 2013;154(1):103–109. doi: 10.1016/j.pain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsao JCI, Seidman LC, Evans S, Lung KC, Zeltzer LK, Naliboff BD. Conditioned pain modulation (CPM) in children and adolescents: effects of sex and age. J Pain. 2013 Mar 26; doi: 10.1016/j.jpain.2013.01.010. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32(1):55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz LF, Seidman LC, Zeltzer LK, Tsao JCI. Mother-child concordance for pain location in a pediatric chronic pain sample. J Pain Manag. [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao JCI, Li N, Parker D, Seidman LC, Zeltzer LK. Pubertal status moderates the association between mother and child laboratory pain tolerance. Pain Res Manag. doi: 10.1155/2014/390368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 32.Tanner JM. Growth at Adolescence: With A General Consideration of the Effects Of Hereditary and Environmental Factors upon Growth and Maturation From Birth to Maturity. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 33.Tanner JM. Growth and endocrinology of the adolescent. In: Gardner LJ, editor. Endocrine and Diseases of Childhood. 2nd ed. Philadelphia: WB Saunders; 1975. pp. 14–64. [Google Scholar]
- 34.Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- 35.Tarvainen MP, Niskanen J. Kubios HRV Version 2.0 User’s Guide. Biosignal Analysis and Medical Imaging Group (BSAMIG), Department of Physics, University of Kuopio; Finland: 2008. [Google Scholar]
- 36.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 37.Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol Psychol. 2007;74(2):243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Gockel M, Lindholm H, Niemisto L, Hurri H. Perceived disability but not pain is connected with autonomic nervous function among patients with chronic low back pain. J Rehabil Med. 2008;40(5):355–358. doi: 10.2340/16501977-0172. [DOI] [PubMed] [Google Scholar]
- 40.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum. 2000;29(4):217–227. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 41.Mostouf SM, Afari N, Ahumada SM, Reis V, Wetherell JL. Health and distress predictors of heart rate variability in fibromyalgia and other forms of chronic pain. J Psychosom Res. 2012;72(1):39–44. doi: 10.1016/j.jpsychores.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Hipwell AE, Keenan K, Marsland A. Exploring psychophysiological markers of vulnerability to somatic illnesses in females. J Pediatr Psychol. 2009;34(9):1030–1039. doi: 10.1093/jpepsy/jsp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen AL, Johnsen BH, Sollers JJ, 3rd, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. Eur J Appl Physiol. 2004;93(3):263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- 44.Wilson AC, Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. J Pain. 2012;13(2):121–130. doi: 10.1016/j.jpain.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R, Seckl J. Minireview: stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152(12):4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- 47.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 48.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes. 2002;51(12):3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]