INTRODUCTION TO EMOTIONAL PROCESSING
Mood and anxiety disorders are characterized by a variety of neuroendocrine, neurotransmitter, and neuroanatomical disruptions. Identifying the most functionally relevant differences is complicated by the high degree of interconnectivity between neurotransmitter- and neuropeptide-containing circuits in limbic, brain stem, and higher cortical brain areas. Furthermore, a primary alteration in brain structure or function or in neurotransmitter signaling may result from environmental experiences and underlying genetic predisposition; such alterations can increase the risk for psychopathology.
Functional Anatomy
Symptoms of mood and anxiety disorders are thought to result in part from disruption in the balance of activity in the emotional centers of the brain rather than in the higher cognitive centers. The higher cognitive centers of the brain reside in the frontal lobe, the most phylogenetically recent brain region. The prefrontal frontal cortex (PFC) is responsible for executive functions such as planning, decision making, predicting consequences for potential behaviors, and understanding and moderating social behavior. The orbitofrontal cortex (OFC) codes information, controls impulses, and regulates mood. The ventromedial PFC is involved in reward processing1 and in the visceral response to emotions.2 In the healthy brain, these frontal cortical regions regulate impulses, emotions, and behavior via inhibitory top-down control of emotional-processing structures (eg,3).
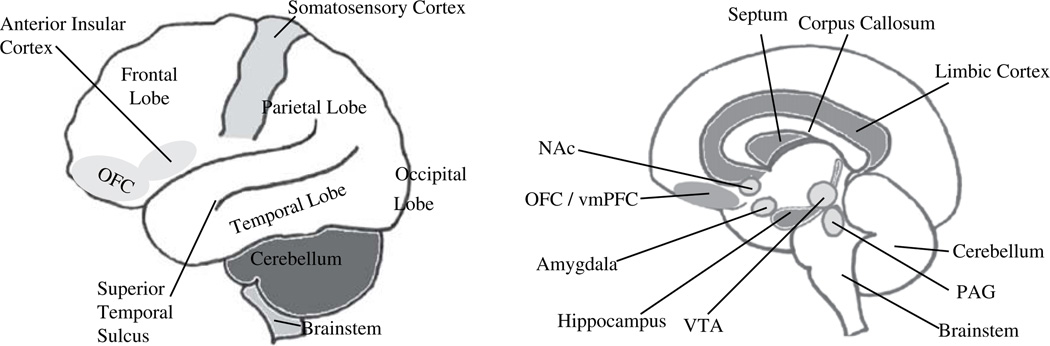
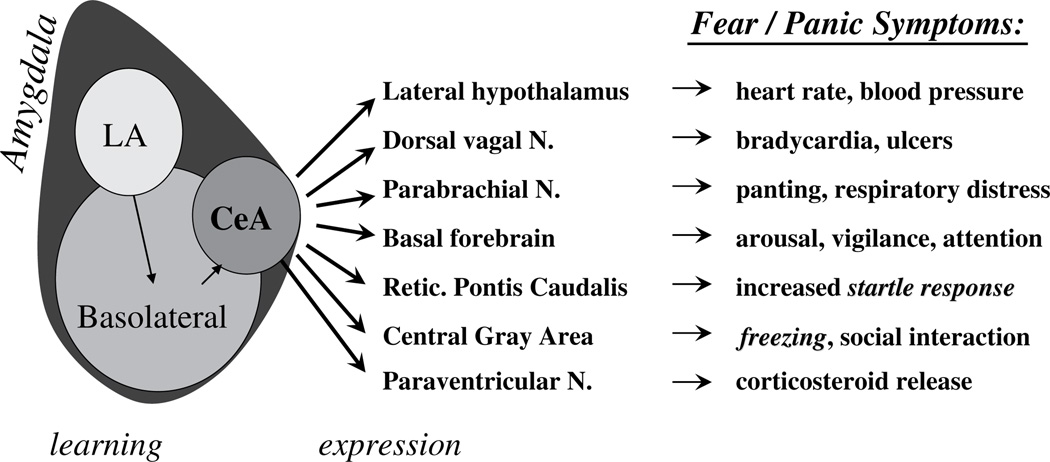
The emotional-processing brain structures historically are referred to as the “limbic system” (Fig. 1). The limbic cortex is part of the phylogenetically ancient cortex. It includes the insular cortex and cingulate cortex. The limbic cortex integrates the sensory, affective, and cognitive components of pain and processes information regarding the internal bodily state.4,5 The hippocampus is another limbic system structure; it has tonic inhibitory control over the hypothalamic stress-response system and plays a role in negative feedback for the hypothalamic–pituitary–adrenal (HPA) axis. Hippocampal volume and neurogenesis (growth of new cells) in this structure have been implicated in stress sensitivity and resiliency in relationship to mood and anxiety disorders. An evolutionarily ancient limbic system structure, the amygdala, processes emotionally salient external stimuli and initiates the appropriate behavioral response. The amygdala is responsible for the expression of fear and aggression as well as species-specific defensive behavior, and it plays a role in the formation and retrieval of emotional and fear-related memories. (Fig. 2 depicts the amygdala’s involvement in fear circuitry). The central nucleus of the amygdala (CeA) is heavily interconnected with cortical regions including the limbic cortex. It also receives input from the hippocampus, thalamus, and hypothalamus.
Fig. 1.
The limbic system. (A) Lateral view of cortex. (B) Sagittal view of slice through midline. NAc, nucleus accumbens; OFC, orbital frontal cortex; PAG, periaqueductal gray, VTA, ventral tegmental area.
Fig. 2.
The fear response is a hardwired process involving the amygdala. (Adapted from Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci 1992;15:356; with permission.)
Neuroendocrine and Neurotransmitter Pathways
In addition to the activity of each brain region, it also is important to consider the neurotransmitters providing communication between these regions. Increased activity in emotion-processing brain regions in patients who have an anxiety disorder could result from decreased inhibitory signaling by γ-amino-butyric-acid (GABA) or increased excitatory neurotransmission by glutamate.
Well-documented anxiolytic and antidepressant properties of drugs that act primarily on monoaminergic systems have implicated serotonin (5-hydroxytryptamine, 5-HT), norepinephrine (NE), and dopamine (DA) in the pathogenesis of mood and anxiety disorders. Genes whose products regulate monoaminergic signaling have become a prime area of research in the pathophysiology of mood and anxiety disorders, and they are thought to be critical for the mechanism of action of antidepressant drugs. Monoaminergic regulators include transmitter receptors; vesicular monoamine transporter (vMAT), which packages these neurotransmitters into vesicles; the vasopressin (AVP), oxytocin, and vasopressin (AVP), oxytocin, and transmitter-specific reuptake transporters serotonin transporter (SERT), neurotonin transporter, and dopamine transporter; the enzyme monoamine oxidase, which degrades 5-HT, DA, and NE; and the enzyme catecholamine-O-methyltransferase (COMT), which degrades DA and NE.
In the central nervous system, classic neurotransmitters often are packaged and co-released with neuropeptides, many of which are expressed in limbic regions where they can influence stress and emotion circuitry (Table 1). The functional implications of these limbic co-localizations have been addressed in numerous reviews (eg,6–12). Neuropeptides with particularly strong links to psychopathology include cholecystokinin (CCK), galanin, neuropeptide Y (NPY), vasopressin (AVP), oxytocin, and corticotropin-releasing factor (CRF), among others. CCK is found in the gastrointestinal system and vagus nerve and is located centrally in numerous limbic regions (reviewed in13). Galanin is co-localized with monoamines in brainstem nuclei. It influences pain processing and feeding behavior and also regulates neuroendocrine and cardiovascular systems.14–16 NPY is known for its orexigenic effects and is expressed abundantly in the central nervous system, where it is co-localized with NE in the hypothalamus, hippocampus, and amygdala (reviewed in13). Centrally, oxytocin regulates reproductive, maternal, and affiliative behavior.17,18 Central AVP regulates fluid homeostasis but also can co-localize with oxytocin to influence affiliative behavior19 or with CRF to regulate the HPA axis.
Table 1.
Neuropeptides in stress and psychopathology
| Neuropeptide | Role in Stress-neurobiology | Role in Psychopathology |
|---|---|---|
| Cholecystokinin (CCK) (Brawman-Mintzer et al., 1997; Koszycki et al., 2004) |
Weak ACTH secretagogue | Anxiogenic Exogenous CCK evokes anxiety; patients who have anxiety disordersare hypersensitive |
| Galanin (Gal) (Barrera et al., 2005; Karlsson and Holmes, 2006) |
Increased by physiological and psychological stress and pain |
Depressogenic Galanin antagonists are being developed and possess antidepressant properties |
| Neuropeptide Y (NPY) (Hashimoto et al., 1996; Heilig, 2004; Martin, 2004; Sajdyk et al., 2004; Hou et al., 2006; Yehuda et al., 2006; Karl and Herzog, 2007) |
Increased during stress Endogenous alarm system Stress-induced increase in feeding Modulate behavior to cope with chronic stress. |
Antidepressant and anxiolytic in laboratory animals Depressed patients have low plasma concentrations of NPY, especially in first episode Plasma NPY concentration is normalized by antidepressants |
| Oxytocin (OT) Gimpl and Fahrenholz, 2001) |
Weak ACTH secretagogue |
Low OT in CSF is associated with depression in women |
| Vasopressin (AVP) (van Londen et al., 1997; Ma et al., 1999; Wigger et al., 2004; Goekoop et al., 2006) |
Increased by stress Moderate ACTH secretagogue synergize to stimulate ACTH production and release |
Potentially elevated in depression |
| Corticotropin-releasing factor |
Increased by stress Primary ACTH secretagogue |
Elevated in MDD, PD, PTSD; associated with HPA axis hyperactivity in MDD and HPA axis hypoactivity in PTSD |
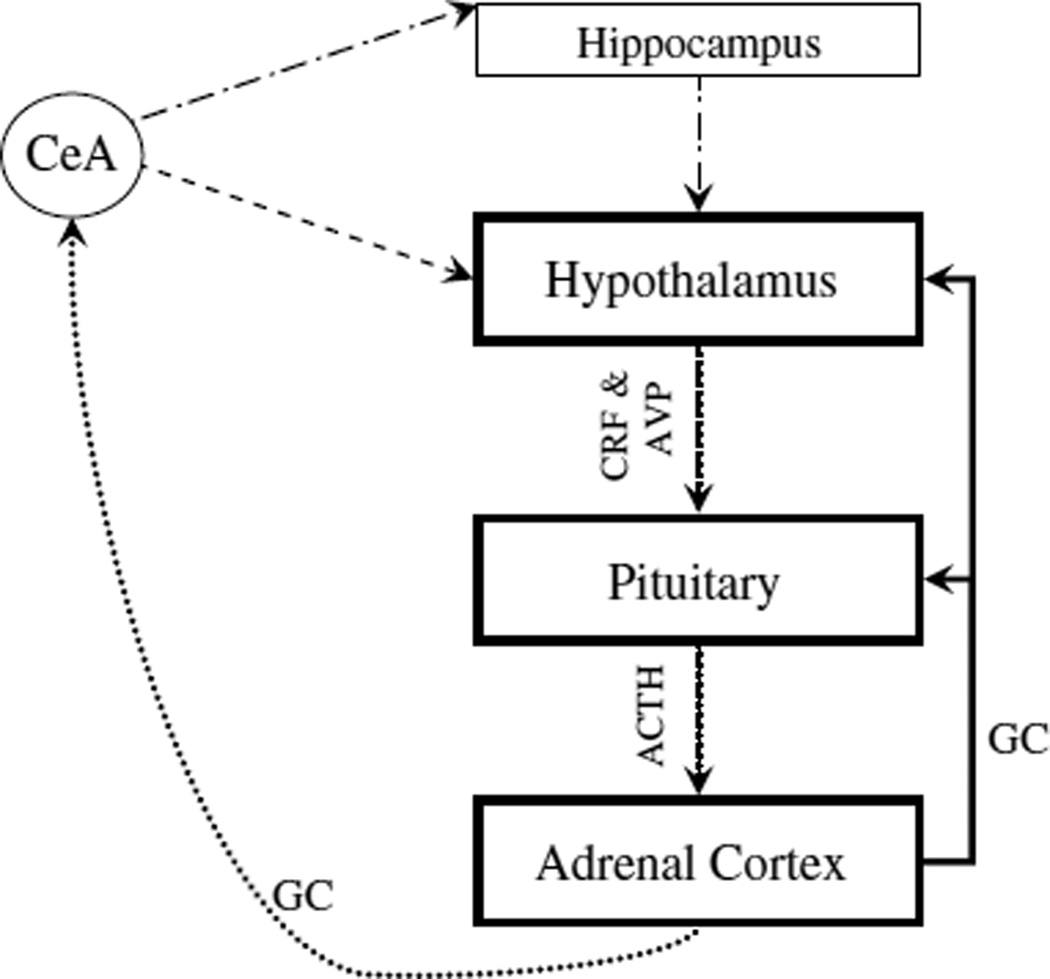
CRF in parvocellular neurons of the hypothalamic paraventricular nucleus is the primary secretagogue for the HPA axis in response to a threatening stimulus. AVP synergizes with CRF in HPA axis activation. In the HPA axis, CRF is released from the paraventricular nucleus and acts on receptors in the anterior pituitary to elicit production and release of adrenocorticotropic hormone (ACTH), which is released systemi-cally and activates production and release of glucocorticoids from the adrenal cortex. In humans, the main stress steroid is cortisol; in rats it is corticosterone. HPA axis activity is regulated by numerous other limbic system structures, including the amygdala, which enhances HPA axis activity, and the hippocampus, which suppresses HPA axis activation (Fig. 3).
Fig. 3.
The HPA axis. Black line- Suppression connection; dotted line- Facilitory connection; dots and dashes line- Suppression connection indirect pathway (via BNST and other limbic regions); and dashed lines- Facilitory connection indirect pathway (via BNST and other limbic regions).
Standardized endocrine challenge tests to assess HPA axis activity include the dexamethasone suppression test and the CRF stimulation test. In the dexamethasone suppression test, systemic administration of dexamethasone, a synthetic glucocorticoid, decreases (ie, suppresses) plasma ACTH and cortisol concentrations via negative feedback at the level of the pituitary gland. In the CRF stimulation test, intravenously administered CRF (which does not enter the central nervous system) elevates plasma ACTH and cortisol concentrations by stimulating CRF1 receptors in the anterior pituitary. A combination of the dexamethasone suppression test and the CRF stimulation test, the Dex/CRF test, developed by Holsboer and colleagues, generally is considered to be the most sensitive measure of HPA axis activity.
Genetic Contribution to Emotionality
Each anxiety disorder, as well as major depressive disorder (MDD), has both genetic and environmental contributions to vulnerability. In attempts to identify the genetic contribution for psychopathology, the candidate genes have largely been the same across diagnoses. Researchers have tended to concentrate on the genes whose products regulate the HPA axis and monoaminergic signaling. Ongoing research supports the hypothesis that a genetic predisposition may be shared among mood and anxiety disorders, with the individual clinical manifestation being a product of both genetic and environmental influences. In particular, epigenetic factors may permit a remarkably complex range of gene–environment interactions.
Among the limited longitudinal studies available, there is much support for a “developmental dynamic pattern” regarding the influence of genetic factors on individual differences in symptoms of depression and anxiety. In this model, the impact of genes on psychopathology changes so that different developmental stages are associated with a unique pattern of risk factors. This model is in sharp contrast to a “developmental stable model” in which the genetic contribution to psychopathology is mediated by one set of risk factors that do not change with the age of the subject.20
Another approach for assessing the impact of genes on risk for psychopathology focuses not on diagnostic class but on more circumscribed phenotypic characteristics. A recent study assessed anxious behavioral characteristics in children between 7 and 9 years of age. They found shared and specific genetic effects on anxiety-related behavior but no single underlying factor, supporting the hypothesis that genes are involved in the general predisposition for anxiety-related behavior and also for specific symptom subtypes.21
PANIC DISORDER
Anatomical and Neuroimaging Findings in Panic Disorder
Neuroimaging in patients who have panic disorder (PD) under resting conditions and under anxiety- or panic-provoking conditions has identified neuroanatomical alterations associated with symptom severity or treatment response.
Single-photon emission computed tomography (SPECT) identified lower metabolism in the left inferior parietal lobe and overall decreased bilateral cerebral blood flow (CBF) in patients who had PD as compared with control subjects, and this decrease corresponded with symptom severity.22 Other studies, however, have demonstrated elevated glucose uptake in the amygdala, hippocampus, thalamus, midbrain, caudal pons, medulla, and cerebellum as measured by positron emission tomography (PET). These elevations normalize after successful pharmacological or behavioral therapy, suggesting that the increased glucose uptake in these regions is state dependent. Patients who had PD had decreased frontal activity bilaterally but increased activity in the right medial and superior frontal lobe in SPECT studies. Interestingly, the CBF asymmetry and shift to the right hemisphere correlated with disorder severity in individual patients (reviewed in23).
After administration of the respiratory stimulant doxapram, patients who had PD exhibited a greater decrease in PFC activity but a larger increase in cingulate gyrus and amygdala activity while experiencing panic than control subjects. In patients who had PD who were administered sodium lactate to provoke a panic attack, functional MRI (fMRI) demonstrated elevated CBF in the right OFC and left occipital cortex but decreased CBF in the hippocampus and amygdala (reviewed in23). Other studies have shown that patients who do not experience a panic attack after sodium lactate infusion show no differences in CBF compared with control subjects. Interestingly, when a spontaneous panic attack was observed in an fMRI study, the panic was associated with significantly increased activity in the right amygdala.24
Imaging analyses of patients who have PD who are in an anxious (but not panicked) state also have provided important data. Upon presentation of threatening words in fMRI studies, the left posterior cingulate and left medial frontal cortex were activated in these patients.25 Others have shown that presentation of negative emotional words elicits activations in the right amygdala and right hippocampus in patients who have PD.26 When patients who have PD are presented with anxiety-provoking visual stimuli, they exhibit increased activity in the inferior frontal cortex, hippocampus, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and OFC.27 Compared with healthy control subjects, patients who had PD exhibited less activation in the ACC and amygdala when shown pictures of angry faces. These latter results were interpreted as a blunted response caused by chronic hyperactivity in these circuits in patients who had PD.28
Neuroendocrine and Neurotransmitter Signaling in Panic Disorder
Amino acid neurotransmitters
Decreased inhibitory signaling has been hypothesized to play an important pathophysiological role in PD. In drug-free patients who had PD, increased benzodiazepine binding in the temporal cortex and right lateral frontal gyrus29 but decreased binding in the left hippocampus30,31 has been observed. In patients who have PD and comorbid MDD treated with antidepressant medications, benzodiazepine binding was decreased in the lateral temporal lobes, left medial inferior temporal lobe, and bilateral OFC. Binding in the insular cortex bilaterally was negatively correlated with panic severity and with comorbid depression.32
Magnetic resonance spectroscopy (MRS) has demonstrated decreased GABA concentrations in the occipital cortex,33 ACC, and basal ganglia34 in patients who have PD compared with control subjects. Although there is no evidence for differences in plasma or cerebrospinal fluid (CSF) GABA concentrations in patients who have PD,33 low baseline CSF GABA concentrations did correlate with a poor therapeutic response to the triazolobenzodiazepine alprazolam or the tricyclic antidepressant imipramine. Interestingly, patients who have PD and who have a family history of mood and anxiety disorders exhibit decreased cortical GABA concentrations (reviewed in35).
Elevated excitatory glutamatergic signaling is associated with panicogenicity, and drugs that reduce glutamate availability are hypothesized to possess anxiolytic properties. For example, LY354740, an agonist on presynaptic metabotropic glutamate receptors (mGluR II), leads to decreased release of glutamate. This drug decreases anxiety-like behavior in the fear-potentiated startle paradigm in experimental animals.36 LY354740 and other presynaptic metabotropic glutamate agonists also exert neuroprotective effects. In human studies, LY354740 and related drugs decrease subjective anxiety in a conditioned-fear paradigm in healthy volunteer subjects. In patients who have PD, mGluR II agonists are protective against panicogenic agents such as carbon dioxide inhalation (reviewed in37).
Monoamines
Monoaminergic drugs, including tricyclic antidepressants and selective serotonin-reuptake inhibitors (SSRIs), are effective in the treatment of PD. Two SSRIs, fluvoxamine and paroxetine, had a more rapid onset of action and a better therapeutic response on PD symptoms than achieved with cognitive behavioral therapy (CBT) (reviewed in38). The dose of paroxetine needed to treat PD optimally is higher than that required for MDD, suggesting that the mechanism by which SSRIs reduce panic symptoms may be distinct from their mechanism of antidepressant action.39 Patients who have PD exhibit an increased anxiogenic response to administration of the 5-HT2c/5-HT3 agonist meta-chlorophenylpiperazine (mCPP).40 In PET studies, 5HT1A receptor binding is decreased in the cingulate cortex and raphe nucleus of patients who have PD. SPECT studies have revealed decreased SERT binding in the midbrain, bilateral temporal lobe, and thalamus. The magnitude of the decrease correlates with symptom severity and also normalizes in patients who have PD in remission (reviewed in35). Together, these data support a role for serotonergic circuits in the pathogenesis of PD.
Noradrenergic involvement in PD is evidenced by challenge with the α2 antagonist yohimbine. Yohimbine-elicited panic-like anxiety in patients who have PD is associated with elevated cardiovascular activity and increased serum NE concentrations. There is some evidence that the α2 agonist clonidine has an anxiolytic effect. Patients who have anxiety disorders, including PD, often exhibit a blunted growth hormone response to clonidine administration, suggesting that presynaptic NE autoreceptors are supersensitive (reviewed in35). Overall, these data suggest that patients who have PD have alterations in NE circuits, and this system therefore may represent a target for novel treatment development.
Neuropeptides
Although CCK is a well-known panic-inducing agent even in healthy volunteers, few studies have specifically addressed the role of CCK in panic disorder. Chronic imipramine treatment decreases the acute anxiety-inducing effects of CCK, but this finding does not speak to a role for endogenous CCK systems in PD (reviewed in13).
A recent study also identified an association between galanin and symptom severity in female patients who had PD but had no effect on risk for PD. The associated single-nucleotide polymorphisms (SNPs) were within CpG dinucleotides of the galanin promoter, suggesting that epigenetic factors could explain the influence of galanin on PD severity.41
Corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis
Patients who have PD have been reported to exhibit increased baseline plasma cortisol concentration, which is positively correlated with the risk for a panic attack after lactate administration. These data suggest that elevated baseline plasma cortisol represents a state of anticipatory anxiety, but not panic itself. The underlying biology of elevated basal cortisol concentrations may be related to increases in CRF concentrations in the CSF of patients who have PD (reviewed in35).
The HPA axis in patients who have PD has been assessed at rest over a full circadian cycle, before and after activation by a panicogenic agent that does not independently activate the HPA axis (doxapram) and before and after administration of a panicogenic agent that does activate the HPA axis (the CCK-B agonist pentagastrin). Increased overnight plasma cortisol concentrations corresponding to sleep disruption have been noted in subjects who have PD; this increase is a trait rather than a statedependent marker of PD. In the doxapram challenge study, an exaggerated increase in plasma ACTH was observed in the patients who had PD. Compared with healthy control subjects, plasma ACTH concentrations were elevated following pentagastrin administration in patients who had PD. Taken together, these data support the hypothesis that patients who have PD are hypersensitive to the HPA axis–activating effects of situations that are novel, threatening, and uncontrollable. After the basal state was established reliably, the ACTH response to CRF administration was not altered in patients who had PD, suggesting that the previous studies were confounded by the effects of the novel environment on the HPA axis (reviewed in42).
Genetic Contribution to Panic Disorder
PD is thought to be the most heritable of the anxiety disorders. First-degree relatives of proband patients who have PD have a sevenfold increased likelihood for PD and also have an increased risk for phobic disorders.43–45 Twin studies suggest that 30% to 40% of the variance in vulnerability for PD is derived from genetic factors and the remainder from individual-specific, but not shared, environment/life experiences.43
Linkage studies in families that have PD have been hampered by non-replication and small numbers.45,46 A large analysis including 120 pedigrees with more than 1500 individuals revealed two loci with genome-wide significance on chromosomes 2q and 15q, but these results await further replication.47 A large number of genetic association studies for PD have been published, implicating many genes. A recent review compiled the genes that have been associated with PD in more than one study thus far, although in some cases different polymorphisms within these genes have been associated with PD in different studies, complicating any attempt to draw causal conclusions from these data (reviewed in45). The genes associated with PD in multiple studies are:
COMT
Adenosine 2A receptor
CCK
CCK Receptor B
5HT2A receptor
Monoamine oxidase-A
In addition to the aforementioned target genes, polymorphisms in SLC6A4, the gene for the serotonin transporter, also have been associated with PD. The association, however, is not with the well-studied promoter-length polymorphism.48 Rather, SNPs within the serotonin transporter gene show association with PD and comorbid PD/social anxiety disorder (SAD). Subjects who have at least one copy of haplotype A-A-G from rs3794808, rs140701, and rs4583306 have 1.7 times the odds of PD than subjects with no copy of this haplotype.49 In combination with associations of other genes within the monoamine system mentioned earlier in this article, these data support the hypothesis that monoaminergic systems are involved in anxiety disorders as a group; their exact role may be disorder specific.
Although most genetic-association studies have investigated only single polymorphism contributions, it is very likely that a combination of polymorphisms in sets of candidate genes act in concert to increase the risk for this disorder. In fact, a recent study investigating the contribution of genetic variants in the CRF and AVP system reported that the strongest results were the combined effects of rs878886 in CRF1 and rs28632197 in the gene encoding the vasopressin 1B receptor (AVP1B).50 A model with two SNPs showed significant associations with PD in both samples separately, and significance improved to P 5 .00057 in the combined sample of 359 cases and 794 controls. Both SNPs are of potential functional relevance, because rs878886 is located in the 3′ untranslated region of the CRF1 gene, and rs28632197 leads to an arginine-to-histidine amino acid exchange at position 364 of AVP1B, which is located in the intracellular C-terminal domain of the receptor and probably is involved in G-protein coupling. These genetic data support the large body of evidence demonstrating interactions of AVP and CRF systems in anxiety. Another family-based study failed to find an association of four polymorphisms in the CRF1 locus with PD, but fewer CRF1 polymorphisms and no AVP1B polymorphisms were tested in this study.51
POSTTRAUMATIC STRESS DISORDER
Anatomical and Neuroimaging Findings in Posttraumatic Stress Disorder
Activation of the amygdala is important for the fear learning associated with PTSD symptoms and with extinction learning associated with PTSD treatment. Amygdala hyperresponsiveness has been identified in numerous studies of patients who have PTSD (reviewed in37). Greater activation of the amygdala in response to viewing fearful faces corresponded with poor prognosis in CBT;52 other studies have shown that severity of PTSD symptoms predicts the magnitude of amygdala activation when encoding memories unrelated to the traumatic event.53
A recent study examined the neural correlates of responsiveness to CBT in Iraq war veterans who had PTSD. Avoidance symptoms of PTSD are thought to result from conditioned fear-like encoding of the environment surrounding a traumatic event. CBT in PTSD attempts to override the conditioned fear with extinction learning. In patients who had recently diagnosed PTSD, rostral ACC volume predicted a successful CBT response. It is possible that decreased rostral ACC volume results in a decreased ability for extinction learning. Thus, patients who have PTSD and who have a smaller ACC volume may be less able to regulate fear during therapy, rendering the CBT process less effective.54 Functional imaging studies have shown that greater activation of the ventral ACC in response to viewing fearful faces corresponded with a poorer response to CBT.52
It has been hypothesized that symptoms of PTSD, including intrusive thoughts and re-experiencing trauma, result from an inability of higher cognitive structures to repress negative emotional memories. This imbalance is obvious in functional imaging studies with tasks that require interrelated executive and emotional processing systems. In healthy subjects and in recently deployed veterans of war who have PTSD, presentation of emotional stimuli, as compared with neutral stimuli, elicits activation in ventral frontolimbic brain regions, including the ventromedial PFC, inferior frontal gyrus, and ventral anterior cingulate gyrus. In patients who have PTSD, the magnitude of ventral activation is positively correlated with symptom severity. Furthermore, compared with neutral stimuli, combat-related stimuli produced enhanced activation of this ventral emotional system. The amplitude of this increase also correlated with the severity of PTSD symptoms.55 During executive tasks, healthy controls and patients who have PTSD activate a dorsal executive network that includes the middle frontal gyrus, dorsal anterior cingulate gyrus, and inferior parietal lobule. In patients who have PTSD, reduced activation of the dorsal executive network correlates with symptom severity. The middle frontal gyrus, a component of the dorsal executive network, also is activated when patents who have PTSD view combat-related images. These results suggest that brain areas that are restricted to executive functioning in healthy subjects are used for emotional/affective processing in patients who have PTSD, thereby diminishing the capacity of executive control.55
Similarly, sensory gating deficits in patients who have PTSD may result from information processing systems being overpowered by hypervigilance for threat-related stimuli and hyperarousal. A task requiring subjects to inhibit a primed motor response has demonstrated deficits of inhibitory control in patients who have PTSD. In control subjects, inhibitory processing activated the right frontotemporoparietal cortical network. In patients who had PTSD, the left ventrolateral PFC (vlPFC) was activated, and the frontotemporoparietal cortical network was less active. In terms of the behavioral response, increased error correlated with PTSD symptom severity. Increased symptom severity may result in increasingly overwhelmed inhibitory networks. Conversely, decreasing ability to recruit inhibitory control networks may result in more intense symptoms.56
Neurotransmitter and Neuroendocrine Signaling in Posttraumatic Stress Disorder
Amino acid neurotransmitters
Glutamate plays a critical role in hippocampal-dependent associative learning and in amygdala-dependent emotional processing in stressful conditions or following stress exposure. Inappropriate glutamate signaling therefore could contribute to the processing distortion experienced by many patients who have PTSD. In support of the glutamate hypothesis of PTSD, the N-methyl-D-aspartic acid receptor antagonist ketamine is well known for its ability to induce dissociative and perceptual distortions, similar to the processing distortion in patients who have PTSD (reviewed in37).
Recent research has explored the possible therapeutic potential of glutamatergic targets in PTSD. One such drug is the anticonvulsant topiramate. Topiramate inhibits excitatory transmission at kainate and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors and has demonstrated anxiolytic properties at lower doses than required for anticonvulsant effects, suggesting a unique mechanism of action. Open-label studies using topiramate as either adjunctive or monotherapy have demonstrated some efficacy in diminishing nightmares and flashbacks and in improving overall PTSD symptoms.37
Monoamines
There are numerous reports of hyperactive noradrenergic signaling in PTSD. For example, NE is robustly secreted after exposure to acute physiological stress, and CSF concentrations of NE are tonically elevated in PTSD veterans. There is no evidence of a correlation between NE concentration and symptom severity, however (reviewed in57). As with patients who have PD, yohimbine elicits panic-like anxiety associated with cardiovascular symptoms and increased serum NE in patients who have PTSD relative to healthy control subjects (reviewed in35). Furthermore, patients who have PTSD have been shown to exhibit elevated 24-hour urinary catecholamine excretion.58 Some of the effects of NE on PTSD symptoms may be mediated by interactions between NE and glucocorticoids (eg,59). Drugs targeting the NE system have been assessed in PTSD with varying degrees of success for individual PTSD symptoms (see57 for a thorough review).
SSRIs have been demonstrated to be of moderate efficacy in PTSD, and sertraline is approved by the Food and Drug Administration to treat this disorder. In patients who had non–combat-related PTSD, paroxetine treatment improved hyperarousal and avoidance symptoms by 8 weeks and improved re-experiencing symptoms by the end of the 12-week study.60 The Institute of Medicine report on treatment of PTSD did not consider the efficacy data on SSRIs to be sufficient when compared with the psychotherapy data.61
Neuropeptides
In healthy soldiers during intense military training, interrogation stress led to an increase in plasma NPY concentrations; plasma NPY concentrations were correlated with cortisol concentrations and with behavioral performance. Combat-exposed men who did not develop PTSD tended to have higher concentrations of plasma NPY than combat-exposed men who had PTSD. These data suggest that NPY could be a neural correlate of resiliency.62
A recent review article identified a potential role for neurokinins in PTSD.35 Neurokinin 2 antagonists did not exhibit anxiolytic properties in preclinical tests in which benzodiazepines were active. The latter are of limited use in PTSD, however. Expression of galanin has been demonstrated to be stress responsive, in that it is decreased by acute stress but returns to normal within several days. If the stress continues and becomes chronic, galanin expression increases. It has been suggested that elevated galanin expression induced by chronic stress leads to increased autoinhibition of NE cell bodies in the locus coeruleus (LC); decreased tonic LC activity could contribute to depressive symptoms in patients who have PTSD (reviewed in35).
Corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis
Numerous studies have identified HPA axis disruption in patients who have PTSD.63–68 Compared with healthy control subjects, and in contrast to patients who have MDD, cortisol concentration is decreased in plasma, in saliva upon awakening, and in 24-hour urinary measures in combat-exposed patients who have PTSD.69 In a more recent study, a mixed population of civilian patients who had PTSD also exhibited decreased cortisol concentrations; lower plasma cortisol corresponded with greater symptom severity.70 Importantly, there also have been studies showing no difference in circadian salivary or 24-hour urinary cortisol concentrations (eg,71,72).
As in patients who have MDD, CSF concentrations of CRF were found to be higher in patients who had PTSD than in comparison subjects in two studies.73,74 Patients who have MDD typically exhibit a blunted HPA axis response in the CRF-stimulation test, and in veterans of the Vietnam or Korean wars hospitalized for PTSD, the ACTH response to ovine CRF injection also was blunted relative to control subjects and was independent of comorbid MDD diagnosis.75 In contrast, although dexamethasone non-suppression often is observed in patients who have MDD, patients who have PTSD exhibit greater suppression of plasma ACTH and cortisol concentrations.76 Negative findings also have been reported.77 Dexamethasone hypersuppression in patients who have PTSD may result from sensitized central glucocorticoid receptors (GRs) secondary to chronic elevations in CRF. This finding is in sharp contrast to patients who have MDD, in whom chronic CRF overexpression is thought to result eventually in GR desensitization and reduced negative feedback (reviewed in35). Alterations in CRFergic signaling and the HPA axis could result from insufficient glucocorticoid signaling caused by decreased hormone bioavailability or from decreased hormone receptor sensitivity.78
Genetic Contribution to Posttraumatic Stress Disorder
The heritability for PTSD has an estimated range of 30% to 40%, probably resulting from a variety of genes, each with relatively small contributions to the genetic predisposition for this disorder.79–83 Because of the importance of the environmental impact for this disorder, linkage studies in pedigrees cannot be conducted easily. Candidate gene association studies also are confounded by the problem of matching for environmental exposure and largely have been limited by small sample size (n < 100); therefore these studies would able to detect only large genetic effects.
Because PTSD is the only anxiety diagnosis requiring a prior traumatic event, much research has been devoted to examining gene-by-environment interactions in patients who have PTSD.A complex-repeat polymorphism in the 5′ upstream region of SLC6A4, the gene encoding the serotonin transporter (serotonin transporter-linked polymorphic region, 5-HTTLPR), has been studied in depth by numerous groups. This polymorphism consists of a repetitive region containing 16 imperfect repeat units of 22 bp, located approximately 1000 bp upstream of the transcriptional start site.48,84 The 5-HTTLPR is polymorphic because of the insertion/deletion of units 6 through 8, which produces a short (S) allele that is 44 bp shorter than the long (L) allele. The 5-HTTLPR has been associated with different basal expression and functional activity of the transporter, most likely related to differential transcriptional activity.48,84 The L-allele of this polymorphism has been shown to lead to a higher serotonin reuptake by the transporter and thus less serotonin in the synaptic cleft. The shortSERT allele has been shown to interact with stressful life events (including abuse in childhood)to increase the risk for depression later in life.85–91 This polymorphism recently has been shown to play a role in the genetic underpinnings of PTSD. In hurricane victims, the SERT polymorphism interacts with severity of trauma and level of social support toward the development of PTSD.92
Other genes interacting with early-life stress (ELS) also are strong candidates for influencing susceptibility for PTSD. Preclinical studies indicate that the persistent hyperactivity of the HPA axis associated with ELS is mediated by a hyperactive CRF1 system, with chronic overactivity of CRF1 in limbic brain regions.93,94 In fact, the authors have shown that a haplotype within the gene encoding CRF1 interacts with child abuse to predict depression severity in adults.95 These polymorphisms, however, did not interact with ELS to predict PTSD symptoms.96
Polymorphisms in genes regulating GR activity may alter sensitization of the stress-response pathway during development so that victims of ELS have increased risk for PTSD following traumatic events in adulthood. FKBP5, a co-chaperone of heat shock protein 90, plays a role in regulating the expression of glucocorticoid-responsive genes.97 Increased expression of FKBP5 has been shown to reduce glucocorticoid binding affinity98 and to reduce nuclear translocation of the GR,99 resulting in resistance to glucocorticoid activation. In humans, the rare alleles of the FKBP5, SNPs rs4613916, rs1360780, and rs3800373, were associated with higher FKBP5 expression in blood monocytes as well as with a stronger induction of FKBP5 mRNA by cortisol.100 As an important candidate gene in trauma-related HPA axis disturbances, the putative functional SNPs in FKBP5 are hypothesized to moderate the development of PTSD and/or to alter the impact of early trauma or PTSD on GR.100–102
In support of this hypothesis, there seems to be a positive correlation between the upregulation of FKBP5 mRNA in peripheral blood mononuclear cells induced by acute trauma and the development of the PTSD 4 months later.103 Furthermore, when exposed to medical trauma, pediatric patients who had the rs3800373 and rs1360780 alleles were more likely to exhibit peritraumatic dissociation,104 a strong predictor of PTSD in adulthood.105 In the largest genetics study in PTSD conducted thus far, the authors’ group showed that the same alleles increased the risk for adult PTSD symptom severity in adults who had been exposed to child abuse but not to trauma as adults.96 Additional research will be necessary to clarify the gene–environment relationship between early-life trauma versus adult trauma.
SOCIAL ANXIETY DISORDER
Anatomical and Neuroimaging Findings in Social Anxiety Disorder
As with PD and PTSD, amygdala activation has been implicated in symptoms of SAD. Social-cue tasks, such as the viewing of harsh faces, were associated with hyperreactivity in the amygdala and other limbic areas in patients who had SAD. Similarly, in response to viewing negative (but not neutral or positive) affective faces, patients who have SAD exhibited bilateral amygdala activation, which positively correlated with symptom severity and which reversed upon successful treatment. In anticipation of public speaking, subcortical, limbic, and lateral paralimbic activity is increased in patients who have SAD, suggesting elevations in automatic emotional processing. Decreased activity in the ACC and PFC in these subjects suggests a decreased ability for cognitive processing (reviewed in23).
In contrast to the social-cue studies, activity in the left hippocampus and right amygdala was decreased during script-guided mental imagery tasks that provoke social anxiety. This decrease may reflect active blunting of the emotional and autonomic response to improve overall functioning during social situations that provoke anxiety.106 Furthermore, anxiety-provoking imagery (compared with neutral imagery) was associated with increased activation in the left postcentral gyrus and putamen and in the right inferior frontal and middle temporal gyri. Relative decreased activity was observed in the right middle temporal gyrus, left precuneus, and posterior cingulate gyrus. After 8 weeks of treatment with nefazodone, both remitted and partially improved social anxiety was associated with decreased regional CBF (rCBF) in the lingual gyrus, left superior temporal gyrus, and right vlFC and with increased rCBF in the left middle occipital gyrus and inferior parietal cortex. In subjects who achieved remission following nefazodone treatment, posttreatment testing revealed decreased rCBF in the ventral and dorsal ACC, left vlPFC, dorsolateral PFC, and brainstem and increased rCBF in the middle cingulate cortex, left hippocampus, parahippocampal gyrus, subcallosal orbital, and superior frontal gyri.106
The combined results of imaging analysis in subjects who have SAD suggest dysfunction of a cortico-striato-thalamic network: hyperactivity in the right PFC, striatal dysfunction, and increased hippocampal and amygdala activity with left lateralization. It has been suggested that hyperactivity in the frontolimbic system, including the ACC, which processes negative emotional information and anticipation of aversive stimuli, could result in misinterpretation of social cues (reviewed in23,107).
Neurotransmitter and Neuroendocrine Signaling in Social Anxiety Disorder
Amino acid neurotransmitters
Increased excitatory glutamatergic activity has been reported in patients who have SAD. Compared with matched control subjects, patients who had SAD had a 13.2% higher glutamate/creatine ratio in the ACC as measured by MRS. The glutamate/creatine ratio correlated with symptom severity, suggesting a causal role between excitatory signaling in the ACC and psychopathology (reviewed in37).
Monoamines
The Neurobiology of Anxiety Disorders In addition to benzodiazepines, SSRIs, SNRIs, and monoamine oxidase inhibitors are effective in the treatment of SAD. That SSRI treatment is successful in treating SAD symptoms and reversing some brain abnormalities (eg, elevated amygdala activity) has been cited as evidence for a serotonergic role in the etiology of SAD.107 Data supporting the hypothesis of disrupted monoaminergic signaling in patients who have SAD include decreased 5HT1A receptor binding in the amygdala, ACC, insula, and dorsal raphe nucleus (DRN). Moreover, trait and state anxiety is elevated in patients who have SAD who have one or two copies of the short SERT allele, and this patient population exhibits amygdala hyperactivity in anxiety-provocation paradigms. Neuroimaging analyses also have revealed decreased density of the dopamine transporter and decreased binding capacity for the D2 receptor (reviewed in23). A role for DA in SAD is supported by the finding that patients who have Parkinson’s disease have high rates of comorbid SAD (reviewed in107). This co-morbidity, however, could result from insecurity regarding display of the physical symptoms of this movement disorder rather than a common etiology of DA malfunction.
A recent study assessed whether a DA agonist (pramipexole, 0.5 mg) or antagonist (sulpiride, 400 mg) influenced response to anxiogenic challenge such as verbal tasks and autobiographical scripts in patients who had SAD. The anxiogenic effect of the behavioral challenges was significantly increased in patients who had untreated SAD following administration of either drug. After successful treatment with SSRIs, however, administration of pramipexole seemed to dampen the behavioral provocation-induced anxiety, whereas sulpiride administration continued to enhance the anxiogenic effects of these tasks. These authors suggested that instability in the dopaminergic response to social stress contributes to anxiety severity and is normalized only partly by successful treatment, perhaps via SSRI-induced desensitization of postsynaptic D3 receptors.108
Neuropeptides
As key effectors of social behavior, the neuropeptides oxytocin and vasopressin are of particular interest in SAD and autistic spectrum disorders. Recently direct oxytocin administration to the amygdala in laboratory animals was shown to decrease activation in this region and to dampen amygdala–brainstem communications, which are known to play a role in the autonomic and behavioral components of fear. Furthermore, preliminary data have shown that genetic variants in the central vasopressin and oxytocin receptors (AVP1A and OXTR, respectively) influence amygdalar activity. These data support the hypothesis of amygdala hyperactivity in SAD. Future research in this area may elucidate neural underpinning of human social behavior and the genetic risk for disorders including SAD and autism.18
Corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis
Some evidence indicates sensitization of the HPA axis in patients who have SAD. Psychosocial stress produces a greater increase in plasma cortisol, but not ACTH, in patients who have SAD than in control patients despite similar baseline cortisol concentrations.109 Compared with healthy control subjects or patients who have PTSD, subjects who have SAD tend toward an elevated cortisol response in the Trier Social Stress Test (TSST). The degree of cortisol elevation was correlated with increased avoidance behavior in the approach–avoidance task and the predicted stress-induced increased social avoidance above and beyond effects of blood pressure and subjective anxiety.110 Negative findings also have been reported, however (eg,111,112). For example, an earlier study found that adolescent girls who had social phobia and control subjects exhibited an equal elevation in salivary cortisol following the TSST. To the authors’ knowledge, there are no endocrine-challenge studies (Dex-Suppression, CRF-Stimulation, or Dex/CRF) in patients who have SAD.
Genetic Contribution to Social Anxiety Disorder
The Neurobiology of Anxiety Disorders Unfortunately, there are very few studies specifically examining the genetic underpinnings of SAD. Available data suggest that SAD has a high degree of familial aggregation. In a recent meta-analysis in which SAD was grouped with specific phobia and agoraphobia, an association between phobia in probands and their first-degree relatives was identified.43
Twin studies in social phobics suggest that additive genetics is responsible for increased incidence of SAD in monozygotic compared with dizigotic twins and suggest no role for common environmental experiences. Adult twin studies of combined phobia diagnoses (including social phobics) suggest that the additive genetics accounts for 20% to 40% of the variance in diagnosis. This result corresponds with a population-based twin study of adolescents diagnosed with social phobia, MDD, and alcoholism, in which genetics accounted for 28% of the risk variance for SAD. Again, the remaining risk was derived from non-shared environmental experiences. Unlike MDD and PTSD, there is little evidence that early-life trauma influences the risk for developing SAD in adulthood.43
The one genome-wide linkage analysis of SAD implicated a region on chromosome 16 near the gene encoding the norepinephrine transporter. Other genes associated with SAD include (1) a functional variant in ADRB1, the gene encoding the β1-adrenergic receptor, and (2) two SNPs and a 3-SNP haplotype in the gene for COMT in female patients who have SAD (reviewed in107). Because SAD is such a complex phenotype, it has been suggested that it may be more fruitful to search for susceptibility genes by examining intermediate phenotypes, quantitative traits, and comorbidity with other illnesses. In fact, SAD heritability includes disorder-specific but also nonspecific genetic factors. SAD is associated with behavioral inhibition in childhood, low extroversion, and high neuroticism. These personality traits are not SAD specific but are hypothesized to contribute to a spectrum of psychopathology inclusive of mood and anxiety disorders. Furthermore, behavioral inhibition, low extroversion, and high neuroticism are each known to be highly heritable and may largely account for the genetic contribution to SAD.
Genes associated with high behavioral inhibition include CRF and SERT. Internalizing neuroticism is associated with the gene encoding glutamic acid decarboxylase, the rate-limiting enzyme in the synthesis of GABA from glutamate (reviewed in107).
GENERALIZED ANXIETY DISORDER
Anatomical and Neuroimaging Findings in Patients who Have Generalized Anxiety Disorder
Structural imaging studies have shown high ratios of gray matter to white matter in the upper temporal lobe of pediatric patients who have generalized anxiety disorder (GAD).113 Pediatric patients who have GAD also exhibit increased amygdala volume, which may correspond to the stress-induced amygdalar hypertrophy observed in laboratory animal studies (reviewed in37).
In functional imaging studies of adolescent patients who have GAD, resting vlPFC activity is elevated relative to healthy control subjects. Because the vlPFC activity correlates negatively with symptom severity, the elevation in vlPFC metabolism is interpreted as a compensatory response rather than an underlying cause of GAD.114 Because of observed hypermetabolism in the PFC of patients who have GAD, neuronal viability has been assessed in this region as measured by the ratio of N-ace-tylasparate to creatine using proton MRS. For patients who had GAD, neuronal viability was increased in the right dorsolateral PFC in those without early-life stress but was decreased in those who self-reported early-life trauma.115
Functional brain imaging results obtained under resting conditions in patients who have GAD have tended to be inconsistent; provocative anxiety-inducing tasks have produced more robust and interpretable fMRI results. The pattern of brain activity in anxious patients who have GAD correlates well with results from laboratory animal studies in which limbic circuits, particularly the amygdala, play an important role in the fear response (eg,116,117; see118 for a review). In fact, many imaging studies of patients who have GAD show elevated amygdala and insula activation during negative emotional processing (reviewed in23,119,120). In response to viewing angry faces, adolescent patients who had GAD exhibited an elevated right amygdala response; this activation correlated positively with symptom severity. The overactivity in the right amygdala also was correlated negatively with activity in the right vlPFC, suggesting top-down disinhibition as a potential mechanism for elevated amygdala activity.121 Interestingly, strong pretreatment activation of the left amygdala in pediatric patients who had GAD predicted a positive therapeutic response to fluoxetine or CBT.122 These results have been interpreted to suggest that a greater amygdaloid response to negative emotions represents a healthier signal-to-noise ratio. When adult patients who have GAD view fearful faces, lower pretreatment amygdala activity and higher ACC activity predict a positive treatment response to venlafaxine.123 Additional studies will be crucial in determining whether amygdala activation has clinical utility in predicting treatment outcome.
Interconnectivity with brain regions responsible for interpreting social behavior may be one mechanism by which the amygdala plays a substantial role in anxiety disorders. The brain regions responsible for interpreting social behavior include the superior temporal gyrus, thalamus, and PFC. Amygdala hyperactivity may mediate the inaccurate interpretations of social behavior in patients who have GAD.120
Neurotransmitter and Neuroendocrine Signaling in Generalized Anxiety Disorder
Amino acid neurotransmitters
The observed limbic overactivity in patients who have GAD could result from decreased inhibitory neurotransmission, increased excitatory neurotransmission, or a combination of these two processes. Dysregulation of GABA inhibitory neurotransmission has been documented in several anxiety disorders (reviewed in124). GABAA receptor downregulation is observed in patients who have GAD and has been hypothesized to play a role in the etiology of this illness (reviewed in68). In support of this hypothesis is the finding that symptoms of GAD, including excessive worry, hypervigilance, and psychomotor agitation, are treated effectively with GABAA facilitators such as benzodiazepines and barbiturates (reviewed in124). Furthermore, treatment with riluzole, an anti-glutamatergic agent, seems to improve GAD symptoms.125,126
Monoamines
Although all the SSRIs have shown efficacy in GAD, the drug most frequently studied in anxiety is paroxetine, which decreases symptoms of harm avoidance. It is important to note that GAD often is comorbid with other disorders, including MDD, PD, and SAD, each of which also has shown responsiveness to SSRI treatment.39
More concrete evidence supporting a role for 5-HT circuitry in GAD includes challenge with the 5-HT2c/5-HT3 agonist mCPP, which elicits anxiety and anger in patients who have GAD (reviewed in68).
Further evidence for a serotonergic component of GAD is provided by functional brain imaging studies that have found that midbrain SERT density correlates negatively with symptom severity.127,128 Recent studies have replicated the negative correlation between SERT density and anxiety symptoms in GAD, but there is no difference in SERT density in subjects who have GAD as compared with controls.127
Neuropeptides
Patients who have GAD are hypersensitive to exogenously administered CCK agonists,129,130 leading to the study of CCK receptor–selective antagonists as a putative novel class of anxiolytics. One such drug was developed but was not demonstrated to possess anxiolytic efficacy.131 Additional research and development of unique CCK antagonists will be an important step in clarifying the role of CCK in anxiety and its potential as a therapeutic target.
To the authors’ knowledge, no studies have specifically examined the role of NPY in GAD. NPY does possess anxiolytic effects in laboratory animals (reviewed in132). These anxiolytic effects may be caused by NPY–CRF interactions; these two neuro-peptides are co-localized in numerous limbic regions and exert opposing effects on the amygdala, LC, and periaqueductal gray matter, the last region is responsible for the motor output for the behavioral stress response.133
Corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis
Although very few studies have specifically examined HPA axis reactivity in patients who have GAD, there is no evidence of hypercortisolism, dexamethasone non-suppression, or increased CSF CRF concentrations.67,68 That CRF and the HPA axis seem to play a less prominent role in GAD than in other anxiety disorders and MDD is perhaps surprising given that CRF antagonists have been demonstrated to possess anxiolytic effects (134–136; reviewed in137). It is possible that the lack of evidence for a pathophysiological role for CRF circuits in GAD is an artifact of the paucity of endocrine studies in these patients. It is equally likely, however, that the difference in CRF/HPA axis observations in patients who have MDD and patients who have GAD represents a critical biological distinction between these two syndromes.
Genetic Contribution to Generalized Anxiety Disorder
Overall the genetic contribution is thought to be less substantial in GAD than in other anxiety disorders. Studies have shown that first-degree relatives of GAD probands have elevated rates of mood and anxiety disorders in general138 and perhaps have a specifically increased risk for GAD.43 A recent study of more than 3000 twin pairs found modest familial aggregation of GAD with equal heritability in males and females in same-sex or opposite-sex twin pairs; there was no evidence for gender-specific genetic underpinnings of GAD.139 Results from twin studies estimate that approximately 32% of the variance for liability to GAD is caused by additive genetics in male and female twins and that the remaining variance is explained by environment specific to the individual, rather than the shared environment of the twin pair (reviewed in43). Only a handful of genetic-association studies specific for GAD have been reported, and all are thus far unreplicated (eg,140–142).
SUMMARY AND GUIDANCE FOR THE DIAGNOSTIC AND STATISTICAL MANUAL OF MENTAL DISORDERS, EDITION FIVE
Functional Neuroanatomy
The Neurobiology of Anxiety Disorders Commonalities in anxiety disorders include functional hyperactivity in limbic regions, particularly the amygdala, and the inability of higher cortical executive areas to normalize the limbic response to stimuli (Table 2). In contrast to MDD, in which amygdala hyperactivity is observed under resting conditions, provocation paradigms are required to identify amygdalar hyperactivity in patients who have an anxiety disorder.
Table 2.
Functional anatomy of normal and pathological sadness and anxiety
| Anatomic Area | Normal and Pathological Sadness | Normal and Pathological Anxiety |
|---|---|---|
| Insular cortex | Acute sadness activates dorsal insula |
Acute anxiety activates ventral insula |
| Cingulate cortex | Pregenual ACC deactivated in euthymic MDD Pregenual ACC activated in acute MDD Subgenual ACC normal in acute MDD but hypoactive in patients who have remitted MDD ACC and PCC activated by acute sadness |
Acute anxiety has no effect on ACC but deactivates the PCC |
| Amygdala | Overactive at rest in primary mood disorders Magnitude of activity correlates to severity Overactivity without conscious perception Normal activity after treatment Smaller volume of left amygdale versus controls |
Not overactive at rest Overactive during symptom provocation Right amygdala most relevant to anxiety |
Additional neuroimaging studies must focus not on individual brain regions but on corticolimbic circuits. Between-laboratory consistency must become a priority throughout the research community to allow interpretation of results across studies. Perhaps most importantly, neuroimaging research must place more emphasis on hypothesis-driven studies. It is hoped that such increased consistency and clear goals will lead to more reliable and robust observations that finally can piece together the diagnosis-specific clinical implications of functional and structural alterations in patients who have mood and anxiety disorders.
Neurotransmitter and Neuroendocrine Signaling
Disruption in neurotransmitter, neuropeptide, and neuroendocrine signaling is not unique to mood and anxiety disorders; a great deal of overlap between diagnostic syndromes should be expected. For example, dysregulation of the generalized stress response is common to numerous medical and psychiatric diagnoses. Repeated, prolonged, or particularly severe stress could increase the magnitude and duration of CRF, glucocorticoid, and catecholaminergic signaling, and these three signaling classes can explain the psychiatric, circulatory, metabolic, and immune manifestations of stress-related illness. In contrast, hypoactivation of the HPA axis as a compensatory mechanism for chronic/severe stress exposure may occur also. HPA axis hyperactivity is seen in MDD, OCD, PD, anorexia, and alcoholism (to name a few), whereas HPA axis hypoactivity is observed in chronic fatigue, fibromyalgia, nicotine withdrawal, PTSD, and the postpartum period. Importantly, the direction of the HPA axis disruption depends on the nature, duration, predictability, and severity of the stressor and also on the age of the subject, individual genetic background, and previous experiences (reviewed in58). The clinical implications of altered monoaminergic signaling probably are influenced by an equally long list of factors. A closer relationship between preclinical and clinical research is essential before it will be possible to begin to piece together the relationship between each of these factors.
Genetic Contribution
The Neurobiology of Anxiety Disorders When attempting to identify the genetic contribution toward susceptibility for psychopathology, the candidate genes are largely the same across diagnoses and tend to be genes whose products regulate the HPA axis and monoaminergic signaling. These similarities, however, do not preclude important clinical distinctions between diagnostic classes within anxiety disorders or between anxiety disorders and MDD. Some genetic factors are nonspecific but influence the risk for psychopathology in general. Others are diagnosis specific. Moreover, the impact of individual diagnosis-specific genetic risk factors may vary over time, depending on the developmental stage and previous experience of each subject.
Overall, the decision to classify MDD, PD, PTSD, SAD, and GAD as distinct disorders must be based not only on clinical phenomenology but also on pathophysiology, genetics, course of illness, and treatment response data. Neuroendocrine, neurotransmitter, and neuroanatomical differences between patients who have mood or anxiety disorders and healthy control subjects must be interpreted with care (Table 3). Brain regions and neurotransmitter systems implicated in mood and anxiety disorders have wide-ranging functions, many of which may be unrelated to the etiology of psychiatric disorders. Finally each of these disorders clearly represents the result of complex gene–environment interactions. The clinical phenotype may well be determined largely by individual differences in multiple genes that exhibit functional polymorphisms. It is hoped that continued research will begin to uncover more consistent findings across laboratories, methodologies, and subjects. At that point, a new discussion of diagnostic criteria may be relevant.
Table 3.
Summary of select neurotransmitter abnormalities in MDD, GAD, and normal sadness and anxiety
| Neurotransmitter | Normal and Pathological Sadness | Normal and Pathological Anxiety |
|---|---|---|
| GABA | Inconsistent GABA-A agonists not approved for MDD by the Food and Drug Administration |
Decreased GABA-A receptor density in GAD; GABA-A agonists are anxiolytic Affinity for GABA-A predicts efficacy of benzodiazepines |
| Serotonin | Decreased 5HIAA CSF concentrations in suicide victims Normal in non-suicidal MDD patients Blunted prolactin response to 5-HT agonists |
Decreased 5HIAA CSF concentrations in some studies |
| SERT | Decreased density in midbrain Density correlates negatively with anxiety symptoms in MDD |
Density correlates negatively with anxiety symptoms in GAD |
| 5HT1A | — | Anxiolytic as DRN autoreceptors Anxiogenic as hippocampus postsynaptic receptors |
| 5HT2 | Desensitized by antidepressants | Anxiogenic Antagonists are anxiolytic |
| Norepinephrine | Elevated in CSF and plasma of patients who have severe melancholic MDD Unchanged in patients who have non-melancholic MDD Blunted growth hormone response to clonidine Blunted rapid-eye-movement response to clonidine |
Unchanged in GAD |
Acknowledgments
This work was supported by National Institute of Health (NIH) grants MH-541380, MH-77083, MH-69056, MH-58922, MH-42088, MH071537, and DA-019624; the Doris Duke Clinical Scientist Award, and the Burroughs Wellcome Fund. K.J.R. has received awards and/or funding support from Lundbeck, Burroughs Wellcome Foundation, Pfizer, the National Alliance for Research in Schizophrenia and Depression (NARSAD), the National Institute of Mental Health (NIMH), and the National Institute on Drug Abuse and has a consulting agreement with Tikvah Therapeutics for N-methyl-D-as-partic acid–based therapeutics. C.B.N currently serves on the scientific advisory boards of American Foundation for Suicide Prevention (AFSP), AstraZeneca, NARSAD, Quintiles, Janssen/Ortho-McNeil, and PharmaNeuroboost. He holds stock/equity in Corcept; Revaax, NovaDel Pharma, CeNeRx, and PharmaNeuroboost. He is on the board of directors of the AFSP, George West Mental Health Foundation, NovaDel Pharma, and Mt. Cook Pharma, Inc. He holds a patent on the method and devices for transdermal delivery of lithium (US 6,375,990 B1) and the method for estimating serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum (provisional filing April, 2001). In the past year, he also served on the Scientific Advisory Board for Forest Laboratories, received grant support from the NIMH, NARSAD, and AFSP, and served on the Board of Directors of the American Psychiatric Institute for Research and Education. E.B. is co-inventor on the following patent applications: FKBP5: a novel target for antidepressant therapy, international publication number: WO 2005/054500; and Polymorphisms in ABCB1 associated with a lack of clinical response to medicaments, international application number: PCT/EP2005/005194. She receives grant support from NARSAD and the Doris Duke Charitable Foundation. In the past 2 years, she has received grant support from Pfizer Pharmaceuticals (Young Investigator award) and GlaxoSmithKline.
REFERENCES
- 1.Keedwell PA, Andrew C, Williams SC, et al. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 3.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Treede RD, Kenshalo DR, Gracely RH, et al. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 5.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 6.Gysling K, Forray MI, Haeger P, et al. Corticotropin-releasing hormone and urocortin: redundant or distinctive functions? Brain Res Brain Res Rev. 2004;47:116–125. doi: 10.1016/j.brainresrev.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Barrera G, Echevarria DJ, Poulin JF, et al. One for all or one for one: does cotransmission unify the concept of a brain galanin “system” or clarify any consistent role in anxiety? Neuropeptides. 2005;39:289–292. doi: 10.1016/j.npep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Honkaniemi J, Pelto-Huikko M, Rechardt L, et al. Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology. 1992;55:451–459. doi: 10.1159/000126156. [DOI] [PubMed] [Google Scholar]
- 9.Palkovits M. Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur J Pharmacol. 2000;405:161–166. doi: 10.1016/s0014-2999(00)00549-5. [DOI] [PubMed] [Google Scholar]
- 10.Watts AG. The impact of physiological stimuli on the expression of corticotropinreleasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- 11.Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes A, Heilig M, Rupniak NM, et al. Neuropeptide systems as novel therapeutic targets fordepressionand anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Schatzberg AF, Nemeroff CB, editors. Textbook of psychopharmacology. ed. 3. Washington (DC): The American Psychiatric Publishing; 2004. pp. 717–765. 847–68, 913–35. [Google Scholar]
- 14.Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Liu HX, Hokfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23:468–474. doi: 10.1016/s0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- 16.Bedecs K, Berthold M, Bartfai T. Galanin 10 years with a neuroendocrine peptide. Int J Biochem Cell Biol. 1995;27:337–349. doi: 10.1016/1357-2725(95)00008-d. [DOI] [PubMed] [Google Scholar]
- 17.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Lindenberg A. Impact of prosocial neuropeptides on human brain function. Prog Brain Res. 2008;170:463–470. doi: 10.1016/S0079-6123(08)00436-6. [DOI] [PubMed] [Google Scholar]
- 19.Egashira N, Tanoue A, Matsuda T, et al. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychol Med. 2008;38:1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallett V, Ronald A, Rijsdijk F, et al. Phenotypic and genetic differentiation of anxiety-related behaviors in middle childhood. Depress Anxiety. 2009;26:316–324. doi: 10.1002/da.20539. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Hwang J, Kim SJ, et al. Decreased blood flow of temporal regions of the brain in subjects with panic disorder. J Psychiatr Res. 2006;40:528–534. doi: 10.1016/j.jpsychires.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Engel K, Bandelow B, Gruber O, et al. Neuroimaging in anxiety disorders. J Neural Transm. 2009;116:703–716. doi: 10.1007/s00702-008-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfleiderer B, Zinkirciran S, Arolt V, et al. fMRI amygdala activation during a spontaneous panic attack in a patient with panic disorder. World J Biol Psychiatry. 2007;8:269–272. doi: 10.1080/15622970701216673. [DOI] [PubMed] [Google Scholar]
- 25.Maddock RJ, Buonocore MH, Kile SJ, et al. Brain regions showing increased activation by threat-related words in panic disorder. Neuroreport. 2003;14:325–328. doi: 10.1097/00001756-200303030-00006. [DOI] [PubMed] [Google Scholar]
- 26.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 27.Bystritsky A, Pontillo D, Powers M, et al. Functional MRI changes during panic anticipation and imagery exposure. Neuroreport. 2001;12:3953–3957. doi: 10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- 28.Pillay SS, Gruber SA, Rogowska J, et al. fMRI of fearful facial affect recognition in panic disorder: the cingulate gyrus-amygdala connection. J Affect Disord. 2006;94:173–181. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Malizia AL, Cunningham VJ, Bell CJ, et al. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–720. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- 30.Bremner JD, Innis RB, Southwick SM, et al. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am J Psychiatry. 2000;157:1120–1126. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 31.Bremner JD, Innis RB, White T, et al. SPECT [I-123]iomazenil measurement of the benzodiazepine receptor in panic disorder. Biol Psychiatry. 2000;47:96–106. doi: 10.1016/s0006-3223(99)00188-2. [DOI] [PubMed] [Google Scholar]
- 32.Kaschka W, Feistel H, Ebert D. Reduced benzodiazepine receptor binding in panic disorders measured by iomazenil SPECT. J Psychiatr Res. 1995;29:427–434. doi: 10.1016/0022-3956(95)00019-2. [DOI] [PubMed] [Google Scholar]
- 33.Goddard AW, Mason GF, Appel M, et al. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–2193. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- 34.Ham BJ, Sung Y, Kim N, et al. Decreased GABA levels in anterior cingulate and basal ganglia in medicated subjects with panic disorder: a proton magnetic resonance spectroscopy (1H-MRS) study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:403–411. doi: 10.1016/j.pnpbp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Kent JM, Mathew SJ, Gorman JM. Molecular targets in the treatment of anxiety. Biol Psychiatry. 2002;52:1008–1030. doi: 10.1016/s0006-3223(02)01672-4. [DOI] [PubMed] [Google Scholar]
- 36.Helton DR, Tizzano JP, Monn JA, et al. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabo tropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- 37.Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10:820–830. doi: 10.1017/s1092852900010427. [DOI] [PubMed] [Google Scholar]
- 38.Pull CB, Damsa C. Pharmacotherapy of panic disorder. Neuropsychiatr Dis Treat. 2008;4:779–795. doi: 10.2147/ndt.s1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 40.Neumeister A, Young T, Stastny J. Implications of genetic research on the role of the serotonin in depression: emphasis on the serotonin type 1A receptor and the serotonin transporter. Psychopharmacology (Berl) 2004;174:512–524. doi: 10.1007/s00213-004-1950-3. [DOI] [PubMed] [Google Scholar]
- 41.Unschuld PG, Ising M, Erhardt A, et al. Polymorphisms in the galanin gene are associated with symptom-severity in female patients suffering from panic disorder. J Affect Disord. 2008;105:177–184. doi: 10.1016/j.jad.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Abelson JL, Khan S, Liberzon I, et al. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- 43.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 44.Hettema JM, Prescott CA, Myers JM, et al. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 45.Smoller JW, Gardner-Schuster E, Covino J. The genetic basis of panic and phobic anxiety disorders. Am J Med Genet C Semin Med Genet. 2008;148:118–126. doi: 10.1002/ajmg.c.30174. [DOI] [PubMed] [Google Scholar]
- 46.Finn CT, Smoller JW. The genetics of panic disorder. Curr Psychiatry Rep. 2001;3:131–137. doi: 10.1007/s11920-001-0010-5. [DOI] [PubMed] [Google Scholar]
- 47.Fyer AJ, Hamilton SP, Durner M, et al. A third-pass genome scan in panic disorder: evidence for multiple susceptibility loci. Biol Psychiatry. 2006;60:388–401. doi: 10.1016/j.biopsych.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 49.Strug LJ, Suresh R, Fyer AJ, et al. Panic disorder is associated with the serotonin transporter gene (SLC6A4) but not the promoter region (5-HTTLPR) Mol Psychiatry. 2008 doi: 10.1038/mp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keck ME, Kern N, Erhardt A, et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1196–1204. doi: 10.1002/ajmg.b.30750. [DOI] [PubMed] [Google Scholar]
- 51.Hodges LM, Weissman MM, Haghighi F, et al. Association and linkage analysis of candidate genes GRP, GRPR, CRHR1, and TACR1 in panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:65–73. doi: 10.1002/ajmg.b.30773. [DOI] [PubMed] [Google Scholar]
- 52.Bryant RA, Felmingham K, Kemp A, et al. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med. 2008;38:555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- 53.Dickie EW, Brunet A, Akerib V, et al. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46:1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Bryant RA, Felmingham K, Whitford TJ, et al. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33:142–146. [PMC free article] [PubMed] [Google Scholar]
- 55.Morey RA, Petty CM, Cooper DA, et al. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq war veterans. Psychiatry Res. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falconer E, Bryant R, Felmingham KL, et al. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33:413–422. [PMC free article] [PubMed] [Google Scholar]
- 57.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharma-cology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 58.Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. J Neuroendocrinol. 2008;20:632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 59.Hurlemann R. Noradrenergic-glucocorticoid mechanisms in emotion-induced amnesia: from adaptation to disease. Psychopharmacology (Berl) 2008;197:13–23. doi: 10.1007/s00213-007-1002-x. [DOI] [PubMed] [Google Scholar]
- 60.Marshall RD, Schneier FR, Fallon BA, et al. An open trial of paroxetine in patients with noncombat-related, chronic posttraumatic stress disorder. J Clin Psychopharmacol. 1998;18:10–18. doi: 10.1097/00004714-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Committee on Treatment of Posttraumatic Stress Disorder, Institute of Medicine. Treatment of posttraumatic stress disorder: an assessment of the evidence. Washington (DC): National Academies Press; 2008. [Google Scholar]
- 62.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 63.Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(17):41–46. [PubMed] [Google Scholar]
- 64.Yehuda R, Giller EL, Southwick SM, et al. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry. 1991;30:1031–1048. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 65.de Kloet CS, Vermetten E, Geuze E, et al. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fossey MD, Lydiard RB, Ballenger JC, et al. Cerebrospinal fluid corticotropinreleasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry. 1996;39:703–707. doi: 10.1016/0006-3223(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 68.Nutt DJ. Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry. 2001;62(11):22–27. [discussion: 28] [PubMed] [Google Scholar]
- 69.Yehuda R, Southwick SM, Nussbaum G, et al. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Olff M, Guzelcan Y, de Vries GJ, et al. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:1220–1230. doi: 10.1016/j.psyneuen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Rasmusson AM, Lipschitz DS, Wang S, et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- 72.Lipschitz DS, Rasmusson AM, Yehuda R, et al. Salivary cortisol responses to dexamethasone in adolescents with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 2003;42:1310–1317. doi: 10.1097/01.chi.0000084832.67701.0d. [DOI] [PubMed] [Google Scholar]
- 73.Baker DG, Ekhator NN, Kasckow JW, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- 74.Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith MA, Davidson J, Ritchie JC, et al. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biol Psychiatry. 1989;26:349–355. doi: 10.1016/0006-3223(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 76.Yehuda R, Halligan SL, Grossman R, et al. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:393–403. doi: 10.1016/s0006-3223(02)01357-4. [DOI] [PubMed] [Google Scholar]
- 77.Kudler H, Davidson J, Meador K, et al. The DST and posttraumatic stress disorder. Am J Psychiatry. 1987;144:1068–1071. doi: 10.1176/ajp.144.8.1068. [DOI] [PubMed] [Google Scholar]
- 78.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 79.Segman RH, Shalev AY. Genetics of posttraumatic stress disorder. CNS Spectr. 2003;8:693–698. doi: 10.1017/s1092852900008889. [DOI] [PubMed] [Google Scholar]
- 80.Yehuda R, Halligan SL, Bierer LM. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J Psychiatr Res. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 81.Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev Psychopathol. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- 82.Koenen KC, Lyons MJ, Goldberg J, et al. A high risk twin study of combatrelated PTSD comorbidity. Twin Res. 2003;6:218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- 83.Stein MB, Jang KL, Taylor S, et al. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 84.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 85.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 86.Eley TC, Sugden K, Corsico A, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 87.Grabe HJ, Lange M, Wolff B, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- 88.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kendler KS, Kuhn JW, Vittum J, et al. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 90.Gillespie NA, Whitfield JB, Williams B, et al. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 91.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 92.Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 93.Ladd CO, Huot RL, Thrivikraman KV, et al. Long-term behavioral and neuroendo-crine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 94.Plotsky PM, Thrivikraman KV, Nemeroff CB, et al. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 95.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 98.Denny WB, Valentine DL, Reynolds PD, et al. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 99.Wochnik GM, Ruegg J, Abel GA, et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 100.Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 101.Newport DJ, Heim C, Bonsall R, et al. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 102.Yehuda R, Golier JA, Halligan SL, et al. The ACTH response to dexamethasone in PTSD. Am J Psychiatry. 2004;161:1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- 103.Segman RH, Shefi N, Goltser-Dubner T, et al. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:500–513. doi: 10.1038/sj.mp.4001636. 425. [DOI] [PubMed] [Google Scholar]
- 104.Koenen KC, Saxe G, Purcell S, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- 105.Ozer EJ, Best SR, Lipsey TL, et al. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 106.Kilts CD, Kelsey JE, Knight B, et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 107.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 108.Hood S, Potokar J, Davies S, et al. Dopaminergic challenges in social anxiety disorder: evidence for dopamine D3 desensitisation following successful treatment with serotonergic antidepressants. J Psychopharmacol. 2008 Oct 6; doi: 10.1177/0269881108098144. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 109.Condren RM, O’Neill A, Ryan MC, et al. HPA axis response to a psychological stressor in generalised social phobia. Psychoneuroendocrinology. 2002;27:693–703. doi: 10.1016/s0306-4530(01)00070-1. [DOI] [PubMed] [Google Scholar]
- 110.Roelofs K, van Peer J, Berretty E, et al. Hypothalamus-pituitary-adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biol Psychiatry. 2009;65:336–343. doi: 10.1016/j.biopsych.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 111.van Veen JF, van Vliet IM, Derijk RH, et al. Elevated alpha-amylase but not cortisol in generalized social anxiety disorder. Psychoneuroendocrinology. 2008;33:1313–1321. doi: 10.1016/j.psyneuen.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 112.Martel FL, Hayward C, Lyons DM, et al. Salivary cortisol levels in socially phobic adolescent girls. Depress Anxiety. 1999;10:25–27. doi: 10.1002/(sici)1520-6394(1999)10:1<25::aid-da4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 113.De Bellis MD, Keshavan MS, Shifflett H, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002;51:553–562. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- 114.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 115.Mathew SJ, Mao X, Coplan JD, et al. Dorsolateral prefrontal cortical pathology in generalized anxiety disorder: a proton magnetic resonance spectroscopic imaging study. Am J Psychiatry. 2004;161:1119–1121. doi: 10.1176/appi.ajp.161.6.1119. [DOI] [PubMed] [Google Scholar]
- 116.Herry C, Ciocchi S, Senn V, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 117.Izumi T, Inoue T, Kato A, et al. Changes in amygdala neural activity that occur with the extinction of context-dependent conditioned fear stress. Pharmacol Biochem Behav. 2008;90:297–304. doi: 10.1016/j.pbb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 118.Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- 119.Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- 120.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 121.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McClure EB, Adler A, Monk CS, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 123.Whalen PJ, Johnstone T, Somerville LH, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol Bull. 2003;37:133–146. [PubMed] [Google Scholar]
- 125.Mathew SJ, Amiel JM, Coplan JD, et al. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162:2379–2381. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- 126.Pittenger C, Coric V, Banasr M, et al. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs. 2008;22:761–786. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- 127.Maron E, Kuikka JT, Ulst K, et al. SPECT imaging of serotonin transporter binding in patients with generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:392–396. doi: 10.1007/s00406-004-0520-3. [DOI] [PubMed] [Google Scholar]
- 128.Malison RT, Price LH, Berman R, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 129.Koszycki D, Copen J, Bradwejn J. Sensitivity to cholecystokinin-tetrapeptide in major depression. J Affect Disord. 2004;80:285–290. doi: 10.1016/S0165-0327(03)00110-1. [DOI] [PubMed] [Google Scholar]
- 130.Brawman-Mintzer O, Lydiard RB, Bradwejn J, et al. Effects of the cholecystokinin agonist pentagastrin in patients with generalized anxiety disorder. Am J Psychiatry. 1997;154:700–702. doi: 10.1176/ajp.154.5.700. [DOI] [PubMed] [Google Scholar]
- 131.Pande AC, Greiner M, Adams JB, et al. Placebo-controlled trial of the CCK-B antagonist, CI-988, in panic disorder. Biol Psychiatry. 1999;46:860–862. doi: 10.1016/s0006-3223(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 132.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 134.Lelas S, Wong H, Li YW, et al. Anxiolytic-like effects of the corticotropin-releasing factor1 (CRF1) antagonist DMP904 [4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyrimidine] administered acutely or chronically at doses occupying central CRF1 receptors in rats. J Pharmacol Exp Ther. 2004;309:293–302. doi: 10.1124/jpet.103.058784. [DOI] [PubMed] [Google Scholar]
- 135.Millan MJ, Brocco M, Gobert A, et al. Anxiolytic properties of the selective, nonpeptidergic CRF(1) antagonists, CP154,526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology. 2001;25:585–600. doi: 10.1016/S0893-133X(01)00244-5. [DOI] [PubMed] [Google Scholar]
- 136.Chaki S, Nakazato A, Kennis L, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 137.Holsboer F, Ising M. Central CRH system in depression andanxiety—evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 138.Ninan PT. Recent perspectives on the diagnosis and treatment of generalized anxiety disorder. Am J Manag Care. 2001;7:S367–S376. [PubMed] [Google Scholar]
- 139.Hettema JM, Prescott CA, Kendler KS. A population-based twin study of generalized anxiety disorder in men and women. J Nerv Ment Dis. 2001;189:413–420. doi: 10.1097/00005053-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Tadic A, Rujescu D, Szegedi A, et al. Association of a MAOA gene variant with generalized anxiety disorder, but not with panic disorder or major depression. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:1–6. doi: 10.1002/ajmg.b.10013. [DOI] [PubMed] [Google Scholar]
- 141.You JS, Hu SY, Chen B, et al. Serotonin transporter and tryptophan hydroxylase gene polymorphisms in Chinese patients with generalized anxiety disorder. Psychiatr Genet. 2005;15:7–11. doi: 10.1097/00041444-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 142.Koenen KC, Amstadter AB, Ruggiero KJ, et al. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2009;26:309–315. doi: 10.1002/da.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]