Abstract
Background
A challenge in the delivery of intensive obesity treatment is making care scalable. Little is known about whether the outcome of clinician-directed weight loss treatment can be improved by adding mobile technology.
Methods
We conducted a 2-arm, 12-month study (between October, 2007 and September, 2010). Seventy adults (body mass index [BMI] >25 and ≤ 40 kg/m2) were randomly assigned to either standard of care group treatment alone (Standard) or Standard + connective mobile technology system (+Mobile). Participants attended biweekly weight loss groups held by the VA outpatient clinic. The +Mobile group was provided personal digital assistants (PDAs) to self-monitor diet and physical activity; they also received biweekly coaching calls for 6 months. Weight was measured at baseline, 3, 6, 9, and 12 months follow-up.
Results
Sixty-nine adults received intervention (mean age 57.7 years, 85.5% male). A longitudinal intent-to-treat analysis indicated that the +Mobile group lost on average 8.6 more pounds (representing 3.1% more weight loss relative to the control) than the Standard group at each post-baseline time point, 95% CI [4.9, 12.2]. As compared to the Standard group, the +Mobile group had significantly greater odds of having lost 5% or more of their baseline weight at each post-baseline time point [OR= 6.5; 95% CI = 2.3, 18.6].
Conclusions
The addition of a PDA and telephone coaching can enhance short-term weight loss in combination with an existing system of care. Mobile connective technology holds promise as a scalable delivery mechanism to augment the impact of clinician-delivered weight loss treatment.
Intensive multicomponent behavioral treatment has been shown to produce clinically significant weight loss (> 5% of initial body weight) in overweight and obese adults.1,2 The U.S. Preventive Services Task Force recommends intensive behavioral treatment for all obese adults, citing evidence that more contact within the first year produces greater weight loss.3 Treatment involves behavioral self-management activities such as setting weight loss goals, self-monitoring, improving diet and physical activity, addressing barriers to change, and strategizing how to maintain lifestyle changes.4,5
A challenge to obesity management is the need to implement access to intensive treatment within existing systems of care. Many physicians describe lack of time and training as barriers to delivering behavioral treatment.6 The Institute of Medicine suggests that interprofessional health care teams have the diverse expertise needed for integrated care.7 However, recent evidence suggests that intensive lifestyle interventions may not need in-person delivery. Among patients referred from primary care, Appel and colleagues8 demonstrated that telephone and internet-based treatment omitting in-person contact produced weight loss comparable to an in-person intervention. Hybrid interventions that use technology and remote intervention components to augment existing in-person treatment programs could prove readily scalable. We tested the additive benefit of augmenting a system-wide group obesity program with a connective technology system that provided mobile decision support (i.e., calorie and activity feedback). The technology allowed participants to transmit data to a behavioral coach who monitored their uploads and provided scheduled telephone coaching.
Self-monitoring of diet and physical activity is associated with weight loss success9 and can be performed conveniently using handheld technology.10-12 Mobile technologies afford in-the-moment decision support by enabling users to check the energy value of foods and activities and track energy balance in real time.13,14 Studies of technology-supported weight loss interventions indicate that digital tools are more effective and acceptable to participants when they supplement rather than replace contact with providers.15,16 Therefore, the current trial tested whether a connective mobile technology system, telephone coaching, and the standard of care obesity treatment improved weight loss outcomes, as compared to standard of care group obesity treatment alone. The standard of care was the MOVE! group weight loss program, offered at all Veteran’s Affairs Medical Centers. 17
Design and Methods
The study design and methods are presented in detail elsewhere18 and will be described briefly.
Setting and Participants
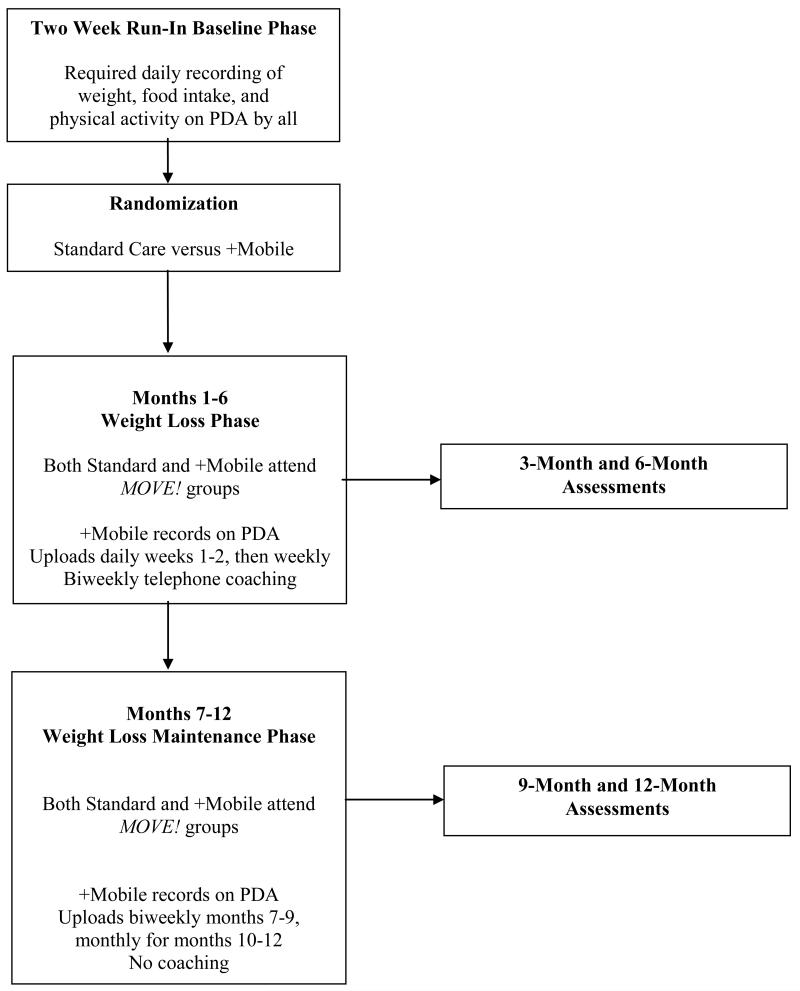
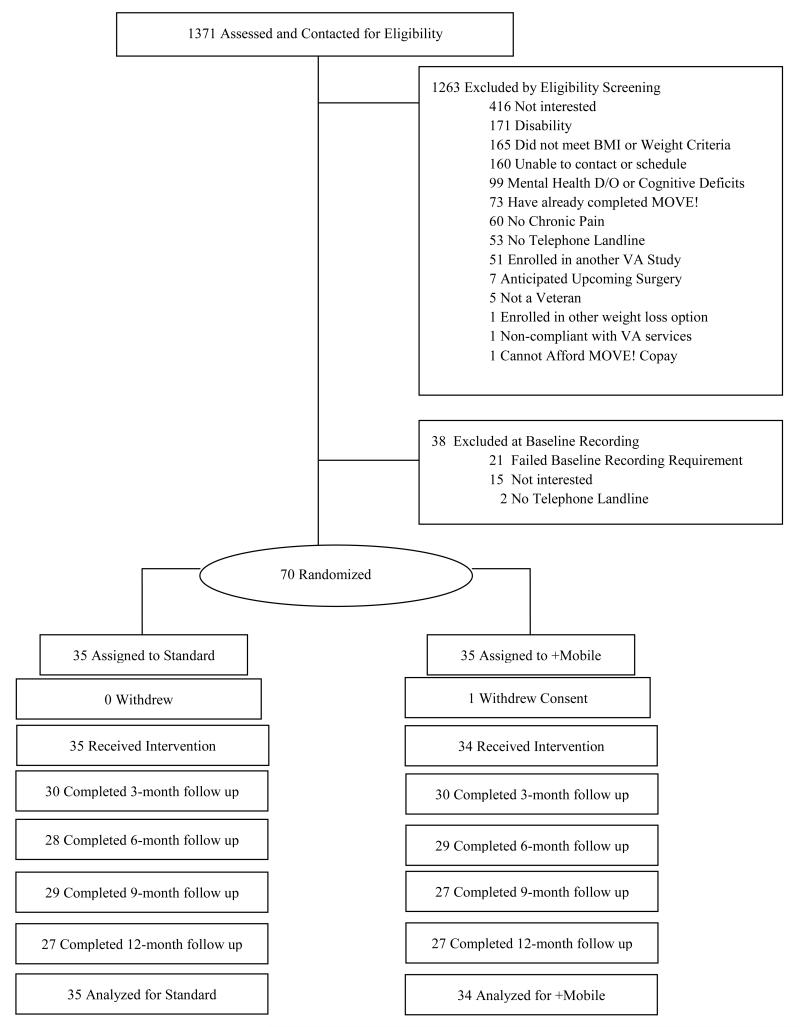
Between October, 2007 and September, 2010, we recruited overweight and obese adults at a Midwestern VA Hospital from those recently referred to MOVE! Inclusion criteria included a body mass index [BMI] >25 and ≤ 40 kg/m2, weight <400 pounds, and being able to participate in moderate-intensity physical activity. Recent psychiatric hospitalization, current substance abuse, binge eating disorder, or a severe mood disorder were exclusion criteria. FIGURE 1 depicts the phases of the trial and FIGURE 2 shows participant flow. All study procedures were approved by the Institutional Review Board at the VA Hospital.
Figure 1. Trial phases.
PDA indicates personal digital assistant.
Figure 2. Participant flow.
BMI indicates body mass index and VA, Veterans Affairs. Chronic pain was eliminated as a study entry criterion 1 year after the study began.
Randomization
Participants completed a technology fluency assessment19, and received a brief (15 minute) training session on how to use a personal digital assistant (PDA) to record food intake, weight, and physical activity. They were loaned a PDA for two weeks and asked to upload their data daily. Those who entered their weight and ≥ 2 meals (with ≥2 items per meal) per day for at least 7 days underwent an equipoise induction which detailed the procedures and highlighted the pros and cons of both groups to equalize their desirability and prevent dropout after randomization.20 Participants were then randomly assigned to either standard of care group treatment alone (Standard) or Standard + connective mobile technology system (+Mobile). Randomization, stratified by age (<65 vs. >65), BMI (<35 vs. >35), and gender, was computer generated using the method of randomly permuted blocks.
Intervention (Weight Loss) Phase
Participants assigned to the Standard group returned the PDA when the 6-month intervention phase began; those assigned to +Mobile retained the PDA. During months 1-6, both groups attended biweekly MOVE! sessions led by dieticians, psychologists, or physicians. Each session lasted approximately 1.5 hours, and included discussion of nutrition, physical activity, and behavior change.21 Participants were given a 5-10% weight loss goal. They were weighed at each session and encouraged to self-monitor, but personalized feedback was not provided.
For participants assigned to +Mobile, a goal feedback thermometer on the PDA was activated at the start of the intervention phase. By entering their foods throughout the day, the thermometer was automatically updated with current caloric intake, and participants used the PDA as a decision support tool to self-regulate energy intake. Participants uploaded their data every day for the first two weeks of the intervention, and once per week thereafter until the end of month 6. After the first month of treatment, the coach introduced physical activity goals and activated a second goal feedback thermometer to depict progress toward a daily physical activity goal. During the 6-month intervention phase, a paraprofessional coach telephoned participants every two weeks to provide 10-15 minutes of individualized guidance based on the uploaded data and monitored the uploads to respond to technical difficulties.
Calorie goals were tailored according to the participant’s baseline weight; activity goals were calculated using current activity level. Progress through the treatment algorithm was mastery-based (triggered by accomplishment of each prior goal). If, after meeting calorie goals, participants did not lose weight for two consecutive weeks, they were instructed to reduce calories in 100 kcal increments until they reached a calorie intake level that yielded a weight loss rate of .5-1% of their current weight per week. For safety, no participant was given an intake goal below 1200 kcal per day. Conversely, if the rate of weight loss was too rapid (operationalized as weight loss of >3 lbs. /week for 4 consecutive weeks), the calorie intake goal was increased in 100 kcal increments until the goal of 1-2 lbs. weight loss per week was attained. Daily physical activity goals (in minutes) were assigned by increasing self-reported baseline activity level by 25% after one month in the protocol. Subsequent physical activity goals were increased by 25% when participants met their previous goal. Goal activity counts were progressively increased until the criterion of an equivalent of 60 minutes per day of moderate intensity physical activity was reached.
Weight Loss Maintenance Phase
During the maintenance phase (months 7-12), participants in both groups attended monthly MOVE! support group sessions led by hospital staff. From months 7-9, +Mobile participants were asked to record and transmit data biweekly; from months 10-12, they transmitted one week of data per month. Throughout maintenance, coaches telephoned participants only if data were not submitted; they provided no other behavioral feedback.
Outcomes
Weight was measured in light clothing with shoes off on a calibrated balance beam scale at randomization and at 3, 6, 9, and 12 month follow-up. The primary outcome was weight loss at six months; the secondary outcome was weight loss at 12 months. Weight loss was measured as change of weight in pounds (lbs.) and as percent weight loss. Change in waist circumference and the proportion achieving a clinically significant 5% loss of initial body weight were exploratory outcomes. Assuming a SD of 12 pounds, a sample size of 150 (75 per group) was projected to yield power of .80 to detect a 6 pound difference in weight loss between groups.
Statistical Analysis
Weight change over time was analyzed using a longitudinal covariance pattern model, utilizing an unstructured variance-covariance matrix.22 Specifically, weight was modeled at all timepoints (baseline, 3, 6, 9, and 12 months) using a-priori contrasts treating baseline as the reference cell in order to assess weight change, relative to baseline, at the four post-baseline time points. Group effects on these a-priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the post-baseline time points. All stratification variables (age, BMI, gender) were included in the analyses. Weight change was also expressed as a 5% or more weight loss (Y/N), relative to baseline, at 3, 6, 9, and 12 months. These repeated binary outcomes were modeled using a generalized estimating equations (GEE) logistic regression model for longitudinal data23 with an unstructured working correlation matrix, and again, we tested whether the group effect was equal or varied across the post-baseline time points.
Results
Participants
The study sample of 69 adults included 59 males (85.5%), 21 minorities (30.4%), 62.3% with less than a college degree and mean age 57.7 years (SD= 11.9, range = 28-86, median=60). Demographic information is summarized in TABLE 1. Only 25% of the sample (N=36) reported full fluency19 with rudimentary computer skills (e.g. “I can print a document”). At baseline (randomization), males had a mean weight of 252.5 lbs (SD = 32.0) with BMI of 36.3 (SD= 4.7). Females had a mean baseline weight of 212.2 lbs (SD= 28.1) and a BMI of 36.4 (SD= 5.3). No differences in weight existed between treatment groups at baseline (p = .35). The proportion of missing data ranged from 13.0 to 21.7% across post-randomization assessment periods, and the proportion of participants who attended all four outcome assessments was 73.9%.
Table 1.
Participant Demographics*
| PDA+ (n=34) |
Standard Care (n=35) |
Total (n=69) |
|
|---|---|---|---|
| Demographic Characteristic | |||
| Age (y) | 57.7 (13.5) | 57.7 (10.2) | 57.7 (11.9) |
| Male, No. (%) | 29 (42.0) | 30 (43.4) | 59 (85.5) |
| Ethnicity, No. (%) | |||
| Hispanic or Latino | 1 (2.9) | 3 (8.6) | 4(5.8) |
| Not Hispanic or Latino | 33 (97.1) | 32 (91.4) | 65 (94.2) |
| Race, No. (%) | |||
| White | 25 (73.5) | 27 (77.1) | 52 (75.4) |
| Black or African American | 9 (26.5) | 8 (22.9) | 17 (24.6) |
| Education Level, No. (%) | |||
| College Graduate | 10 (29.4) | 14 (40.0) | 26 (37.7) |
| Anthropometry | |||
| Weight (lbs.) | 250.6 (35.5) | 242.9 (33.3) | 246.7 (34.4) |
| BMI (kg/m2) | 36.9 (5.4) | 35.8 (3.8) | 36.3 (4.6) |
| Waist Circumference, inches | 47.4 (5.5) | 47.4 (3.5) | 47.4 (4.6) |
At Randomization. Data are given as mean (SD) unless otherwise indicated. No between-group differences on baseline variables were observed.
+Mobile Treatment
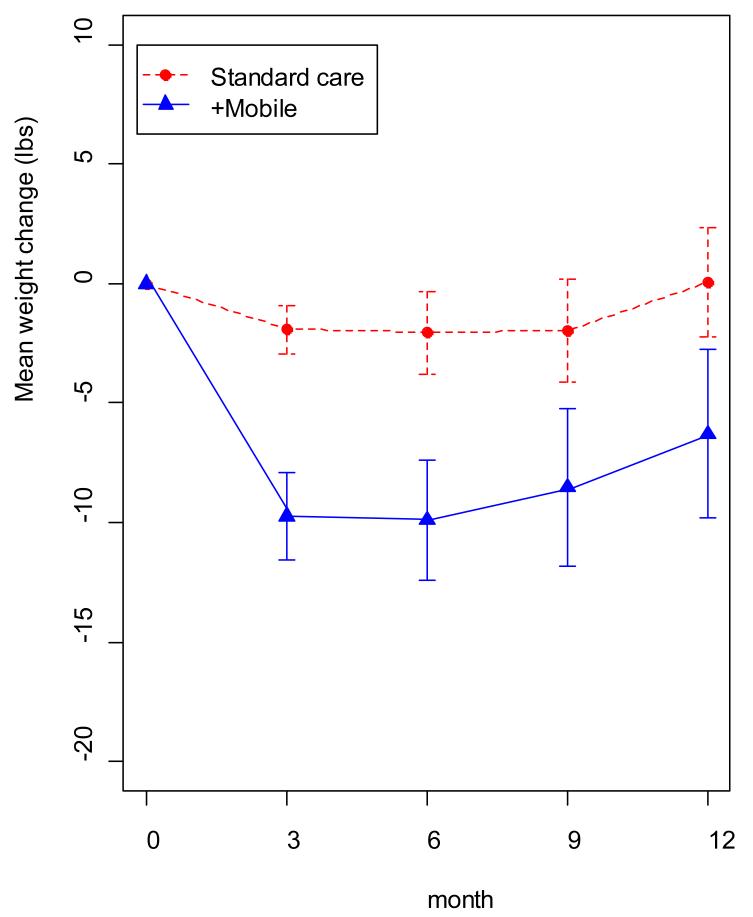
Participants assigned to +Mobile lost on average 8.6 lbs more (3.1% more weight loss relative to the control group) than participants in the Standard group at each post-baseline time point, 95% CI [4.9, 12.2] and there was no evidence that the treatment effect varied across time (p=.44). In terms of the specific time points, weight loss was greater for the +Mobile group (9.7 lbs., 95% CI [6.0, 13.5]) than the Standard group (1.9 lbs., 95% CI [0.1, 4.0]) at 3-months, 6-months (+Mobile: 9.9 lbs., 95% CI [4.7, 15.1]; Standard: 2.1 lbs.,95% CI [−1.5, 5.6]), 9-months (+Mobile: 8.5 lbs., 95% CI [1.8, 15.3]; Standard: 2.0 lbs.,95% CI [−2.5, 6.4]), and at 12-months (+Mobile: 6.3 lbs.,95% CI [−1.0, 13.6]; Standard: −0.05 lbs. 95% CI [−4.7, 4.6]). These data are shown in FIGURE 3. In terms of 5% or more weight loss (Y/N), a significant group effect favoring the +Mobile intervention was observed (OR 6.46, 95% CI [2.5, 18.6]), and this effect did not vary significantly across time (p = .13). The observed proportions were: 3-months (+Mobile 36.7% achieving the 5% criterion versus Standard 0%), 6-months (+Mobile 41.4%, Standard 10.7%), 9-months (+Mobile 33.3%, Standard 10.3%), and 12-months (+Mobile 29.6%, Standard 14.8%).
Figure 3. Weight Change over Time for +Mobile versus Standard Treatment.
Weight loss plotted over time for the connective mobile technology (+mobile) and standard groups. Weight loss was significantly greater for the +mobile group at 3, 6, and 9 months.
+Mobile Treatment and MOVE! Adherence
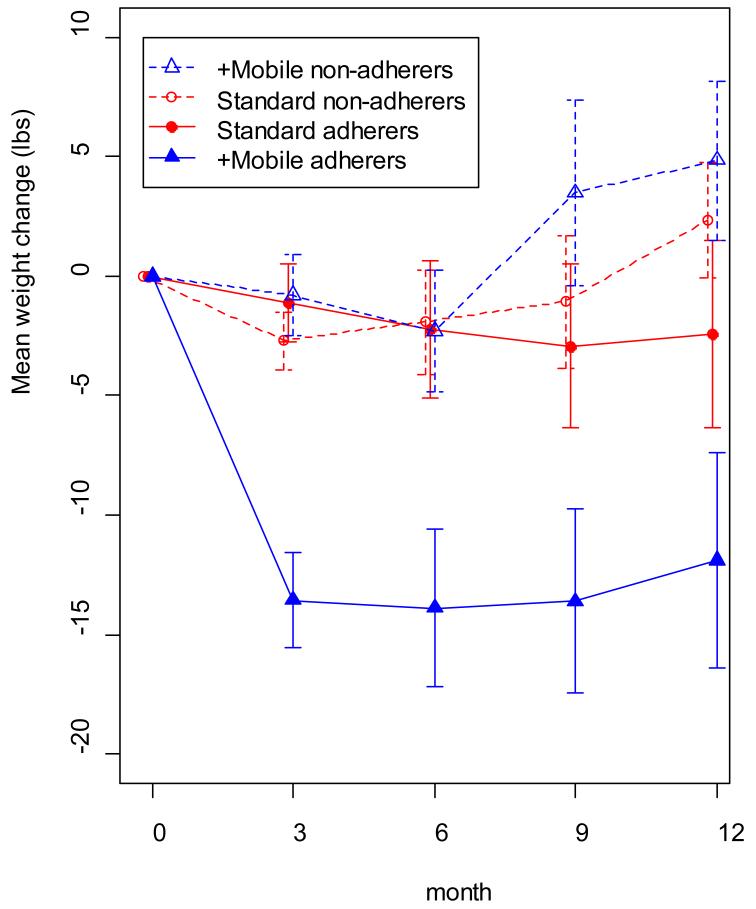
There was no difference in how frequently participants assigned to +Mobile attended MOVE! groups, as compared to those assigned to Standard MOVE! group treatment alone (mean number attended: +Mobile: 6.2 meetings; Standard: 5.9 meetings) [p = .54]. However, participants randomized to +Mobile who were also adherent to MOVE! treatment, as evidenced by attending ≥80% of treatment sessions, lost significantly more weight than less adherent +Mobile participants and adherent or nonadherent Standard participants (see FIGURE 4).
Figure 4. Weight Loss over Time as a function of Treatment Assignment and MOVE! Adherence*.
Participants in the connective mobile technology (+mobile) group who were adherent to MOVE! treatment (ie, attended 80% of treatment sessions) lost significantly more weight than less adherent +mobile participants and either adherent or nonadherent standard-of-care participants. Data were available from the following: standard adherers, 15 participants at baseline and 13 at month 12; standard nonadherers, 20 at baseline and 14 at month 12; +mobile adherers, 21 at baseline and 18 at month 12; and +mobile nonadherers, 13 at baseline and 9 at month 12.
+Mobile Treatment Calls Completed
Of the recommended 12 coaching calls, the average +Mobile participant received 74.4%, a mean of 8.9 calls (SD= 2.8, range = 0-15, median=8) lasting a total duration of 125.6 minutes (SD= 48.8, median= 125) per participant. Total additional time spent on technical support calls averaged a mean of 15.3 minutes (SD= 5.4, median= 10) per participant.
Comment
The current study demonstrates the feasibility of using mobile connective technology to interface with a hospital-based, standard of care weight loss treatment. Adding technology and coaching to the available group obesity treatment significantly enhanced weight loss outcomes at 3, 6, 9, and 12 months. More than 36% of participants utilizing the mobile technology system and coaching, compared to 0% in the Standard of care condition, lost at least 5% of initial body weight at 3 months, a degree of weight loss that has been shown to improve cardiovascular disease risk biomarkers.24,25 These findings indicate that it is possible to implement an intensive behavioral weight loss intervention that is easy to access, effective, and readily integrated into an existing system of care.
The current results are, to our knowledge, the first to demonstrate that use of a mobile technology system and remote coaching can significantly augment weight loss and maintenance when added to an existing standard of care obesity treatment program. Previous PDA-based weight loss interventions have been successful in producing weight losses at 6 months of between 5.5 to 7.3%. However, in those studies the PDA was used in conjunction with an intensive in-person researcher-delivered behavioral weight loss program that required participants to attend 20-24 in-person treatment sessions.12,26 The current study demonstrates that significant, sustained weight loss can be produced when the PDA and up to 12 brief biweekly telephone calls are combined with 12 group sessions over a 6-month period. Instead of requiring participants to attend 8-12 additional treatment sessions, each lasting 90 minutes plus commuting time, those in the current study received an average of 8 coaching calls, each lasting approximately 14 minutes. This very substantial reduction in the cost and travel burden placed on participants would be expected to increase the program’s appeal to the public and its feasibility of implementation. Moreover, unlike prior obesity interventions using mobile technology, the group randomized to +Mobile maintained significant weight loss during the maintenance phase (months 7-12), even though meeting frequency was reduced to monthly and coaching calls were discontinued. These results suggest that the addition of a mobile technology system and coaching calls to clinician-delivered standard of care of obesity treatment can enhance weight loss outcomes at low added burden and cost.
The current mobile connective technology system was unique in that the coach tracked and supportively held participants accountable for self-monitoring.15, 27 Coaches provided timely, tailored, feedback on calls since data had been transmitted and analyzed beforehand. Failure to upload data for several days led the coach to suspect technical difficulties and make outreach. In contrast, prior weight loss interventions mediated by mobile technology sent automated rather than personally crafted text messages and delayed feedback on participants’ hand-delivered data for at least a week because coaches needed time to study it.12,26,28,29 When used to record diet and activity without behavioral support, digital self-monitoring lacks a weight loss advantage over paper and pencil recording.29
Consistent with results of the current study, recent systematic reviews of technology-based weight loss interventions conclude that technology is a promising way to produce clinically significant weight loss in overweight and obese adults. 16,30 In addition to PDAs, a range of technologies has been tested as primary delivery channel or adjunct, including computers,28,31 internet,32-37 text messages,38,39 and physical activity monitors.40,41 Weight loss varied depending upon the specific technology, amount and type of interventionist contact, and duration of the intervention. In general, greater weight loss (5.7 to 8.8 kilograms) occurs when technology is combined with weekly in-person contact.12,40-42 However, since in-person interventionist contact is the most expensive treatment component to deliver and the most burdensome to access, we substituted brief, regularly scheduled telephone coaching to which coaches came prepared by having reviewed participants’ transmitted, analyzed data. Results suggest that connective technology, like that used in the present study, can allow telephone contact to substitute efficiently for face-to-face time. Other intervention components could conceivably be automated for added efficiency.
Strengths of this study include the demonstration that a technology-based intervention can be integrated into the VA, a large system of care, suggesting that the treatment approach is scalable. The demographics of the sample, which consisted primarily of males (85.5%) are also a strength, given that most weight loss studies include predominately women.9,43 Importantly, the sample also represented a population subgroup that has been slow to adopt technology: older adults with less than a college education.44 Our participants used the PDA effectively after a brief training session, which accords with other evidence that older adults increasingly use technology.45,46 For example, 81% of 55-64 year olds and 56% of those who are 65+ own a cell phone.47 Mobile devices and apps have become accessible to a degree that was unimaginable previously47. Therefore, interventions such as the present one that use technology to augment an existing care system hold great potential to advance population health.
Despite the study’s strengths, some limitations warrant consideration. First, the fact that the study was conducted at a VA Medical Center outpatient clinic limits generalizability. The VA system is atypical in having well-established, integrated preventive care programs by virtue of its commitment to population level health care. Nevertheless it is noteworthy that our intervention significantly augmented the weight loss outcomes produced by VA’s standard of care weight loss program.
Our intervention incorporated two main components: a mobile device that provided feedback about diet and activity, and regular telephone coaching that was informed by a knowledge of participants’ self-monitoring behavior. As for most treatment packages, the impact of these two components cannot be disentangled at present. For comparison, Appel and colleagues8 found that adding 21 coaching calls to didactic lessons on the internet produced weight loss comparable to 18 in-person treatment sessions. We augmented an institution’s pre-existing biweekly in-person obesity treatment program with fewer (12) telephone coaching calls, presumably rendered better tailored and more efficient because coaches were pre-informed via participants’ mobile uploads. Our trial illustrates an optimization strategy whereby medical practices can test whether adding or reconfiguring current treatment strategies in their setting would improve local outcomes cost-effectively.48 Although the current study utilized older-generation technology, newer technologies, including smartphones, retain enhanced functionalities of a PDA and are on track to be used by most of the population in the next 3 years.47 These technological advancements, used in conjunction with standard of care obesity treatment, may greatly enhance scalability.
In sum, this study highlights the promise of a mobile technology system as a scalable, cost-effective means to augment the effectiveness of clinician-delivered weight loss treatment. Technology offers new channels to reconfigure the delivery of effective components of behavioral weight loss treatment (i.e. self-monitoring, goal setting, lifestyle counseling, in-person sessions).8,16,49,50 A handheld tool that provides decision support for self-monitoring embraces patient-centered care by helping patients to manage their own behavior change. By enabling trained paraprofessionals to deliver highly personalized treatment remotely, at reduced cost and participant burden, connective technology systems can help to ease the burden on strained care systems.
Acknowledgements
This study was supported by VA Merit Review F442291 RRD-funded study at Hines VA Medical Center, Hines, IL to Dr. Spring. The development of the PDA tool used in this study was funded by an NHBLI grant HL075451.
Footnotes
Author Contributions: Dr. Spring had full access to all of the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and Design: Spring, Janke, Kozak, Hedeker, Epstein
Acquisition of Data: Duncan, Demott, Pictor
Analysis and Interpretation of Data: Hedeker, Spring, Siddique, McFadden, Duncan
Drafting of the Manuscript: Spring, Duncan, Janke, Kozak, Hedeker
Critical Revision of the manuscript for intellectual content: Janke, Kozak, Hedeker, Pellegrini, Buscemi
Obtained Funding: Spring
Administrative, technical, or material support: Duncan, McFadden, Demott, Pictor Study Supervision: Spring, Janke, Kozak
Financial Disclosure: None reported.
Trial Registration: Clinicaltrials.gov Identifier: NCT00371462
References
- 1.Franz MJ, VanWormer JJ, Crain AL, et al. Weight loss outcomes: a systematic review and meta-analysis of weight loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am. 2005 Mar;28(1):151–+. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011 Oct 4;155(7):434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002 Dec;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009 Apr;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995 Nov;24(6):546–552. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 7.DeSilets LD. The Institute of Medicine’s Redesigning Continuing Education in the Health Professions. J Contin Educ Nurs. 2010 Aug;41(8):340–341. doi: 10.3928/00220124-20100726-02. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Clark JM, Yeh HC, et al. Comparative Effectiveness of Weight loss Interventions in Clinical Practice. N Engl J Med Overseas Ed. 2011 Nov 24;365(21):1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011 Jan;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr. 2008 Apr;27(2):280–286. doi: 10.1080/07315724.2008.10719701. [DOI] [PubMed] [Google Scholar]
- 11.Burke LE, Styn MA, Glanz K, et al. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemp Clin Trials. 2009 Nov;30(6):540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011 Feb;19(2):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker RC, Kirschenbaum DS. Self-Monitoring May Be Necessary for Successful Weight Control. Behav Ther. 1993;24(3):377–394. Sum. [Google Scholar]
- 14.Baker RC, Kirschenbaum DS. Weight control during the holidays: Highly consistent self-monitoring as a potentially useful coping mechanism. Health psychol. 1998 Jul;17(4):367–370. doi: 10.1037//0278-6133.17.4.367. [DOI] [PubMed] [Google Scholar]
- 15.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. doi: 10.2196/jmir.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao G, Burke LE, Spring BJ, et al. New and emerging weight management strategies for busy ambulatory settings: a scientific statement from the American Heart Association endorsed by the Society of Behavioral Medicine. Circulation. 2011 Sep 6;124(10):1182–1203. doi: 10.1161/CIR.0b013e31822b9543. [DOI] [PubMed] [Google Scholar]
- 17.Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for Veterans. Prev Chronic Dis. 2009 Jul;6(3):A98. [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan JM, Janke EA, Kozak AT, et al. PDA+: A Personal Digital Assistant for Obesity Treatment - An RCT testing the use of technology to enhance weight loss treatment for veterans. Bmc Public Health. 2011 Apr 11;:11. doi: 10.1186/1471-2458-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunz U. The Computer-Email-Web (CEW) Fluency Scale-Development and Validation. Int J Human-Computer Interaction. 2004;17(4):479–506. [Google Scholar]
- 20.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight loss trial. Health Educ Res. 2005 Aug;20(4):439–447. doi: 10.1093/her/cyg139. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed July, 18, 2012];VHA National Center for Health Promotino & Disesease Prevention, MOVE! Group Sessions (Version 1) http://www.move.va.gov/GrpSessionsV1.asp.
- 22.Hedeker DR, Gibbons RD. Longitudinal data analysis. Wiley-Interscience; Hoboken, N.J.: 2006. [Google Scholar]
- 23.Hardin JW, Hilbe J. Generalized estimating equations. Chapman & Hall/CRC; Boca Raton, Fla.: 2003. [Google Scholar]
- 24.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995 Sep;3(Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 25.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011 Jul;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yon BA, Johnson RK, Harvey-Berino J, Gold BC. The use of a personal digital assistant for dietary self-monitoring does not improve the validity of self-reports of energy intake. J Am Diet Assoc. 2006 Aug;106(8):1256–1259. doi: 10.1016/j.jada.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Spring B, Schneider K, McFadden HG, et al. Multiple behavior changes in diet and activiity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172(10):1–8. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnett KF, Taylor CB, Agras WS. Ambulatory Computer-Assisted Therapy for Obesity - a New Frontier for Behavior-Therapy. J Consult Clin Psychol. 1985;53(5):698–703. doi: 10.1037//0022-006x.53.5.698. [DOI] [PubMed] [Google Scholar]
- 29.Burke LE, Styn MA, Sereika S, et al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med. 2012;43(1):20–26. doi: 10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coons MJ, DeMott A, Buscemi J, et al. Technology interventions to curb obesity: a systematic review of the current literature. Curr Cardiovasc Risk Rep. 2012;6:120–134. doi: 10.1007/s12170-012-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor CB, Agras WS, Losch M, Plante TG, Burnett K. Improving the Effectiveness of Computer-Assisted Weight loss. Behav Ther. 1991 Spr;22(2):229–236. [Google Scholar]
- 32.Maruyama C, Kimura M, Okumura H, Hayashi K, Arao T. Effect of a worksite-based intervention program on metabolic parameters in middle-aged male white-collar workers: a randomized controlled trial. Prev med. 2010 Jul;51(1):11–17. doi: 10.1016/j.ypmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-month outcomes and process evaluation of the SHED-IT RCT: an internet-based weight loss program targeting men. Obesity (Silver Spring) 2011 Jan;19(1):142–151. doi: 10.1038/oby.2010.119. [DOI] [PubMed] [Google Scholar]
- 34.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes - A randomized trial. J Am Med Assoc. 2003 Apr 9;289(14) doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 35.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006 Aug 14-28;166(15):1620–1625. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 36.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. J Am Med Assoc. 2001 Mar 7;285(9):1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 37.Wing RR, Pinto AM, Crane MM, Kumar R, Weinberg BM, Gorin AA. A statewide intervention reduces BMI in adults: Shape Up Rhode Island results. Obesity (Silver Spring) 2009 May;17(5):991–995. doi: 10.1038/oby.2008.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patrick K, Raab F, Adams MA, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11(1):e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr. 2009 Dec;12(12):2382–2391. doi: 10.1017/S1368980009005230. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini CA, Verba SD, Otto AD, Helsel DL, Davis KK, Jakicic JM. The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity (Silver Spring) 2012 Feb;20(2):356–363. doi: 10.1038/oby.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuger SL, Barry VW, Sui X, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev med. 2010 Aug;51(2):123–128. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes. 2005 Oct;29(10):1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 44.Czaja SJ, Charness N, Fisk AD, et al. Factors predicting the use of technology: Findings from the center for research and education on aging and technology enhancement (CREATE) Psychol Aging. 2006 Jun;21(2):333–352. doi: 10.1037/0882-7974.21.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charness N, Fox MC, Mitchum AL. Lifespan cognition and information technology. In: Fingerman CB K, Antonnuci T, Smith J, editors. Handbook of lifespan psychology. Springer; New York: 2010. [Google Scholar]
- 46.US Census Bureau [Accessed July, 18, 2012];Computer and Internet Use in the United States: 2010. http://www.census.gov/hhes/computer/publications/2010.html.
- 47.Smith A. [Accessed April 25, 2012];Pew Internet & American Life Project: Smartphone adoption and usage. 2011 http://pewinternet.org/Reports/2011/Smartphones.aspx.
- 48.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011 Apr;41(2):208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011 Oct;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanovski SZ. Obesity Treatment in Primary Care - Are We There Yet? N Engl J Med. 2011 Nov 24;365(21):2030–2031. doi: 10.1056/NEJMe1111487. [DOI] [PubMed] [Google Scholar]