Abstract
This manuscript will review our current understanding of neutrophilic polymorphonuclear leukocyte (neutrophil) interactions with the endothelium during immune and inflammatory responses, focusing on the molecular mechanisms regulating neutrophil adhesion to and migration through the endothelium in response to infection or tissue injury. This is a complex and dynamic area of research and one that has been the topic of several recent comprehensive reviews to which the interested reader is referred (64, 118, 131). By design, this review will begin with a brief review of some basic aspects of neutrophil biology and endothelial adhesion to provide a foundation. The remainder of the review will focus on selected areas of this complex field, specifically the role of the endothelial glycocalyx in regulating neutrophil adhesion and the mechanisms and consequences of migration of neutrophils between (paracellular) and through (transcellular) endothelial cells during egress from the vasculature.
Neutrophils and Innate Immune Response
Neutrophils have been described as the foot soldiers on the front lines of the innate immune response (88, 89, 140). Neutrophils are a primary and critical component of the immediate response to tissue injury or infection and can sense tissue injury and/or the presence of invading microbial pathogens by virtue of diverse membrane receptors that recognize products of tissue injury, chemoattractants, and microbial compounds that mark the tissue or organ as requiring their attention (6, 12, 88, 141). Neutrophils arrive early (within minutes) during acute inflammatory responses and represent the most abundant immune cells in the inflamed tissues for many hours. In their capacity as the first responders of the innate immune system, the primary roles of these professional phagocytic cells are to recognize, contain, and kill invading microbial pathogens and remove damaged cells and other debris from the vicinity in preparation for repair of the damaged tissues (42, 71, 89, 134, 140). Additionally, an emerging literature suggests that, contrary to current dogma, neutrophils may actively promote repair processes (56, 114, 127).
Neutrophils are well equipped for their role in host defense; they possess a potent anti-microbial arsenal that includes oxidant-generating systems such as the phagocyte NADPH oxidase (NOX2) and nitric oxide synthases (NOSI and NOSIII), as well as an impressive array of potent proteolytic enzymes and antimicrobial peptides contained in membrane-encapsulated vesicles termed granules (32, 38, 42, 86, 91). When neutrophils ingest microbial pathogens, their cytotoxic compounds are typically released directly into the phagosome, compartmentalizing both the pathogen and the cytotoxic products. Recent studies have also illuminated a novel antimicrobial mechanism. Upon activation, neutrophils discharge DNA fibers that form lattices, termed neutrophil extracellular traps (NETs), to which antimicrobial proteins are attached that mediate extracellular killing of microbial pathogens (76). NETs are thought to form via a unique death pathway triggered by reactive oxygen species (ROS) produced by the phagocyte NADPH oxidase (NOX2) (76).
It is paradoxical that these same potent antimicrobial responses can, under pathological circumstances, lead to host tissue damage (90). Teleologically, this may be considered a form of unintended “collateral damage” from rapid mobilization of well armed troops as part of a robust antimicrobial response. In such circumstances, neutrophil-derived antimicrobial compounds may be released into the extracellular space in excess quantities, resulting in inflammatory tissue injury. It is important to bear in mind that neutrophils in the context of an inflammatory response are inherently beneficial; it is only when their responses become excessive or unregulated that injury to host tissues may ensue (86, 88, 89).
The ability of neutrophils to egress from the vasculature and migrate to the site of infection or tissue injury is essential for effective innate immune responses. Indeed, if these processes are defective, either from genetic deficiencies or as a result of iatrogenic immunosuppressive therapy, serious and life-threatening infections ensue (70, 72). During this journey, neutrophils recognize and are activated by specific signals such as chemokines or bacterial products released in the vicinity of infection or tissue injury. In response to these environmental cues, neutrophils adhere to and pass through the endothelial lining and through permissive areas of the basal lamina of the blood vessels supplying the affected area (120, 122). In organs such as the lung, GI tract, and kidney, neutrophils may then transmigrate through the epithelial layer, likely through preexisting holes in the epithelial basal lamina and then between adjacent epithelial cells into the lumen of the organ (122). It is also during this complex series of events that unrestrained activation of neutrophils in response to microbial or host-derived stimuli may result in release of cytotoxic compounds that can injure vicinal host cells. Although it is clear that neutrophils can emigrate from the vasculature into organs such as the lung and GI tract without causing injury (79, 80, 126), there is compelling evidence from observations in both humans and experimental models that an intense influx of neutrophils may incite pathological inflammation in organs including the lung, liver, GI tract, joints, kidney, heart, and brain and may contribute to the pathogenesis of conditions such as acute lung injury, inflammatory bowel disease, nephritis, arthritis, ischemia-reperfusion injury, myocardial infarction, and stroke (1, 10, 13, 33, 40, 51, 67, 90, 95, 102, 110, 132). To understand this transition toward a maladaptive inflammatory state, it is essential to review the physiological processes involved in neutrophil adhesion and transendothelial migration.
Neutrophil Adhesion to Endothelium
We will begin with a brief overview of the common mechanisms involved in neutrophil and other leukocyte adhesion and transendothelial migration to provide context for a more detailed discussion of specific facets of this process. Traditionally, leukocyte adhesion to the endothelium was viewed as involving three discrete phases: selectin-mediated rolling, leukocyte activation triggered by chemokines, and firm attachment (arrest) mediated by integrins (reviewed by Refs. 64, 105). However, recent studies have revealed complexity in these events and defined additional phases including tethering, slow rolling, modulation of adhesion strength, intraluminal crawling, and finally transcellular or paracellular migration (reviewed by Refs. 64, 138). Currently, the initial step in neutrophil and other leukocyte adhesion to the endothelium is believed to involve capture (or tethering) of leukocytes mediated by interactions between L, E, and P-selectins and P-selectin glycoprotein ligand (PSGL1) and α4β1 (VLA4) integrin. L-selectin is expressed by leukocytes, P-selectin is expressed by inflamed endothelium and platelets, E-selectin is expressed by inflamed endothelium, and PSGL1 is expressed by endothelium and some leukocytes. Subsequently, leukocytes roll on the endothelium, a step mediated by interactions between selectins and PSGL-1 and other glycosylated ligands. Importantly, adhesion mediated by L-selectin and P-selectin requires shear stress (53, 135). A phase termed “slow rolling” ensues (mediated by selectin-triggered signaling), followed by arrest of the leukocytes on the endothelial surface, a step that involves β1- and β2-integrins and their respective binding partners.
Integrins are transmembrane glycoproteins expressed on leukocytes that are comprised of α- and β-chains (reviewed by Refs. 68, 105, 131). The β2-integrins consist of a common β-chain (CD18) and a variable α-chain (CD11a, b, c, or d). The phase of leukocyte arrest is mediated by interactions between the β2-integrin CD11a/CD18 (LFA-1) and intercellular adhesion molecule (ICAM)-1, α4β1 (VLA-4) and VCAM1, and α4β7 and MADCAM1. Subsequently, there is strengthening of adhesion followed by spreading of the leukocytes that are mediated by activation of outside-in and inside-out intracellular signaling pathways. The leukocytes then crawl along endothelial cells (“intraluminal crawling”) by a process involving interactions between CD11b/CD18 (αmβ2 or Mac-1) and ICAM-1. Finally, leukocytes transmigrate across the endothelium taking either a paracellular route or a transcellular route, a decision guided by complex and incompletely understood mechanisms discussed in more detail below.
Although the focus of this review is on the endothelium and its glycocalyx, it is important to acknowledge that other cellular and noncellular elements such as pericytes and the basement membrane are involved in regulation of transmigration of neutrophils (16). For instance, regions of the basement membrane that are low in laminins appear to be preferentially used by migrating neutrophils (120, 124). These so-called “low-expression regions” (LER) are also adjacent to gaps between pericytes, an enigmatic cell type of mesodermal origin that surround the endothelial cells of small blood vessels and may be involved in physiological (regulation of capillary tone and homeostasis) and pathological (tissue fibrosis) processes (136). In the lung, fibroblasts have been observed to be in direct continuity with both pulmonary capillary endothelial and alveolar epithelial cells, constituting another potential route along with neutrophils can apparently transmigrate (113, 122).
On, Around, and Through the Endothelium
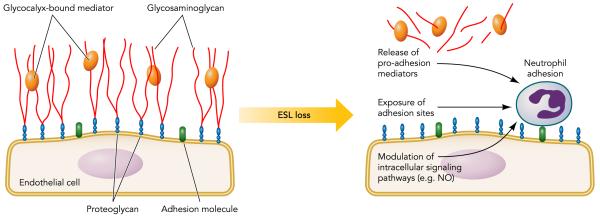
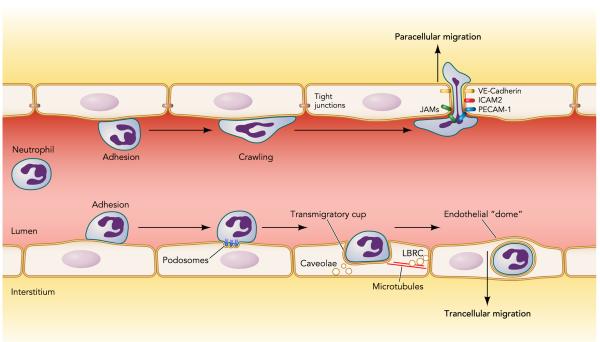
With the previous discussion in mind, we will now examine selected aspects of leukocyte-endothelial interactions in greater detail, focusing primarily on neutrophils but including data from other leukocyte types to illustrate important mechanisms where needed. These include 1) the role of the endothelial glycocalyx in regulating initial neutrophil-endothelial interactions (FIGURE 1) and 2) the mechanisms involved in neutrophil and other leukocyte transendothelial migration via the paracellular and transcellular routes (FIGURE 2).
FIGURE 1. Role of the glycocalyx in regulating neutrophil adhesion to the endothelium.
Endothelial surface layer (ESL) loss induces neutrophil adhesion to endothelial cells. Potential mechanisms include the release of pro-adhesion mediators previously sequestered by the glycocalyx, exposure of previously hidden endothelial surface adhesion molecules, and/or the activation (via proteoglycans) of pro-adhesion endothelial signaling pathways.
FIGURE 2. Paracellular and transcellular migration.
After attaching to the endothelial surface, the neutrophil chooses whether to emigrate from the vascular space using either paracellular or transcellular routes. For paracellular migration, the neutrophil crawls along the endothelial cell surface to the intercellular junctions where it extends lamellopodia between endothelial cells, disrupting the intercellular (tight and adherens) junctions. This is followed by translocation of the entire cell through the inter-endothelial junctions. For transcellular migration, the neutrophil forms actin-rich podosomes that indent the subjacent endothelium. This is followed by the formation of actin-enriched projections by the endothelium that form a so-called “transmigratory cup” around the neutrophil. The cup is formed through the infusions of internal membranes contributed by the lateral border recycling compartment (LBRC) and/or caveolae. The contribution of the LBRC requires microtubules. Despite traversing the cytoplasm of an endothelial cell, the neutrophil causes minimal vascular leak because its route is covered by a dome formed by the endothelium.
Structure and Function of the Endothelial Glycocalyx
The endothelial glycocalyx is a thin (<0.1 μm) layer of glycoproteins and proteoglycans lining the vascular lumen that forms an interface between the blood and the endothelium. These proteins are covalently bound to negatively charged glycosaminoglycans, including heparan sulfate (HS), hyaluronic acid (HA), and chrondroitin sulfate (104). In vivo, these glycosaminoglycans dynamically interact with plasma proteins and become highly hydrated, forming a substantial (>1 μm) endothelial surface layer (ESL) that excludes large molecules (e.g., dextrans) and erythrocytes. Although the glycocalyx has been recognized for decades, its significance in vivo has been underappreciated due to a previously unrecognized loss of ESL thickness in cultured cells (22, 103) as well as during histological fixation and processing (125). Recent advances in in vivo microscopy have allowed for a greater appreciation of ESL thickness and, consequently, renewed interest in the function of the glycocalyx.
With the ability of the ESL to form a charged meshwork overlying the luminal entrance of interendothelial junctions, the glycocalyx serves as a key regulator of paracellular protein and fluid transit (26). Consistent with these observations, it has been demonstrated that enzymatic removal of glycosaminoglycans such as HS or HA from the systemic endothelial glycocalyx increases the permeability of the endothelial barrier in vitro and in vivo (21, 43, 48, 112). Furthermore, there is evidence that the glycocalyx influences endothelial physiology through regulation of cellular response to mechanical stress. Since it is well known that glycosaminoglycans are intimately involved in mechanotransduction, an HS- and HA-replete glycocalyx is necessary for the endothelial-derived nitric oxide (NO) production in response to fluid shear stress (36, 85, 97).
The Glycocalyx as an Interface between Neutrophils and Endothelium
Given its strategic localization along the luminal surface of the vasculature, the glycocalyx is ideally situated to serve as an interface between circulating leukocytes and the endothelium. Specifically, the glycocalyx has been demonstrated to influence neutrophil-endothelial adhesion as well as neutrophil-induced alterations in endothelial physiology.
Regulation of Neutrophil Adhesion
An intact glycocalyx/ESL serves to inhibit neutrophil adhesion to the endothelial surface under basal conditions. Enzymatic degradation of glycocalyx HS in an explanted guinea pig heart model significantly increased neutrophil sequestration within the coronary vasculature (50). Similarly, coronary ischemia-reperfusion rapidly resulted in denudation of the endothelial glycocalyx, leading to increased neutrophil adhesion and consequent myocardial injury within minutes (20, 50, 57, 58). An abrupt loss of ESL thickness induced by the inflammatory cytokine TNF-α has similarly been demonstrated to lead to increased leukocyte adhesion (44).
Although it is apparent that an intact glycocalyx/ ESL limits neutrophil adhesion, the mechanism driving this inhibition remains largely uncharacterized. From a simple architectural standpoint, the thickness of the ESL (>1 μm) greatly exceeds the length of apical-based selectins, and as such it has been proposed that the in vivo glycocalyx forms a “cloak” that conceals endothelial adhesion sites from passing neutrophils (23) (FIGURE 1). Moreover, it has been hypothesized that neutrophils can only access buried endothelial adhesion molecules if the velocity of blood flow is sufficiently slow to allow time for penetration of neutrophil microvilli into the ESL (125). This hypothesis could provide a partial explanation for the observation that, in most organs, neutrophils preferentially adhere to and roll along low-flow venules and not high-flow arterioles (139). Loss of ESL thickness, therefore, could lead to increased neutrophil adhesion within both arterioles and venules, thus predisposing to neutrophil extravasation and increasing the intensity of inflammatory tissue injury across multiple segments of the vascular bed.
Glycocalyx thickness alone, however, is unlikely to fully explain the heterogeneity of neutrophil rolling and adhesion across different vascular segments; other factors undoubtedly influence these events. For example, in postcapillary venules, leukocytes emerging from a narrow capillary are physically deformed, with their cell surfaces held in close contact with the capillary endothelium; these factors could affect their degree of activation (47, 55, 63, 133). In addition, the nature, density, and geographical distribution of adhesion molecules and other receptors on the endothelial cells may differ on endothelial cells in arterioles, capillaries, and postcapillary venules in different organs (14, 30, 108, 109). It is the net effect of these diverse factors that determine the preferential adhesion and rolling of neutrophils in the postcapillary venules in most organs except the lung (see below) (139).
In contrast to the systemic circulation, it is unclear whether the glycocalyx serves a similar cloaking function within the pulmonary circulation, where the main site of neutrophil adhesion and extravasation is the capillary endothelium (15, 29). As described above, the pulmonary capillary lumen is sufficiently small that neutrophils transiting the capillaries are forced to compress during passage, thereby physically altering the capillary endothelial glycocalyx. Presumably, the proximity of neutrophils to endothelial adhesion molecules during capillary passage makes the thickness of the (now compressed) ESL a less significant mechanism promoting or limiting neutrophil/endothelial interactions. To our knowledge, however, there are no studies that have investigated the role of ESL dimensions (e.g., thickness) in governing neutrophil adhesion or activation within the pulmonary circulation.
Another potential mechanism by which the glycocalyx may influence neutrophil adhesion arises from the mechanotransductive functions of glycosaminoglycans. As discussed previously, a glycosaminoglycan-replete glycocalyx is necessary for endothelial production of NO in response to fluid shear stress. As NO inhibits neutrophil-endothelial adhesion (62), diseases marked by loss of glycocalyx could have diminished endothelial NO production, resulting in increased neutrophil adhesion (FIGURE 1). Indeed, loss of NO production occurs within minutes of cardiac ischemia-reperfusion (62, 116), a time course that is consistent with the concordant loss of glycocalyx integrity (58) and neutrophil adhesion (69).
Finally, endothelial cells are known to be a rich source of a variety of bioactive mediators ranging from lipids, such as platelet-activating factor (PAF), to chemokines such as IL-8 and MIP-1α, as well as growth factors such as platelet-derived growth factor (PDGF) and transforming growth factors-α and -β. It is possible that the endothelial glycocalyx binds these mediators, creating a matrix-bound reservoir that can be released in whole or in part, thus influencing key events in the inflammatory cascade including leukocyte adhesion and activation (65, 98). Indeed, both neutrophil adhesion (123) and extravasation (81) were attenuated in transgenic mice featuring modified or truncated HS rendered incapable of efficient chemokine binding. Furthermore, degraded glycocalyx components may themselves be pro-inflammatory, as evidenced by the ability of low-weight HA fragments to function as a damage-associated molecular pattern (DAMP) and trigger innate immune responses (52) (FIGURE 1).
Despite the emerging paradigm of the intact glycocalyx as an anti-adhesive structure, some studies have suggested pro-adhesive roles of individual glycocalyx components. Neutrophil rolling did not slow across TNF-α-stimulated endothelial cells derived from transgenic mice with aberrant heparan sulfation, suggesting that fully sulfated HS is a ligand for L-selectin (123). Interestingly, rolling was not altered in transgenic mice with truncated (but highly sulfated) HS, indicating that sulfation (and not HS size) is essential for L-selectin binding (81). It remains to be seen how acute degradation of the endothelial glycocalyx influences neutrophil rolling.
The Glycocalyx as a Mediator of Neutrophil Effects on Endothelial Function
Another observed function of the endothelial glycocalyx is to serve as a receptor for soluble cationic ligands as mentioned above for lipid mediators and chemokines. Negatively charged glycosaminoglycans, particularly HS, bind positively charged circulating heparan binding proteins (HBPs) such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) (37). These captured ligands may then be presented for endocytosis. Alternatively, HS-HBP complexes may directly trigger intracellular signaling cascades via the HS-associated proteoglycans cores (115). For example, FGF binding to HS attached to the proteoglycan syndecan IV leads to dephosphorylation of the serine 183 residue located within the intracellular domain of this proteoglycan (45). Once dephosphorylated, syndecan IV forms oligomers, activating syndecan intracellular protein kinase C α-domains and subsequently triggering intracellular signaling cascades.
Neutrophils may similarly influence endothelial physiology via the release of cationic HBPs. Dull and colleagues noted that addition of neutrophil-released cations such as polylysine and polyarginine to bovine lung microvascular endothelial cell monolayers induced syndecan clustering and endothelial hyperpermeability; enzymatic degradation of HS attenuated these changes (31). This attenuation occurred in a dose-dependent fashion, in which 40% HS degradation was insufficiently protective, whereas >60% HS loss inhibited syndecan clustering and endothelial permeability (31). This finding, coupled with the previously discussed observations regarding glycocalyx influence on neutrophil adhesion, suggests that neutrophil-induced endothelial hyperpermeability during inflammation requires a specific magnitude of HS loss: an amount of degradation sufficient to allow neutrophil adhesion but below the level required to trigger neutrophil-endothelial signaling.
Paracellular and Transcellular Pathways for Leukocyte Transendothelial Migration
Following initial adhesive events, neutrophils crawl along the surface of endothelial cells and then emigrate from the vascular lumen by passing between (paracellular) or through (transcellular) endothelial cells into inflamed tissues (FIGURE 2). Paracellular transmigration is perhaps the best characterized route for neutrophil egress from the vasculature and can occur quite rapidly (within minutes). On the other hand, transcellular transmigration occurs selectively in specific vascular beds such as in the brain, bone marrow, and pancreas and under conditions where intraluminal crawling is prevented (100). The latter observation provides an important insight into the mechanisms by which transmigration can occur. In essence, neutrophils and other leukocytes do not automatically transmigrate at the site of their initial capture. Rather, they move laterally to permissive sites in the endothelium that are optimal for transmigration, such as at tricellular junctions (15). The process of transmigration is initiated by chemoattractants that are either produced by endothelial cells or diffused from inflamed tissues. Importantly, shear stress imposed by blood flow regulates the efficiency of leukocyte transmigration.
During transmigration, both leukocytes and endothelial cells participate actively in a series of events that guide the leukocytes to specific regions of the endothelium that are permissive to transmigration (reviewed by Ref. 131) (FIGURE 2). When leukocyte integrins bind to their cognate ligands on endothelial cells (such as ICAM-1 and VCAM-1), signaling events are triggered in the endothelial cells that assist leukocyte transmigration. For example, leukocyte adhesion induces alterations in endothelial cells, including the formation of pro-adhesive sites termed endothelial adhesive platforms (EAPs) by a mechanism involving tetraspanin (CD9, CD151, CD81)-enriched microdomains (9). Additionally, endothelial cells form “docking structures” (transmigratory cups) representing projections from the endothelial cell membrane that are rich in ICAM-1 and VCAM-1 as well as in cytoskeletal components and other cytoplasmic molecules such as the ERM proteins ezrin, radixin, and moesin (8, 9). This dynamic endothelial response facilitates subsequent leukocyte transendothelial migration through either the paracellular or the transcellular pathway.
Regulation of Paracellular Migration
The decision making process by leukocytes and their endothelial counterparts as to whether transmigration will occur between or through endothelial cells is not well understood. One important factor in this choice is the pattern of display of specific endothelial cell surface molecules that serve as signposts indicating the most efficient route for transmigration for the specific type of leukocyte under the prevailing conditions. For example, junctional adhesion molecule (JAM)-A, an adhesion molecule expressed by both endothelial cells and leukocytes, appears to participate at early stages in this process. Endothelial JAM-A serves to direct leukocytes toward the inter-endothelial junctions (94, 129), thus facilitating paracellular migration. Interestingly, neutrophil JAM-A also functions in directed migration of leukocytes, perhaps facilitating their movement through the inter-endothelial junctions (25).
Initial interactions of leukocytes with endothelial cell adhesion molecules lead to reduced strength of inter-endothelial junctions via alterations in VE-cadherin that facilitate leukocyte migration through the inter-endothelial junctions (2, 111). This is an active process that involves rapid alterations in the inter-endothelial junctions and the endothelial cytoskeleton, the latter regulated by Rho GTPases and calcium-dependent activation of myosin light chain kinase (46, 84, 87).
The inter-endothelial junctions contain a diverse range of proteins, many of which function to maintain the integrity of the endothelium and regulate its selective permeability to macromolecules (barrier function). During their passage through this intricate space, neutrophils interact with and, indeed, actively regulate (and are regulated by) these molecules. Examples of such molecules include the tight junction-associated proteins ZO-1, claudins, and occludins, as well as the adherens junction proteins E-cadherin and β- and γ-catenin (reviewed by Refs. 28, 119). During trans-endothelial migration, neutrophils disrupt these structures by using a variety of mechanisms including physical disruption (“muscling” their way through) as well as by induction of endothelial signaling pathways. As an example of the latter, ligation of endothelial ICAM-1 by adhering leukocytes stimulates dissociation of thevascular endothelial protein tyrosine phosphatase (VE-PTP) bound to VE-cadherin, resulting in increased tyrosine phosphorylation of VE-cadherin and therefore diminished strength of VE-cadherin-mediated intercellular adhesion (93). Ultimately, this process facilitates leukocyte passage through the inter-endothelial junctions (3, 4, 93). It should be noted that this disruption of inter-endothelial junctions during leukocyte transmigration is transient; the inter-endothelial junctions typically re-assemble rapidly once the process is complete. However, under pathological circumstances in which neutrophils may be activated to release cytotoxic molecules during transmigration, disruption of the junctions may be more profound. This results in increased endothelial permeability leading to interstitial edema and organ dysfunction, such as occurs in sepsis (61).
In addition to these classical junctional molecules, an assortment of additional molecules is present at the endothelial cell junctions that actively facilitate leukocyte passage through the paracellular space. These include endothelial cell molecules such as JAM-1, ICAM-2 (intercellular adhesion molecule-2), CD99, endothelial cell-selective adhesion molecule (ESAM), and JAMs (reviewed by Refs. 64, 118). Although the specific roles of each of these molecules are incompletely understood, recent studies have illuminated certain aspects of their function. For example, ICAM-2 displayed on the luminal surface of endothelial cells may provide a haptotactic gradient that functions to guide neutrophils to the endothelial cell junctions (130). Once the neutrophils have entered the junctions, endothelial JAM-A, possibly via interactions with neutrophil CD11a/CD18, facilitates continued passage of the neutrophils through the junctions (96). PECAM-1 within the endothelial cell junctions interacts with leukocyte PECAM-1 to induce neutrophil surface expression of the α6 β1 integrin (laminin receptor), which, through coordinate actions of ICAM-2 and JAM-2, promotes passage of neutrophils through the junctions and through the basement membrane of the blood vessel (27, 130). The requirement for these molecules in transendothelial migration is dictated in part by the degree of activation of the participating cells. For example, endothelial cell stimulation leads to an increased role for ICAM-2, JAM-A, and ICAM-1 in leukocyte transmigration, whereas direct activation of neutrophils by powerful chemoattractants appears to bypass the requirement for these endothelial cell molecules in certain inflammatory models (130).
The importance of the junctional adhesion molecules (JAMs), members of the immunoglobulin superfamily, as well as ICAM-1 and the Coxsackie virus and adenovirus receptor (CAR) in leukocyte transendothelial migration have been highlighted by recent studies. The role of JAM-A in leukocyte transendothelial migration has been discussed above. In addition to functioning as a guide for leukocytes, JAM-A has a signaling function in endothelial cells where it regulates integrin expression and the activity of Rap-1 (19, 107). JAM-C, via interactions with the leukocyte integrin CD11b/ CD18 (Mac-1), facilitates leukocyte transendothelial migration via the paracellular route (5). Endothelial cell JAM-C can interact with JAM-B, liberating JAM-C from endothelial cell junctions and making it available on the apical surface of vessels to interact with leukocyte CD11b/CD18 (Mac-1). This directs the leukocytes to the inter-endothelial cell junctions and promotes transmigration (59). JAM-L, a molecule related to the JAM family, is strongly expressed by leukocytes, and recent studies have demonstrated its importance in transendothelial migration of monocytes, perhaps via the endothelial cognate ligand, CAR (41, 66). The role of JAM-L in neutrophil migration remains to be clarified.
It has also become apparent that the level of expression and subcellular distribution of endothelial cell junctional proteins can be regulated by inflammatory mediators. For example, PECAM-1 and JAM-A can be redistributed away from the area of endothelial cell junctions in response to certain cytokines (94). Additionally, ICAM-2 expression is diminished in response to cytokine stimulation (82). JAM-A is shed from cytokine-stimulated endothelial cells by a mechanism involving the disintegrin-like metalloproteinase ADAM-17, a process that may serve to suppress neutrophil transmigration via release of soluble antagonists that interfere with neutrophil JAM-A ligands (54). Finally, PECAM-1 is a dynamically regulated molecule that cycles between the endothelial cell junctions and a submembrane network located immediately below the cell border, termed the lateral border recycling compartment (LBRC) (74). This pool of PECAM-1 can be targeted toward the sites of transmigration via homophilic interactions between leukocyte and endothelial PECAM-1 involving kinesin and microtubules (74). This regulation of endothelial junction function by inflammation adds yet another level of complexity to the paracellular migration process.
Regulation of Transcellular Migration
Compared with the passage of emigrating leukocytes between adjacent endothelial cells, much less is known about the importance and regulation of leukocyte transcellular migration. This area was, until recently, studied largely at the descriptive level and was even thought by some investigators to represent an artifact. Part of the reason for the controversy related to technical issues was that most initial studies were done with fixed specimens and electron microscopy (34); dynamic, high-resolution studies in live cells have only been reported within the last decade. Another reason was biological; as discussed below, the frequency of transcellular migration varies markedly between tissue bed types of leukocytes. For instance, human umbilical vein endothelial cells (HUVECs) appear to be the least likely of endothelial cell types to permit transcellular traffic (17) but were the most commonly studied in early investigations. Using modern live-cell imaging techniques, there are now convincing data that transcellular leukocyte migration does occur, with a frequency of 5–60% of total transmigration events depending both on the type of leukocyte and the endothelial tissue bed. This is a rapid, dynamic, and interactive process requiring extensive reorganization of the cytoskeleton and plasma membrane of both leukocytes and endothelial cells (24).
Recognizing that some of the distinctions are somewhat arbitrary, transcellular leukocyte emigration can be conceptually divided into the following steps: 1) attachment and crawling of the leukocyte on the endothelial surface; 2) formation of podosomes by the leukocyte and podoprints by the endothelium; 3) migratory cup formation by the endothelium; and 4) transmigration and membrane closure (FIGURE 2). In this section, we will discuss what is known about each of these steps. We will also discuss the possible physiological implications of this route of emigration, the purpose of which is not yet fully understood.
Attachment and Crawling
Attachment of CD11a/CD18 (LFA-1) and CD11b/ CD18 (Mac-1) on neutrophils to ICAM-1 on the endothelial surface is the first step of neutrophil adhesion, and, as discussed above, modulation of this process affects the pathway of transendothelial migration taken by these leukocytes. For example, overexpression of ICAM-1 by HUVECs leads to an increase in the proportion of neutrophils migrating transcellularly without affecting total neutrophil emigration (137). Pretreatment of HUVECs with TNF-α also increases transcellular emigration attributable to an increase in ICAM-1 expression. This route of emigration requires the cytoplasmic tail of ICAM-1, since expression of a mutant ICAM-1 lacking this moiety ablated transcellular but not paracellular neutrophil emigration. The influence of ICAM-1 on the route of emigration may reflect its impact on neutrophil crawling across the endothelial surface, since high levels of ICAM-1 favor adherence of neutrophils at sites away from intercellular junctions (where paracellular migration usually occurs). This notion is supported by another study in which neutrophils deficient in CD11b/CD18 (Mac-1) were unable to crawl and consequently exhibited a marked increase in transcellular migration (99). Similarly, CD11a/CD18 (LFA-1)-deficient neutrophils were observed to be defective in adhering to the endothelium; however, the few that did adhere exhibited normal crawling and tended to emigrate through paracellular gaps. Together, these observations suggest that transcellular neutrophil migration is favored when neutrophil movement across the endothelial surface is restricted. Whether other factors that regulate leukocyte-endothelial contact, such as selectins, also modulate the route of emigration is not known.
Formation of Podosomes by Leukocytes: Location, Location, Location
Migrating leukocytes have been observed to form and retract actin-rich protrusions along their ventral surface as they crawl along the endothelium. Based on experiments conducted in lymphocytes, these protrusions (known as podosomes) are required for transcellular but not paracellular migration (18). The podosomes indent the subjacent endothelial cells, forming invaginations of the endothelium, termed podoprints. In addition to actin, podosomes are enriched in talin, vinculin, and LFA-1. Lymphocytes extend their podosomes deeply into the subjacent endothelium, effectively bringing the apical and basolateral membranes of the endothelium close together. Inhibition of podosome formation due to deficiency of the actin-binding protein WASP or treatment with a Src kinase inhibitor caused a specific decrease in transcellular migration, leaving paracellular migration unaffected. It is proposed that these podosomes are actively probing the endothelium for a suitable route for transmigration. This is a difficult hypothesis to prove, although its proponents point out that, although podosomes and corresponding podoprints do form over unsuitable migration locations such as the endothelial nucleus, these structures are smaller and more transient than those in locations more suitable to transcellular migration. It would be interesting to examine the formation of leukocyte podosomes over surfaces of varying rigidity (e.g., synthetic bilayers). This would determine whether the migrating leukocyte is indeed capable of sensing its migration partner.
Beyond these essentially descriptive findings, our current understanding of the signaling involved in podosome-podoprint formation is rudimentary. For example, although studies in leukocytes have implicated members of the Rho family of GTPases in actin remodeling required for diapedesis, it is not known whether these molecules are specifically involved in the formation of podosomes. Another area of uncertainty relates to the effect of shear stress (i.e., the effect of blood flow). One group has reported that shear stress induces the formation of podosomes by neutrophils and increases the frequency of transcellular migration (24). By contrast, another group has reported that the frequency of transcellular emigration of lymphocytes is unaffected by flow (18). Clearly, these responses are complex and may depend on many factors, including the type of leukocyte and the state of activation of both the leukocyte and the endothelium.
Endothelial Migratory Cup and Transcellular Pore Formation
After adhesion and podosome protrusion, migrating leukocytes are rapidly surrounded by ICAM-1- and VCAM-1-enriched endothelial projections, which form a structure called a transmigratory cup. These projections, which are reminiscent of phagocytic pseudopodia (49), may serve to anchor the migrating leukocyte in place and to decrease impending disruption of the vascular barrier during transmigration by increasing the area of contact between leukocyte and endothelium. Such projections have been observed by some [but not all (75)] investigators during both paracellular (7) and transcellular (18) leukocyte migration, and it is not known whether they differ between the two routes. Formation of these actin-rich endothelial structures has been reported to require the intermediate filament vimentin (92). Vimentin appears to regulate adhesion molecules, since endothelia deficient in vimentin display a reduced expression of ICAM-1 and VCAM-1, whereas lymphocytes lacking vimentin have lower levels of cell-surface integrin-β1.
Like other pseudopods, the migratory cups require a protrusion of the endothelial cell membrane. Although, in principle, the plasmalemma could simply extend, this would result in a change in shape of the endothelial cell (which might open up paracellular gaps). To avoid this would require an infusion of internal membranes at the site of migration. Based on our knowledge of phagocytosis, there is precedent that internal membranes of cells can be mobilized and that the overall surface area of the cell is increased (instead of decreased) during pseudopod formation (49). In fact, vesicles have been observed to accumulate in endothelia at the site of migratory cup formation. These vesicles are uncoated (i.e., they do not appear to contain clathrin) and are enriched in the SNARE proteins VAMP2 and VAMP3, suggesting that they fuse with the endothelial cell membrane. Inhibition of SNAREs using the nonspecific inhibitor N-ethylmaleimide (NEM) blocks transcellular migration but not the earlier formation of podoprints or leukocyte migration via the paracellular route (18).
Besides simply providing “extra membrane,” it is not known whether the endothelial vesicles bring anything else to the site of migration. It is reasonable to speculate that transmembrane proteins like ICAM-1 and VCAM-1 or other signaling molecules are shuttled by the vesicles to areas in proximity to transmigrating leukocytes. For instance, during phagocytosis, the insertion of vesicles at the base of the phagocytic cup may serve to uncouple signaling molecules in that region so that they can be directed to the tips of the pseudopods where they are needed (60).
The endothelial responses to leukocyte transmigration are determined in part by the type of leukocyte involved. For example, during transmigration of T-lymphocytes across HUVECs, the internal vesicles appear morphologically to be caveolae and are enriched in caveolin-1 (83). In other cases, however, caveolin-1 is not enriched at the site of migration (17). During the migration of monocytes across endothelial monolayers, investigators have described the recruitment to the endothelial membrane of the so-called lateral border recycling compartment (LBRC) (75). This compartment is devoid of caveolin-1, is enriched in PECAM, CD99, and JAM-A, and is also required for leukocyte paracellular migration (73), as discussed above. The redistribution of this compartment (and transcellular migration in general) requires intact microtubules (75). Thus the origin of the internal membranes in endothelial cells during transcellular migration of leukocytes appears to vary depending on the migrating cell. Although the importance of caveolae during transcellular leukocyte migration is unclear, caveolin-1 itself appears to be necessary. Studies by two groups have independently shown that depletion of caveolin-1 inhibits transcellular migration of lymphocytes without affecting their paracellular migration (78, 83).
The transmigratory event requires rapid and dramatic changes in the cytoskeletons of both the leukocytes and the endothelial cells. As discussed above, paracellular migration is known to require endothelial cytoskeletal remodeling and displacement of endothelial junctional proteins like VE-cadherin. In contrast, VE-cadherin does not appear to be involved in transcellular migration (17, 75). Whether changes in the endothelial actin cytoskeleton differ between the two routes of migration is not clear. It is known, for instance, that formation of the migratory cup depends on endothelial RhoG (117). This is activated after engagement of ICAM-1, which leads to activation of SGEF, a guanine nucleotide exchange factor that loads GTP onto RhoG. However, these endothelial events likely occur during both paracellular and transcellular migration. It is also known that in T lymphocytes, lack of the Rac GEF Tiam1 or a functional PKC-ζ enzyme favors transendothelial migration rather than the paracellular route. Reinforcing what was mentioned earlier about CD11b/CD18 (Mac-1)-deficient neutrophils (99), Tiam-1-deficient T lymphocytes are unable to crawl along the surface of the endothelium and exhibit aberrant polarization in response to chemokines (39). This impaired mobility likely accounts for the increase in transcellular traffic.
Transcellular Migration and Membrane Closure
Remarkably little is known about this final and critical stage of transcellular leukocyte migration. The channel or pore that forms in the endothelial cell can be up to 5 μm in size and apparently closes rapidly after exit of the leukocyte (106). One might expect that the migration of one cell through the body of another cell would cause a major disruption of barrier integrity. Remarkably, whether leukocytes transmigrate at cell-cell junctions or through the cytoplasm of individual cells does not appear to affect vascular permeability to fluid and solutes. In both cases, the endothelial monolayer mobilizes internal membranes to seal off discontinuities. For example, endothelial-derived “dome-like” membranous structures have been observed by electron microscopy to completely cover emigrating neutrophils, whether migrating by the transcellular or paracellular route. The result is that vascular permeability is minimally altered (101). The source of this membranous dome is unknown, although the actin-binding protein LSP1 is required for its formation (98a). One possibility is that it represents the cumulative contribution of the numerous vesicles that accumulate at the site of migration, as described earlier. Another (and not mutually exclusive) potential source of the dome is the lateral border recycling compartment (LBRC), the PECAM-1-dependent organelle that is critical for both paracellular and transcellular leukocyte migration.
Physiological Consequences and Role of Transcellular Migration
Although it is now accepted that transcellular leukocyte emigration through endothelial cells occurs in vivo, its physiological raison d’être still remains mysterious. Much of what is known about the process is descriptive in nature, since a systematic molecular characterization remains to be performed. As mentioned earlier, it is known that the frequency of transcellular migration relative to paracellular migration is highly dependent on the type of leukocyte and the type of endothelium (77, 128). At least three general patterns can be discerned. First, transcellular leukocyte emigration is generally much less common than paracellular emigration, at least in cell culture. Its occurrence in cell culture requires activation of the microvascular endothelium by cytokines (18, 35, 75). Second, transcellular migration in vivo appears to be favored in regional circulations that have very tight cell-cell junctions, e.g., the blood-brain barrier (121, 128). Finally, lymphocytes pursue this route of emigration more frequently than neutrophils.
One possible teleological explanation for transcellular migration is the conservation of intercellular junctions, thereby minimizing the disruption of the vascular barrier. However, in the one study that has directly examined this, vascular permeability was only marginally lower for transcellular compared with paracellular migration. The significance of this subtle difference is unclear (101).
In our view, the critical question is whether the route of emigration has an effect on the functional status of the leukocyte. In other words, does a leukocyte that emigrates transcellularly gain (or lose) anything, so to speak, over its paracellular counterpart during its brief sojourn in the endothelial cytoplasm? In the case of antigen presenting cells such as monocytes or dendritic cells, one could imagine that exposure to the endothelial cytoplasm might influence their subsequent responses to the specific antigens that the endothelial cells had previously internalized. Neutrophils might also be conditioned by mediators or ligands present in the endothelial cytoplasm that could be transferred to the transmigrating leukocyte. To our knowledge, this has never been examined. Fortunately, now that investigators in the field are in general agreement that transcellular migration actually occurs, these issues will hopefully be addressed experimentally.
Concluding Remarks
The processes involved in adhesion and transendothelial migration of neutrophils and other leukocytes, although complex, are beginning to be unraveled. It is apparent that the molecular processes involved and the consequences for both the migrating leukocyte and the endothelium are dependent on many factors, including the type of leukocyte, the state of activation of both the leukocyte and the endothelium, unique properties of the organ and its microcirculatory bed, and biophysical factors such as shear stress from blood flow. However, along with the complexity comes flexibility (i.e., neutrophils and other leukocytes have multiple options to egress from the vascular space). Whether the leukocytes migrate via the paracellular or the transcellular route of egress depends on many factors, including biophysical aspects of the endothelium (“tightness” of the intercellular junctions), the presence and potency of leukocyte chemoattractants, whether the endothelium has been activated, the nature of physical forces (shear) exerted by blood flow on the leukocytes and endothelium, the organ involved, and bi-directional communication between the migrating leukocyte and the vicinal endothelial cells. Additionally, what determines whether a physiological process (transient opening of the intercellular junctions) becomes pathological (prolonged disruption of the endothelial barrier function leading to high permeability edema) is uncertain. As these processes become better understood, it is likely that novel pathways and targets will be revealed that might help promote host defense processes and mitigate inflammatory tissue injury in such conditions as acute lung injury, arthritis, ischemia-reperfusion injury, myocardial infarction, and stroke. However, the risk-benefit profile of such interventions must be carefully considered as host defense mechanisms might be compromised.
Acknowledgments
This manuscript was supported by National Heart, Lung, and Blood Institute Grants HL-090669 (to G. P. Downey), HL-105538 (to E. P. Schmidt), and HL-103772 (to R. L. Zemans).
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 2.Alcaide P, Auerbach S, Luscinskas FW. Neutrophil recruitment under shear flow: it’s all about endothelial cell rings and gaps. Microcirculation. 2009;16:43–57. doi: 10.1080/10739680802273892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 5.Aurrand-Lions M, Lamagna C, Dangerfield JP, Wang S, Herrera P, Nourshargh S, Imhof BA. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol. 2005;174:6406–6415. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 6.Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol. 43:5–16. doi: 10.1165/rcmb.2009-0047TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 12.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 13.Birdsall HH. Induction of ICAM-1 on human neural cells and mechanisms of neutrophil-mediated injury. Am J Pathol. 1991;139:1341–1350. [PMC free article] [PubMed] [Google Scholar]
- 14.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 15.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 16.Burton VJ, Butler LM, McGettrick HM, Stone PC, Jeffery HC, Savage CO, Rainger GE, Nash GB. Delay of migrating leukocytes by the basement membrane deposited by endothelial cells in long-term culture. Exp Cell Res. 2011;317:276–292. doi: 10.1016/j.yexcr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cera MR, Fabbri M, Molendini C, Corada M, Orsenigo F, Rehberg M, Reichel CA, Krombach F, Pardi R, Dejana E. JAM-A promotes neutrophil chemotaxis by controlling integrin internalization and recycling. J Cell Sci. 2009;122:268–277. doi: 10.1242/jcs.037127. [DOI] [PubMed] [Google Scholar]
- 20.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 21.Chappell D, Jacob M, Hofmann-Kiefer K, Bruegger D, Rehm M, Conzen P, Welsch U, Becker BF. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–784. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 22.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 23.Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol. 2009;22:155–162. doi: 10.1097/ACO.0b013e328328d1b6. [DOI] [PubMed] [Google Scholar]
- 24.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 25.Corada M, Chimenti S, Cera MR, Vinci M, Salio M, Fiordaliso F, De Angelis N, Villa A, Bossi M, Staszewsky LI, Vecchi A, Parazzoli D, Motoike T, Latini R, Dejana E. Junctional adhesion molecule-A-deficient polymorphonuclear cells show reduced diapedesis in peritonitis and heart ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2005;102:10634–10639. doi: 10.1073/pnas.0500147102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curry FR. Microvascular solute and water transport. Microcirculation. 2005;12:17–31. doi: 10.1080/10739680590894993. [DOI] [PubMed] [Google Scholar]
- 27.Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med. 2002;196:1201–1211. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Downey GP, Worthen GS, Henson PM, Hyde DM. Neutrophil sequestration and migration in localized pulmonary inflammation. Capillary localization and migration across the interalveolar septum. Am Rev Respir Dis. 1993;147:168–176. doi: 10.1164/ajrccm/147.1.168. [DOI] [PubMed] [Google Scholar]
- 30.Doyle NA, Bhagwan SD, Meek BB, Kutkoski GJ, Steeber DA, Tedder TF, Doerschuk CM. Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J Clin Invest. 1997;99:526–533. doi: 10.1172/JCI119189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JG. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285:L986–L995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 32.Evans TJ, Buttery LD, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci USA. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2009;329:123–129. doi: 10.1124/jpet.108.145862. [DOI] [PubMed] [Google Scholar]
- 34.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira AM, McNeil CJ, Stallaert KM, Rogers KA, Sandig M. Interleukin-1beta reduces transcellular monocyte diapedesis and compromises endothelial adherens junction integrity. Microcirculation. 2005;12:563–579. doi: 10.1080/10739680500253493. [DOI] [PubMed] [Google Scholar]
- 36.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 37.Forsten-Williams K, Chu CL, Fannon M, Buczek-Thomas JA, Nugent MA. Control of growth factor networks by heparan sulfate proteoglycans. Ann Biomed Eng. 2008;36:2134–2148. doi: 10.1007/s10439-008-9575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frutos d Sanchez de Miguel L, Farre J, Gomez J, Romero J, Marcos-Alberca P, Nunez A, Rico L, Lopez-Farre A. Expression of an endothelial-type nitric oxide synthase isoform in human neutrophils: modification by tumor necrosis factor-alpha and during acute myocardial infarction. J Am Coll Cardiol. 2001;37:800–807. doi: 10.1016/s0735-1097(00)01185-2. [DOI] [PubMed] [Google Scholar]
- 39.Gerard A, van der Kammen RA, Janssen H, Ellenbroek SI, Collard JG. The Rac activator Tiam1 controls efficient T-cell trafficking and route of transendothelial migration. Blood. 2009;113:6138–6147. doi: 10.1182/blood-2008-07-167668. [DOI] [PubMed] [Google Scholar]
- 40.Gerdin E, Juhlin C, Malmgren M, Gerdin B. Immunohistochemical identification of receptors for epidermal growth factor in tumor endothelium may be affected by cross-reactivity to blood group A antigen. Am J Clin Pathol. 1993;99:28–31. doi: 10.1093/ajcp/99.1.28. [DOI] [PubMed] [Google Scholar]
- 41.Guo J, Wang X, Tao G, Zhang H, Zhu X, Zhang W, Fan E, Han D. Expression of vascular endothelial growth factor and transforming growth factor-beta 1 in nasal polyps. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2001;36:83–86. [PubMed] [Google Scholar]
- 42.Hager M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. J Intern Med. 268:25–34. doi: 10.1111/j.1365-2796.2010.02237.x. [DOI] [PubMed] [Google Scholar]
- 43.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol Heart Circ Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 44.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 45.Horowitz A, Tkachenko E, Simons M. Fibroblast growth factor-specific modulation of cellular response by syndecan-4. J Cell Biol. 2002;157:715–725. doi: 10.1083/jcb.200112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Q, Wu M, Meininger C, Kelly K, Yuan Y. Neutrophil-dependent augmentation of PAF-induced vasoconstriction and albumin flux in coronary arterioles. Am J Physiol Heart Circ Physiol. 1998;275:H1138–H1147. doi: 10.1152/ajpheart.1998.275.4.H1138. [DOI] [PubMed] [Google Scholar]
- 48.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000;278:H1177–H1185. doi: 10.1152/ajpheart.2000.278.4.H1177. [DOI] [PubMed] [Google Scholar]
- 49.Huynh KK, Kay JG, Stow JL, Grinstein S. Fusion, fission, and secretion during phagocytosis. Physiology. 2007;22:366–372. doi: 10.1152/physiol.00028.2007. [DOI] [PubMed] [Google Scholar]
- 50.Jacob M, Paul O, Mehringer L, Chappell D, Rehm M, Welsch U, Kaczmarek I, Conzen P, Becker BF. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–965. doi: 10.1097/TP.0b013e31819c83b5. [DOI] [PubMed] [Google Scholar]
- 51.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastroin-test Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 53.Jung U, Bullard DC, Tedder TF, Ley K. Velocity differences between L- and P-selectin-dependent neutrophil rolling in venules of mouse cremaster muscle in vivo. Am J Physiol Heart Circ Physiol. 1996;271:H2740–H2747. doi: 10.1152/ajpheart.1996.271.6.H2740. [DOI] [PubMed] [Google Scholar]
- 54.Koenen RR, Pruessmeyer J, Soehnlein O, Fraemohs L, Zernecke A, Schwarz N, Reiss K, Sarabi A, Lindbom L, Hackeng TM, Weber C, Ludwig A. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood. 2009;113:4799–4809. doi: 10.1182/blood-2008-04-152330. [DOI] [PubMed] [Google Scholar]
- 55.Kubes P. The role of shear forces in ischemia/ reperfusion-induced neutrophil rolling and adhesion. J Leukoc Biol. 1997;62:458–464. doi: 10.1002/jlb.62.4.458. [DOI] [PubMed] [Google Scholar]
- 56.Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, Loddenkemper C, Heimesaat MM, Grollich K, Zeitz M, Farkas S, Hoffmann JC. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–1892. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 57.Kurzelewski M, Czarnowska E, Beresewicz A. Endothelin in the mechanism of endothelial injury and neutrophil adhesion in the post-ischemic guinea-pig heart. Eur J Pharmacol. 2002;434:95–107. doi: 10.1016/s0014-2999(01)01534-5. [DOI] [PubMed] [Google Scholar]
- 58.Kurzelewski M, Czarnowska E, Beresewicz A. Superoxide- and nitric oxide-derived species mediate endothelial dysfunction, endothelial glycocalyx disruption, and enhanced neutrophil adhesion in the post-ischemic guinea-pig heart. J Physiol Pharmacol. 2005;56:163–178. [PubMed] [Google Scholar]
- 59.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee WL, Mason D, Schreiber AD, Grinstein S. Quantitative analysis of membrane remodeling at the phagocytic cup. Mol Biol Cell. 2007;18:2883–2892. doi: 10.1091/mbc.E06-05-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 62.Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res. 1996;32:743–751. [PubMed] [Google Scholar]
- 63.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 64.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 65.Lortat-Jacob H, Grosdidier A, Imberty A. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci USA. 2002;99:1229–1234. doi: 10.1073/pnas.032497699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luissint AC, Lutz PG, Calderwood DA, Couraud PO, Bourdoulous S. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by alpha4beta1 integrin activation. J Cell Biol. 2008;183:1159–1173. doi: 10.1083/jcb.200805061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lukaszyk A, Bodzenta-Lukaszyk A, Aksiucik A, Gabryelewicz A, Konturek SJ, Bielawiec M. The role of epidermal growth factor in platelet-endothelium interactions. J Physiol Pharmacol. 1998;49:229–239. [PubMed] [Google Scholar]
- 68.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 70.Malech HL. The role of neutrophils in the immune system: an overview. Methods Mol Biol. 2007;412:3–11. doi: 10.1007/978-1-59745-467-4_1. [DOI] [PubMed] [Google Scholar]
- 71.Malech HL. Gallin Current concepts: immunology JI. Neutrophils in human diseases. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 72.Malech HL, Hickstein DD. Genetics, biology and clinical management of myeloid cell primary immune deficiencies: chronic granulomatous disease and leukocyte adhesion deficiency. Curr Opin Hematol. 2007;14:29–36. doi: 10.1097/00062752-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 74.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, Wiedenbauer EM, Krautgartner WD, Stoiber W, Belohradsky BH, Rieber N, Kormann M, Koller B, Roscher A, Roos D, Griese M, Eickelberg O, Doring G, Mall MA, Hartl D. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 77.Marmon S, Cammer M, Raine CS, Lisanti MP. Transcellular migration of neutrophils is a quantitatively significant pathway across dermal microvascular endothelial cells. Exp Dermatol. 2009;18:88–90. doi: 10.1111/j.1600-0625.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 78.Marmon S, Hinchey J, Oh P, Cammer M, de Almeida CJ, Gunther L, Raine CS, Lisanti MP. Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am J Pathol. 2009;174:684–692. doi: 10.2353/ajpath.2009.080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest. 2002;110:1603–1605. doi: 10.1172/JCI17302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Massena S, Christoffersson G, Hjertstrom E, Zcharia E, Vlodavsky I, Ausmees N, Rolny C, Li JP, Phillipson M. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood. 2010;116:1924–1931. doi: 10.1182/blood-2010-01-266072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McLaughlin F, Hayes BP, Horgan CM, Beesley JE, Campbell CJ, Randi AM. Tumor necrosis factor (TNF)-alpha and interleukin (IL)-1beta down-regulate intercellular adhesion molecule (ICAM)-2 expression on the endothelium. Cell Adhes Commun. 1998;6:381–400. doi: 10.3109/15419069809109147. [DOI] [PubMed] [Google Scholar]
- 83.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 84.Millan J, Ridley AJ. Rho GTPases and leucocyte-induced endothelial remodelling. Biochem J. 2005;385:329–337. doi: 10.1042/BJ20041584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–H726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 86.Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol. 2006;13:21–27. doi: 10.1097/01.moh.0000190113.31027.d5. [DOI] [PubMed] [Google Scholar]
- 87.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 88.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 89.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 90.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 91.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 92.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 93.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- 95.Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 96.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 97.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parish CR. The role of heparan sulphate in inflammation. Nature Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 98a.Petri B, Kaur J, Long EM, Li H, Parsons SA, Butz S, Phillipson M, Vestweber D, Patel KD, Robbins SM, Kubes P. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood. 2011;117:942–952. doi: 10.1182/blood-2010-02-270561. [DOI] [PubMed] [Google Scholar]
- 99.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Poon BY, Ward CA, Cooper CB, Giles WR, Burns AR, Kubes P. Alpha(4)-integrin mediates neutrophil-induced free radical injury to cardiac myocytes. J Cell Biol. 2001;152:857–866. doi: 10.1083/jcb.152.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 104.Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol. 2006;176:1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- 105.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 106.Sage PT, Carman CV. Settings and mechanisms for trans-cellular diapedesis. Front Biosci. 2009;14:5066–5083. doi: 10.2741/3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–1925. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shandelya SM, Kuppusamy P, Herskowitz A, Weisfeldt ML, Zweier JL. Soluble complement receptor type 1 inhibits the complement pathway and prevents contractile failure in the postischemic heart. Evidence that complement activation is required for neutrophil-mediated reperfusion injury. Circulation. 1993;88:2812–2826. doi: 10.1161/01.cir.88.6.2812. [DOI] [PubMed] [Google Scholar]
- 109.Shasby DM. Cell-cell adhesion in lung endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L593–L607. doi: 10.1152/ajplung.00386.2006. [DOI] [PubMed] [Google Scholar]
- 110.Shasby DM, Vanbenthuysen KM, Tate RM, Shasby SS, McMurtry I, Repine JE. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982;125:443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- 111.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 112.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 113.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med. 2003;168:1532–1537. doi: 10.1164/rccm.200303-371OC. [DOI] [PubMed] [Google Scholar]
- 114.Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172:7684–7693. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 115.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 116.Tsao PS, Aoki N, Lefer DJ, Johnson G, 3rd, Lefer AM. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990;82:1402–1412. doi: 10.1161/01.cir.82.4.1402. [DOI] [PubMed] [Google Scholar]
- 117.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 119.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 120.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.von Wedel-Parlow M, Schrot S, Lemmen J, Treeratanapiboon L, Wegener J, Galla HJ. Neutrophils cross the BBB primarily on transcellular pathways: an in vitro study. Brain Res. 2011;1367:62–76. doi: 10.1016/j.brainres.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 122.Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res. 1995;50:397–416. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 124.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 126.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Williams IR, Parkos CA. Colonic neutrophils in inflammatory bowel disease: double-edged swords of the innate immune system with protective and destructive capacity. Gastroenterology. 2007;133:2049–2052. doi: 10.1053/j.gastro.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 128.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol (Berl) 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 129.Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110:1848–1856. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- 130.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Woodfin A, Voisin MB, Nourshargh S. Recent developments and complexities in neutrophil transmigration. Curr Opin Hematol. 2010;17:9–17. doi: 10.1097/MOH.0b013e3283333930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Worthen GS, Haslett C, Rees AJ, Gumbay RS, Henson JE, Henson PM. Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis. 1987;136:19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- 133.Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 134.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 135.Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through L-selectin at threshold shear. Biophys J. 2007;92:330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamagishi S, Imaizumi T. Pericyte biology and diseases. Int J Tissue React. 2005;27:125–135. [PubMed] [Google Scholar]
- 137.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zemans RL, Colgan SP, Downey GP. Trans-epithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2008;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]