Abstract
Adolescence is a developmental period in which brain structures involved with stress responses, such as the medial pre-frontal cortex (mPFC), mature. Therefore, exposure to a stressor at this time may have effects that endure the lifespan. The goal of the present study was to determine whether behavioral control over an adolescent stressor mitigates the behavioral and neurochemical consequences of the stressor as occurs in adult rats. Adolescent rats (post natal day 35) were exposed to either inescapable (IS) or escapable tailshocks (ES). As in adults we observed a “stressor controllability effect”; IS reduced social exploration and activated the serotonergic dorsal raphé nucleus while ES did not. Excitotoxic lesions of the medial prefrontal cortex prevented the stressor controllability effect. We also demonstrate that a controllable adolescent stress prevents the behavioral and neurochemical consequences of IS in adulthood. Thus, the controllability of a stressor during adolescence is an important psychological factor.
Keywords: Learned Helplessness, Stress, Adolescence, Serotonin, Social Interaction, Stressor Controllability
1. Introduction
Adolescence is a key developmental period during which adult-like behaviors are established and organisms gain independence. Although the adolescent period is not marked by a specific event or age, it is a period of transition from childhood to adulthood marked by many changes, including an increase in social interaction, novelty seeking and risk-taking behaviors [1], [2] and [3]. Adolescence is also characterized by cortical development, particularly in areas involved in emotional processing and learning, such as the medial pre-frontal cortex (mPFC), which undergoes vast remodeling throughout adolescence [1] and [4]. Accordingly, exposure to stressors during adolescence increases risk for psychiatric disease in adulthood [5] and [6]. Thus, it is important to understand how stressors influence behavioral and neural development.
Control, or perception of control, over stressor onset or offset has been identified in both humans and rats to be an important predictor of many stressor consequences [7], [8], [9] and [10]. In adult rats exposure to inescapable tail shock (IS) produces numerous behavioral (eg. exaggerated fear conditioning, poor shuttle box escape learning, and decreased social interaction) and neural (eg. induction of Fos in numerous brainstem and limbic regions and downregulation of serotonin (5-HT) 1A receptors) consequences that do not occur after equal amounts of escapable tail shock (ES) [11], [12], [13] and [14]. IS and ES differ only in that with ES the subject has control over one aspect of the shocks, their time of termination, whereas IS subjects do not. The effects of IS on later behavior depend upon stress-induced activation of 5-HT containing neurons in the dorsal raphé nucleus (DRN: for review see [15]). Specifically, IS leads to sensitization of the DRN so that it becomes hyper responsive to anxiogenic stimuli [16],with the sensitization being dependent upon downregulation of the 5-HT1A auto receptor [17] and [18]. ES prevents the behavioral consequences of the stressor by blunting DRN activation [19]and 5-HT1A downregulation [17] with this blunting depending on an intact mPFC [13]. All of the aforementioned research was carried out in adult subjects.
Recently, Leussis & Andersen [20] demonstrated an effect of control in adolescent rats given ES at post-natal day (PND) 35. As in adults, adolescent rats that received IS, but not ES, exhibited learning deficits in a shuttle box shortly thereafter [20]. Taking this finding as a base, the present study had three aims. The first was to determine the acute and long term behavioral effects of IS and ES in adolescence on a putative measure of anxiety, social exploration [21]. Since experiential factors have been shown to alter an organism’s vulnerability to subsequent aversive experiences [9], the behavioral impact of adolescent stressor controllability on an uncontrollable stressor in adulthood was assessed. For the second aim, Fos immunohisochemistry (IHC) was used to assess the impact of the stressor within serotonergic neurons of the DRN. This was measured shortly after both adolescent stress and adult stress. Because the mPFC is necessary for the effects of stressor control in adults [13], the final aim was to assess the role of the mPFC in the effects of an adolescent controllable stressor.
2. Materials and Methods
2.1 Subjects
Male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were housed 4/cage in plastic tubs with free access to food and water on a 12 h light/dark cycle (lights on at 7:00 A.M. and off at 7:00 P.M.). Group housing was used to negate the effect of social isolation at this time point and was shown to be necessary for the effect of controllability in adolescence [20]. Cagemates were randomly assigned to groups and exposed to the same experimental conditions. Experiments were conducted during the light phase and all procedures were approved by the University of Colorado Institutional Animal Care and Use Committee.
2.2 Procedures
2.2.1 Escapable and Inescapable Tailshock Procedure
ES and IS were administered in 14 × 11 × 17 cm acrylic wheel turn boxes enclosed in sound-attenuating chambers. Electric shock was delivered by a Precision Regulated Animal Shocker (Coulbourn Instruments, Allentown, PA, USA) through copper electrodes augmented by electrode paste attached 2 and 4 cm from the base of the tail. Eighty tailshocks were administered on a variable interval schedule with a range of 20 to 100 seconds and an average inter-shock interval of 60s (VI-60). Shock intensity was 0.8mA for the first 26 trials, 1.0mA for the following 27 trials, and 1.2 mA for the remaining 27 trials. As with adult rats, turning a wheel at the front of the chamber terminated each tailshock for rats in the ES condition [13]. The escape response requirement began with ¼ of a full turn of the wheel. If the rat performed the response requirement within 5 s, the response doubled on the next trial until a maximum of 4 full wheel turns was reached. If the rat did not did not escape within 30 s the shock was terminated and the response requirement reset to ¼ turn. These parameters help to insure that the rat learns an operant response contingency and does not simply perform a reflexive behavior. IS animals were yoked to animals receiving ES so that they received the same number and duration of shocks. Animals were returned to the colony room immediately following the tail-shock procedure. Stress naïve control rats remained in their home cages (HC).
To examine the long-term “immunizing” stress protective effects of ES, a separate set of rats received ES or IS on PND 35 and then 100 trials of IS 35 days later. For this second stressor rats were placed in a clear acrylic restraint tube that measured 17 cm in length and 7 cm in diameter. The restraint tube contained a small platform extending from the rear to which the tail was fixed with cloth tape and electrodes. Here IS consisted of 100, 5 s shocks on a VI-60 schedule at an intensity of 1.0 mA for the first 10 shocks, 1.3 mA for the second 10 shocks and 1.6 mA for all subsequent trials as previously reported [22] and [16].
2.2.2 Social Exploration Test
Social exploration tests were conducted exactly as described previously [14]. Each experimental rat was allocated a separate transparent plastic cage with shaved wood bedding and a wire lid located in a brightly lit testing room; food and water were not available in the testing cages. After 60 minutes a stimulus rat (PND 28) was added to the cage. Investigative behaviors, including sniffing, pinning, and allogrooming, initiated by the experimental rat were timed by an observer who was blind to group membership. After 3 min, the juvenile was removed and the experimental animal was returned to the homecage. As in our published methods [16], juvenile stimulus rats were used multiple times during a single social exploration test.
2.2.3 Dorsal Raphé Nucleus Serotonin and Fos Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital approximately 2 h after the last tailshock and perfused with 50 ml of cold physiological saline and 200 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Extracted brains were post-fixed in the same 4% paraformaldehyde overnight and then transferred to 30% sucrose until sectioning. Sections measuring 30 μm were obtained in a cryostat and were stored at 4C in cryoprotectant.
Serial immunohistochemical staining for Fos and 5-HT was conducted as described previously [13]. Briefly, staining for Fos was conducted first using rabbit polyclonal Fos primary antibody (1:15,000; Santa Cruz Biotechnology, Santa Cruz, CA) and biotinylated goat anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) and visualized with avidin-biotin horseradish peroxidase (ABC Kit, Vector Labs) and nickel enhanced diaminobenzidine (DAB). 5-HT containing neurons were identified with rabbit 5-HT primary antibody (1:10,000; ImmunoStar, Hudson, WI) and nonbiotinylated goat anti-rabbit IgG (Jackson ImmunoResearch) secondary antibody and were visualized with peroxidase anti-peroxidase and DAB to produce a brown cytoplasmic precipitate. After staining, sections were floated onto glass slides in .15% gelatin, dehydrated, defatted in Histoclear (Fisher Scientific), and cover-slipped with Permount (Fisher Scientific).
The number of 5-HT-stained cells and the number of cells double-labeled for both 5-HT and Fos were quantified by an observer naïve to experimental treatment. Fos-stained nuclei were identified by black ovoid particles. Larger reddish-brown particles, with or without Fos-stained nuclei, were counted as 5-HT-positive cells. Caudal DRN sections were taken at approximately −8.3 mm from bregma. Three sections from this caudal position were taken and averaged for each rat for a cumulative mean score of double-labeled cells.
2.2.4 Excitotoxic mPFC lesions
All surgeries were conducted under inhalational anesthesia with 2–3% isoflurane in oxygen. A rat was placed in a stereotaxic frame (Kopf Instruments) with a bite bar and nose-cone for gas delivery. The skull was adjusted so that bregma and lambda were in the same horizontal plane. Each rat received an injection of 0.5 μL of 5% NMDA (Sigma, St Louis, MO) through a 25μl Hamilton syringe into the border between infralimbic and prelimbic cortices (AP +2.5, DV −2.9). The Hamilton syringe remained in place for 5 min after the injection to permit diffusion. After surgery each rat received prophylactic antibiotic, 25ml Twin-Pen (AgriLabs). At the end of each experiment rats were overdosed with sodium pentobarbital (60 mg/kg i.p.) and brains were removed and flash frozen in −60°C isopentane. Frozen sections (30μm) were cut in a − 20°C cryostat, mounted onto glass slides and stained for cresyl violet in order to verify lesion location.
2.3 Experimental procedures
2.3.1 Experiments 1 and 2: The short and long term effects of stressor controllability during adolescence on later social exploration
The first set of experiments assessed the short and long-term effects of adolescent stressor controllability on social exploration. Animals arrived to the vivarium at post-natal day (PND) 26 and received HC, ES or IS treatment at PND 35. Social exploration tests were conducted on PND 36. The long-term effect of adolescent stressor controllability was assessed using a separate set of animals that received either HC, ES, or IS at PND 35 and remained undisturbed until social exploration tests on PND 70. PND 70 was used because it is well outside the range known to be adolescence (weaning to PND 60) [1] and comparable to prior research using these methods [20]. To assess whether adolescent ES produces a long lasting resistance to later IS animals received ES, IS, or HC at PND 35. ES, IS and half of the HC subjects then received IS on PND 70, while the other half of the HC group received HC again to provide a baseline. Social exploration tests were conducted 24 hours later.
2.3.2 Experiments 3 and 4: The effect of adolescent stressor controllability on DRN 5-HT activation
As in Experiment 1, animals were received at PND 26 and given either HC, ES, or IS at PND 35. In experiment 3, rats were sacrificed 2 h after the end of stress for 5-HT and Fos analysis. In experiment 4, rats returned to the vivarium and were sacrificed on PND 70 2 h after IS. Fos and 5-HT immunohistochemistry was performed as described by Grahn et al., 2003 [23].
2.3.3 Experiment 5: The role of the mPFC in the behavioral effects of adolescent stressor controllability
To assess whether the mPFC is necessary for the short-term effects of ES, excitotoxic lesions were made prior to adolescent stress. Lesion was used rather than muscimol microinjection because of the difficulty inherent in canulation at PND 35 while the skull continues to grow rapidly. Rats arrived on PND 24 and received NMDA lesions on PND 28 followed by either ES, IS, or HC at PND35 and were tested in a social exploration test 24 hours later.
2.4 Statistical analyses
Wheel-turn escape data was analyzed by repeated-measures ANOVA. One-way ANOVA tests were run for all experiments except for the lesion experiment in which a multi-way ANOVA was used. Significant main effects and interactions were followed by Fisher’s protected least significant difference (PLSD) test post hoc comparison (set p < 0.05).
3 Results
3.1 Adolescent Controllable Stress has Short and Long Term Behavioral Effects
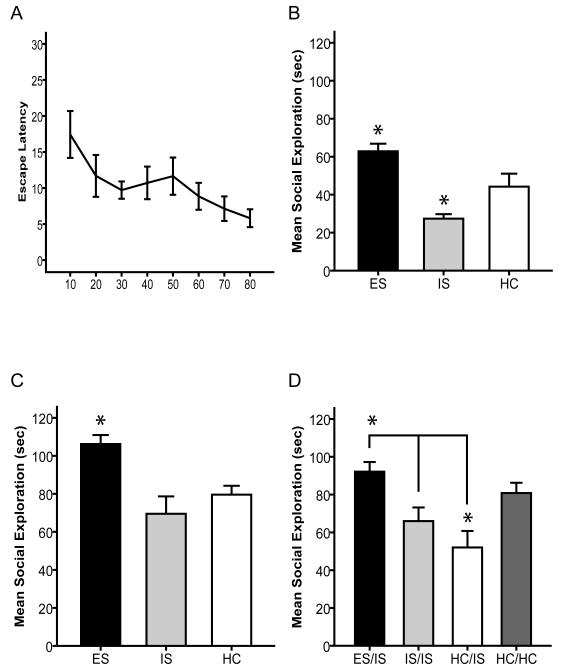
24 (8 per group) male Sprague Dawley adolescent rats were administered either ES, IS or HC on PND 35 followed by a social exploration test on PND 36. Adolescent animals successfully learned to escape shock by turning the wheel as shown by a decrease in escape latencies across trials (Figure 1A). Social investigation is shown in Fig. 1B. ANOVA identified a significant main effect of treatment group on social exploration at PND 36, F(2,21)=12.31, p < .001. Post-Hoc analysis revealed that ES animals exhibited elevated (p<.05) social exploration relative to HC controls, while IS animals exhibited lower (p<.05) social exploration compared to controls (Figure 1B). To assess the long-term effect of adolescent stressor controllability a separate set of 24 (8 per group) male Sprague Dawley adolescent rats were administered either ES, IS, or HC on PND 35 followed by a social exploration test on PND 70. One ES animal and its yoked IS partner were eliminated because the ES animal failed to turn the wheel to escape the shock bringing both groups to 7 animals per group. ANOVA identified a significant main effect of treatment group on social exploration, F(2,19)= 8.46, p<.01. Post-hoc analysis revealed that ES subjects engaged in greater social exploration (p<.05) than did IS or HC subjects (Figure 1C). As discussed below, we have not previously determined whether a stress immunization effect occurs over this interval in adults. Thus, a separate set of 24 animals (n=8/group) received ES, IS or HC in adulthood on (PND 70) and tested in social exploration 35 days later on PND 105. No significant behavioral effect of prior stress was found in this set of animals (data not shown).
Figure 1.
A, Average time required by ES animals (for blocks of 5 trials) to meet wheel turning criteria to terminate shock. B, Mean (+/- SEM) time spent exploring the juvenile conspecific 24 h after 80 tail shocks (PND 35) in a 3 min social exploration test on PND 36 (* denotes significant (p<.05) difference from HC). C, Mean (+/- SEM) time spent exploring the juvenile conspecific 35 days after 80 tail shocks (PND 35) in a 3 min social exploration test on PND 70 (* denotes significant difference from HC). D, Mean (+/- SEM) time spent exploring the juvenile conspecific after receiving ES, IS, HC during adolescence (PND 35) followed by IS or HC 35 days later (PND 70) in a 3 min social exploration test on PND 71 (* denotes significant (p<.05) difference from HC). Brackets denote a significant difference between ES/IS and both IS/IS and HC/IS.
To assess the behavioral effect of adolescent controllability on later response to IS in adulthood, 29 male Sprague Dawley adolescent rats were administered either ES (n=7), IS (n=7) or HC (n=15) on PND 35. All of the animals except half of the HC were given IS at PND 70 followed by a social exploration test at PND 71. The HC subjects not given IS were given the social exploration test to provide a no stress comparison group. ANOVA revealed a significant effect of treatment group F(3, 27) 6.10, p<.01. Post-hoc analysis revealed that IS alone in adulthood (ie HC/IS) significantly reduced (p<.05) social exploration, as is typical (Figure 1D). Post-hoc analysis also revealed that the ES/IS group displayed significantly (p<.05) greater social exploration than did IS/IS or HC/IS groups. That is, ES on Day 35 blocked the effects of IS administered 35 days later. The IS/IS group was not significantly different than either the HC/IS or HC/HC group. The HC/IS group exhibited significantly less (p<.05) social exploration than did either the ES/IS or HC/HC groups.
3.2 Adolescent Controllable Stress has Acute and Long-Term Stress Mitigating Neurochemical Effects
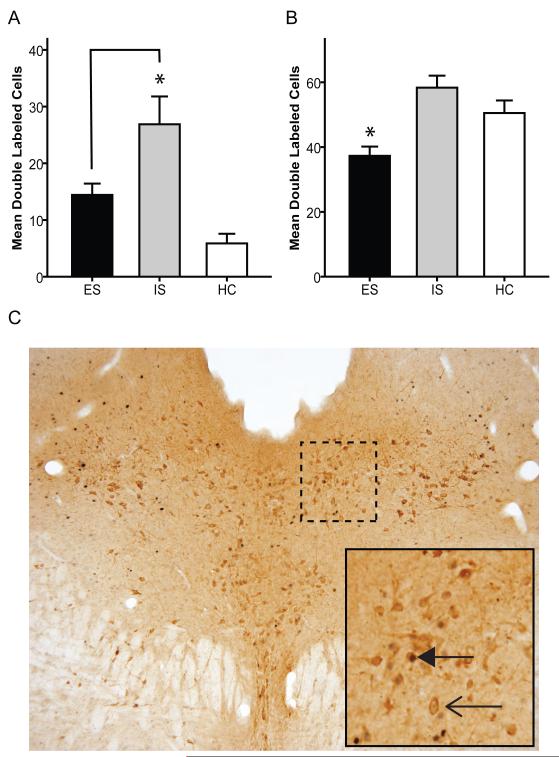
We have previously reported that in adults IS produces greater activation of DRN 5-HT neurons (as assessed by Fos protein expression in 5-HT–labeled neurons) than does ES [23]. This effect of controllability was especially prominent in middle and caudal regions of the DRN. Using the same procedure after adolescent stress at PND 35, ANOVA confirmed a significant effect of treatment group, F(2, 21)= 12.31, p<.001 on the mean number of double-labeled cells in the caudal DRN. Similar to the results found in adults, post-hoc analysis found that adolescent IS significantly increased (p<.05) fos labeled 5- HT neurons compared to ES and HC (Figure 2A). ANOVA also confirmed a significant effect of treatment group F(2, 21)= 9.66, p<.01 in the paradigm in which animals were given ES, IS or HC in adolescence and IS in adulthood. As with the behavioral endpoint, exposure to ES on PND 35 prevented the IS-induced activation of serotonergic neurons in the caudal DRN following adult IS, compared to adolescent HC or IS groups (Figure 2B). Post-hoc analysis revealed that ES produced significantly (p<.05) less activation of serotonergic neurons in the DRN than did IS or HC following the second stressor. Also consistent with the behavioral data, a separate experiment determined that if the first stressor is given in adulthood (PND 70), with IS 35 days later (PND 105) neither prior ES or IS protected from the effects of IS (data not shown).
Figure 2.
A, Mean (+/− SEM) number of neurons double-labeled for Fos and 5-HT in the caudal (−8mm from Bregma) DRN 2 hours after IS on PND 35(* denotes significant (p<.05) difference from HC). Brackets denote significant difference between IS and HC. B, Mean (+/− SEM) number of neurons double-labeled for Fos and 5-HT in the caudal DRN 2 hours after IS on PND 70. Animals receiving ES in adolescence had less adult IS induced serotonergic DRN activation than animal receiving IS or HC in adolescence (* denotes significant (p<.05) difference from HC). C, Caudal (−8mm) DRN section of an IS animal. Bold arrowhead denotes double (fos and 5-HT) labeled cell. Open arrowhead denotes single (5-HT) labeled cell.
3.3 The mPFC is Necessary for the Acute Behavioral Effects of Stressor Controllability
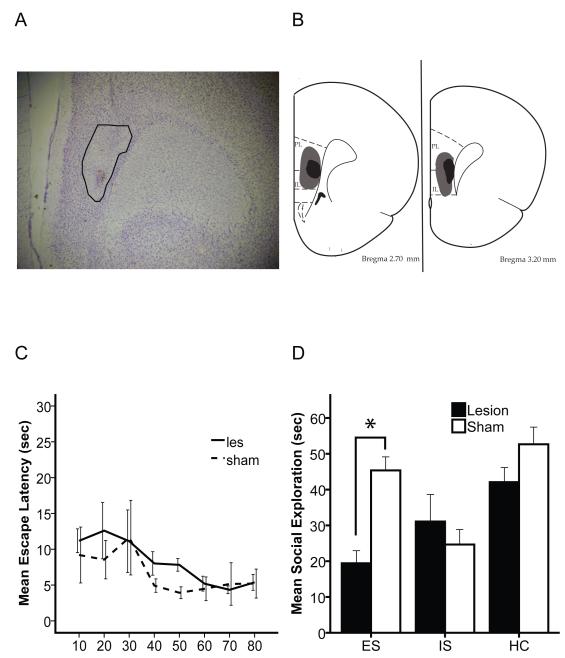
48 rats (n=8/group) were divided into 6 groups with 3 of the groups receiving an NMDA lesion of the mPFC at PND 28 and 3 groups receiving sham surgery. Rats received either ES, IS or HC on PND 35 followed by a social exploration test on PND 36. Lesions were located between the infralimbic and prelimbic region of the PFC (Fig 3A, 3B). The lesion did not alter baseline social exploration on PND 34 (data not shown). The lesion also did not alter latency to escape during ES (Fig 3C). However, the mPFC lesion blocked the beneficial effects of ES. Now, ES subjects showed reduced social investigation, as did IS rats (Fig 3D). Two way ANOVA revealed a significant main effect of lesion, F(1, 46)= 7.08, p<.05, and of group F(2, 45)= 9.19, p<.01. There was also a significant interaction between lesion and group F(2, 45)= 5.93, p<.01. Post-hoc analysis showed that mPFC lesioned animals receiving ES performed as though they had received IS. Social exploration of both lesioned and sham IS animals were significantly lower than sham/HC, however there was no significant reduction in social exploration between of Les/IS compared to Les/HC.
Figure 3.
A, Cresyl Violet stain of lesioned mPFC. Lesion is outlined. B, 2 levels of mPFC (3.2 and 2.7 mm from Bregma) showing the smallest (Black) and largest (Gray) lesion area for each level. C, Average time required by lesion and sham animals (for blocks of 10 trials) to meet wheel turning criteria to terminate shock. D, Mean (+/− SEM) time spent exploring the juvenile conspecific on PND 36 in a 3 min social exploration test. (* denotes significant (p<.05) difference between sham and lesion animals).
4 Discussion
The data from the present series of experiments indicates that the controllability of adolescent stressors is one factor that can help predict both the acute and long lasting behavioral and neurochemical consequences of that stressor. ES increased social exploration, relative to IS, both 24 hours and 35 days following the stressor. Control also prevented the stress-induced Fos induction in the caudal DRN on PND 35, and when exposed to subsequent IS on PND 70 in adulthood. Thus, controllable stress exposure produced resistance to subsequent stress over a time-course much longer than previously reported (eg. [14]). To our surprise, uncontrollable stress in adolescence did not cause any long lasting anxiogenic effects. Consistent with findings in adult rats, the short-term beneficial effect of ES required an intact medial prefrontal cortex. These results provide compelling evidence that adolescence is a critical developmental period that is sensitive to the dimension of stressor controllability.
The first point to note is that adolescent subjects were sensitive to the behavioral control dimension. Indeed, there was some a priori reason to doubt that there would be such sensitivity since the mPFC is not fully developed at that time [4] and [24], and is critical for the effects of stressor control in adults [13]. However control in adolescence was shown to reduce the anxiety produced by tailshock in a social exploration test, both 24 hours and 35 days following the stressor. It is interesting to note that the ES induced increase in social exploration observed in the short term social exploration experiment was not replicated in this lesion experiment. Perhaps this was caused by surgery lowering the maximum time that the animals will explore, and thus creating a ceiling effect. Interestingly, the long-term increase in social exploration following an adolescent stressor is in accordance with findings by Kendig et al. [25], using chronic exposure to uncontrollable predator odor stress in adolescence. The mechanism(s) of this long-term increase in social exploration remains unknown.
In adults, exposure to ES prevents the behavioral and neurochemical effects of IS delivered a week later [16]. In the current study adolescent ES at PND 35 blocked the effects of subsequent IS occurring 35 days later. Thus, the effect of ES lasted much longer than previously observed in adults. This corresponds to other studies which have also shown long term behavioral effects of stress at PND 35 (eg. [26], [20] and [27]). The novelty here is that controllable stress at this time blocked the effects of subsequent IS 35 days later. However, this effect is difficult to interpret since ES, by itself, increases later social exploration and therefore the subsequent IS could just be lowering an elevated baseline level of social exploration. The lack of long-term effect of adolescent IS here is in contrast to many reports, in both humans and rats, that traumatic stressors in adolescence enhance anxiety in adulthood [28], [29], [30] and [31]. However, there are examples of chronic mild stress and social defeat during adolescence producing resilience [32] and [33]. Thus taken together, these findings demonstrate that adolescent stressors can produce dramatically different long-term effects on anxiety (as reviewed in [34]) and perhaps shape the way an organism reacts to subsequent stressors throughout an entire lifespan.
The mPFC, which is crucial to the mediation of the behavioral effects of stressor controllability in adults [13], was also key to the short-term effects of stressor controllability in adolescence. Activation of the mPFC prevents DRN 5-HT activation and the behavioral consequences of tailshocks [13] and [35]. DRN activation is closely tied to anxiety [36] and chronic social isolation in adolescence has lasting effects on the sensitivity of the DRN to alter stimulation and anxiety behaviors[37] and [38]. Whereas a sustained uncontrollable stressor such as social isolation potentiates later anxiety, a single acute stress that is controllable mitigates later stress and anxiety effects. While the number of stressors used in experiments involving adolescent subjects is large, the controllability of the stressor has only been manipulated here and in the study of Leussis & Andersen [20]. Both reports of stressor controllability show that the ability to manipulate the termination of stress during adolescence will mitigate both the acute and long-term consequences of stress exposure. These long lasting effects suggest plasticity within structures such as the mPFC which will be investigated in future studies.
The results presented here suggest that adolescence is a key developmental phase in which experiential factors can have long lasting effects. They also implicate the DRN in the short and long term effects of the controllable stressor during adolescence. The mPFC was implicated in the short-term effects and its involvement in the long-term effects waits to be explored. Finally, the data suggest that experiences with control during adolescence may be especially beneficial.
Highlights.
A rat model to study the effects of controllable stress in adolescence was tested.
Adolescent subjects were sensitive to the behavioral control dimension.
Adolescent controllable stress blunted the effects of adult uncontrollable stress.
Stressor control during adolescence decreased DRN 5-HT activity after stress.
The mPFC is critical for the acute effects of an adolescent controllable stressor.
Acknowledgements
This research was supported by National Institute of Health Grants MH050479 and MH082453.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [2].Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Developmental psychobiology. 1989;22:633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- [3].Toledo-Rodriguez M, Sandi C. Stress during Adolescence Increases Novelty Seeking and Risk-Taking Behavior in Male and Female Rats. Frontiers in behavioral neuroscience. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, et al. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. The Journal of comparative neurology. 2010;518:2693–709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- [6].Burke KC, Burke JD, Jr., Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Archives of general psychiatry. 1990;47:511–8. doi: 10.1001/archpsyc.1990.01810180011002. [DOI] [PubMed] [Google Scholar]
- [7].Seligman ME, Maier SF. Failure to escape traumatic shock. Journal of experimental psychology. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- [8].Minor TR, Hunter AM. Stressor controllability and learned helplessness research in the United States: sensitization and fatigue processes. Integr Physiol Behav Sci. 2002;37:44–58. doi: 10.1007/BF02688805. [DOI] [PubMed] [Google Scholar]
- [9].Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual review of clinical psychology. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- [10].Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. The American journal of psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- [11].Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. Journal of experimental psychology Animal behavior processes. 1990;16:137–49. [PubMed] [Google Scholar]
- [12].Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacology, biochemistry, and behavior. 1993;45:827–35. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- [13].Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature neuroscience. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- [14].Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behavioural brain research. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and biobehavioral reviews. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- [16].Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, et al. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biological psychiatry. 2011;70:458–64. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14107–15. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain research. 1998;783:115–20. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- [20].Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- [21].Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–50. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, et al. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13703–11. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain research. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- [24].Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. The Journal of comparative neurology. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- [25].Kendig MD, Bowen MT, Kemp AH, McGregor IS. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behavioural brain research. 2011;225:405–14. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- [26].Klein ZA, Padow VA, Romeo RD. The effects of stress on play and home cage behaviors in adolescent male rats. Developmental psychobiology. 2010;52:62–70. doi: 10.1002/dev.20413. [DOI] [PubMed] [Google Scholar]
- [27].Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacology, biochemistry, and behavior. 2012;100:440–50. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–72. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- [29].Callaghan BL, Richardson R. Early-life stress affects extinction during critical periods of development: An analysis of the effects of maternal separation on extinction in adolescent rats. Stress. 2012 doi: 10.3109/10253890.2012.667463. [DOI] [PubMed] [Google Scholar]
- [30].Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depression and anxiety. 2005;22:103–13. doi: 10.1002/da.20082. [DOI] [PubMed] [Google Scholar]
- [31].Tsoory M, Guterman A, Richter-Levin G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–93. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- [32].Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–32. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- [33].Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behavioral neuroscience. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Buwalda B, Geerdink M, Vidal J, Koolhaas JM. Social behavior and social stress in adolescence: a focus on animal models. Neuroscience and biobehavioral reviews. 2011;35:1713–21. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- [35].Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in clinical neuroscience. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, et al. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Annals of the New York Academy of Sciences. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- [37].Bledsoe AC, Oliver KM, Scholl JL, Forster GL. Anxiety states induced by post-weaning social isolation are mediated by CRF receptors in the dorsal raphe nucleus. Brain Res Bull. 2011;85:117–22. doi: 10.1016/j.brainresbull.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain research. 2012;1443:1–17. doi: 10.1016/j.brainres.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]