Abstract
Stem cells are fascinating because of their potential in regenerative medicine. Stem cell homeostasis has been thought to be mainly regulated by signals from their adjacent micro-environment named the “stem cell niche”. However, recent studies reveal that there can be multiple layers of environmental controls. Here we review these environmental controls using the paradigm of hair stem cells, because to observe and analyze the growth of hair is easier due to their characteristic cyclic regeneration pattern. The length of hair fibers is regulated by the duration of the growth period. In the hair follicles, hair stem cells located in the follicle bulge interact with signals from the dermal papilla. Outside of the follicle, activation of hair stem cells has been shown to be modulated by molecules released from the intra-dermal adipose tissue as well as body hormone status, immune function, neural activities, and aging. The general physiological status of an individual is further influenced by circadian rhythms and changing seasons. The interactive networks of these environmental factors provide new understanding on how stem cell homeostasis is regulated, inspiring new insights for regenerative medicine. Therapies do not necessarily have to be achieved by using stem cells themselves which may constitute a higher risk but by modulating stem cell activity through targeting one or multiple layers of their micro- and macro-environments.
Keywords: regeneration, niche, macro-environment, stem cell, Bmp, Sfrp, Dkk, hair, alopecia, adipose cell
1. Introduction
Stem cells are fascinating because of their unique potential to differentiate into different cell types and regenerate tissues and organs. This has great promise for the dreams of therapeutic possibility in degenerative disorders. From embryonic stem cells, they differentiate into all derivatives of the three primary germ layers: ectoderm, endoderm, and mesoderm that form different organs. In the adult, some adult organs still contain dormant multipotent or unipotent somatic stem cells which can be activated under changing physiological conditions or in response to injury. How this activation can be regulated is the key to the success of regenerative medicine.
The regeneration time, period, and potential of each organ stem cell varied from one to another (Fig. 1). In some organs, such as the hair follicle, gut and bone marrow, the stem cells can divide frequently and regularly to replenish the exhausted or damaged cells by either natural course or injury throughout the whole life. However, in other organs, such as pancreas and heart, they are not activated spontaneously until a special situation occurrs. Stem cells were first thought to be regulated by the micro-environment, called stem cell niche. More recent studies revealed that the stem cell homeostasis can also be modulated by the so called extra-niche macro-environments (Fig. 2). In the skin, there is intradermal adipose tissue. Then there are systemic factors at the level of an individual which include input from hormones, immune and nervous systems, and aging. Stem cell activity is also modulated by the external environment which includes circadian rhythms and seasonal changes (Fig. 4). Different mammals have different robustness in their ability to grow and regenerate hairs (Fig. 5). There have been many reviews on the micro-environment of stem cells. In this review, we will focus on developing the concept on how the macro-environment affects stem cell activation. We mainly use hair stem cells for discussion, but will refer to other organ stem cells as well.
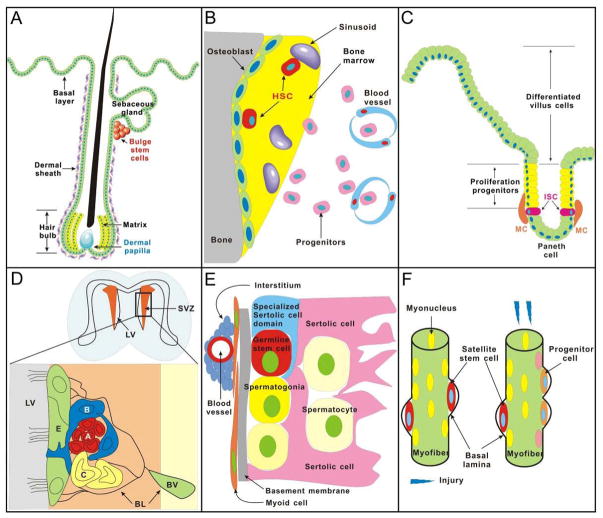
Figure 1. Adult stem cells and their microenvironmental niches.
Examples of several organs are shown. Recent works show the activities of these niches and stem cells can be furter affected by ”macro-environments“ (Please see Fig. 4).
(A) Hairs. Bulge is the place where hair stem cell resides. Hair stem cell can receive signals from either the dermal papilla to form a new hair shaft or wounded skin to replace inter-follicular epidermis. (B) Bone marrow. Endosteal osteoblast and vessel are two major niches of hematopoietic stem cells (HSCs). The endosteum plays a more stimulatory role under conditions of stress to trigger the proliferation of HSCs, however, central vascular regions play a homeostatic role during steady state conditions. (C) Intestinal villi. Intestinal stem cell (ISCs) which could give rise to four different lineages of cells: columnar enterocytes, mucin-producing goblet cells, Paneth cells, and entero-endocrine cells located at the 4th or 5th position from the bottom of the crypt. Mesenchymal cells separated from the crypt epithelium by the basal lamina serve as a niche to release signals to regulate ISCs. (D) Brain. The sub-ventricular zone (SVZ), which is separated from the lateral ventricle (LV) by ependymal cells (E), is the area where neural stem cells (NSCs), the astrocytes (B), are identified. The basal lamina (BL), which is extracellular matrix (ECM) rich and directly contacts NSCs, is the niche that regulates the homeostasis of NSCs. (E) Testis. The inner surface of the tubules or the seminiferous epithelium is the place where spermatogenesis takes place. Specialized sertoli cells, blood vessels, basement membrane, myoid cells and interstitium are thought to function as the niche required to maintain the homeostasis of undifferentiated spermatogonia or germ line stem cells (GSCs). (F) Muscle. Under the basal lamina of myofibers is the place where muscle stem cells, satellite cells, reside. The satellite cell niche is composed of three major components: host muscle fiber, basal lamina and the microvasculature. When injured, activated satellite cells will produce progenitor cells to migrate from beneath the basal lamina.
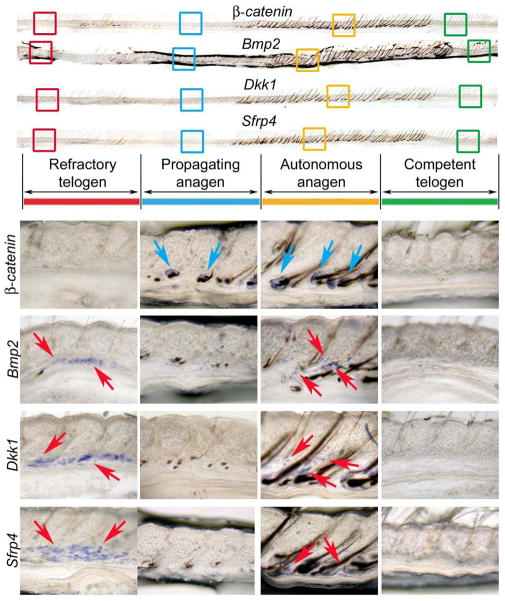
Figure 2. Extra-follicular macro-environmental modulators in the intra-dermal adipose tissue.
A group of new extra-niche modulators on hair stem cells are recently found in intra-dermal adipose tissue (Plikus et al., 2008, 2011). Similar to Bmp2, Wnt signaling antagonists Dkk1 and Sfrp4 are expressed during refractory telogen (R) and autonomous anagen (A) phases but not competent telogen (C) and propagating anagen (P) phases. Adopted from Plikus et al., 2011.
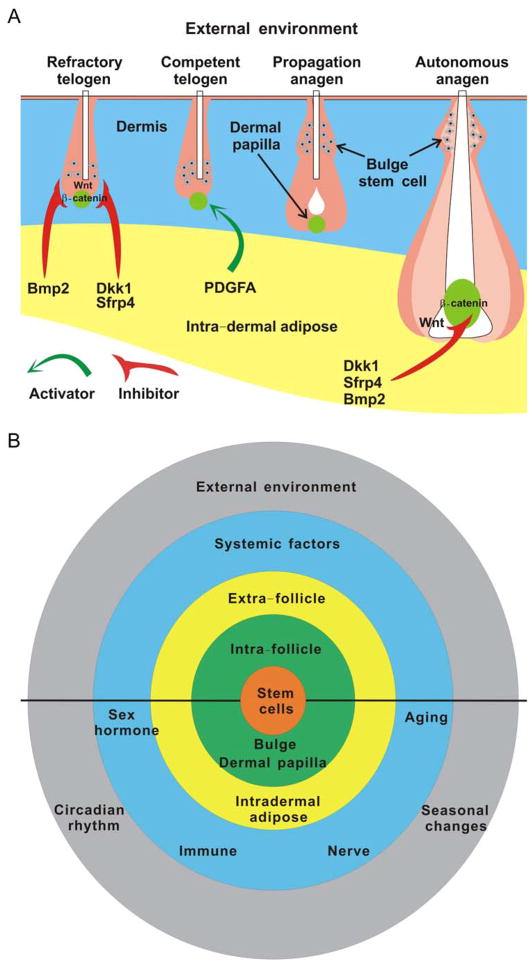
Figure 4. Concept diagram showing multi-layered environmental regulation of adult organ stem cells.
Outside of the micro-environmental niche of stem cells (green), we categorically named the rest of environments as “macro-environment”.
A. Regulation of regenerative hair cycling by the intra-dermal adipose modulators.
Extra-follicular inhibitors, including BMP-2, DKK-1 and SFRP are not only expressed during refractory telogen (R) to inhibit anagen re-entry but also present in autonomous anagen (A) to stop hair wave propagation. In contrast, intradermal adipocyte precursor cells could secret PDGFA to activate hair follicular stem cell activity during competent telogen (C) or propagating anagen (P).
B. Regulation of stem cells by multi-layered environments.
External environment (including circadian rhythm and seasonal change) and systemic factors (including sex hormone, aging, immune and nervous system) can regulate the homeostasis of stem cells directly or through modulating the niche and/or extra-niche macro-environment. Correspondingly, stem cell could also be modified by the niche and extra-niche macro-environment, which forms a regulation network to determine the fate of stem cells by summing all activator and inhibitor signals coming from both intrinsic and extrinsic environments. The upper half is meant for the situation for all organs. The lower half is meant for hair stem cells.
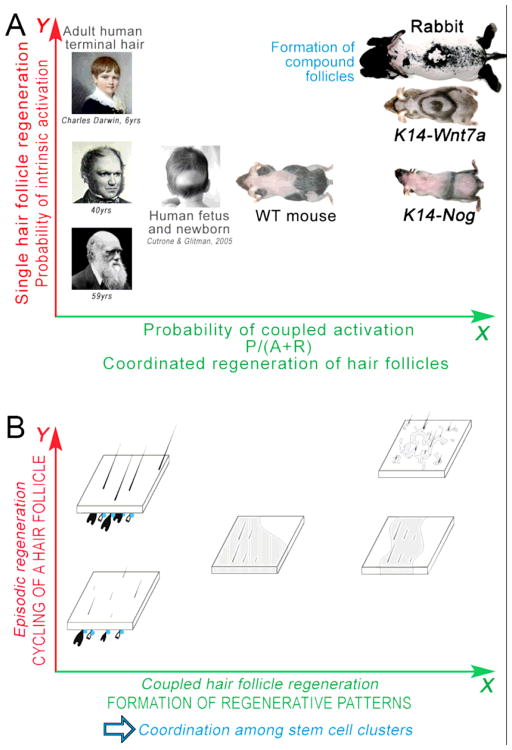
Figure 5. A unifying model of stem cell regeneration in a large population of hair follicles.
A. The spectrum of hair regeneration patterns in different animals with robust (rabbit), medium (mouse) or weak (human) hair regeneration ability.
B. The topology of stem cells and cycling stages of hair follicles underlying the hair wave patterns in panel A.
Hair stem cells (blue) can become activated by the intrinsic hair cycle clock (Y axis on B). Sufficient intrinsic activators are required to trigger such kind of hair follicle regeneration, like adult human scalp (Y axis on A), which can become deficient easily when the intrinsic activation drops, such as upon alopecia. Alternatively, diffusible signaling molecules used for regulating hair stem cell activities within each hair follicle can be co-opted to mediate interactions between neighboring hair follicles. Such signaling couples activation events among many stem cell clusters at once (X axis on B), which represent the characteristic hair regeneration patterns like waves in mice and rabbits (X axis on A). Thus, in mouse and rabbit, hairs can be activated to enter anagen either by factors intrinsic or extrinsic to follicles. In human adult, hairs can only be activated by factors intrinsic to the follicle. Therefore, alopecia is prone to develop when such mechanism is weakened. Adopted from Plikus et al., 2011.
2. Stem cell niche in adult organs
The concept of “niche” is known to be a specialized microenvironment where stem cells reside, which was first proposed by Schofield [1]. The niche not only emits signals to maintain the homeostasis of stem cells but also functions as a shelter to filter extrinsic stimuli that induce apoptosis, differentiation and so on. It is more difficult to identify the stem cell niche in mammalian tissues due to their complicated anatomic structures as compared to Drosophila and Caenorhabditis elegans. However, using lineage tracing methods by uptake and long-term retention of bromo-deoxyuridine (BrdU) or incorporation of fluorescently labeled histone 2B during DNA synthesis, slow cycling or so called “label-retaining” stem cells and their niche could be identified within mammalian tissues.
Different organs have different strategies to regulate their stem cells. In the skin, multi-potent hair stem cells reside in the bulge area which is located below the sebaceous gland. Hair stem cells receive cues from the dermal papilla, a population of specialized mesenchymal cells surrounded by hair matrix cells, to activate into transit-amplifying (TA) cells migrating downward to replenish matrix cells. After a couple of divisions, TA cells differentiate to form a new hair shaft. Alternatively, hair stem cells can accept signals from wounded skin to become epidermal progenitor cells and replace inter-follicular epidermis (Fig. 1A).
Hematopoietic stem cells (HSCs) are regulated by two types of niches, endosteal osteoblastic and vascular niches, which are located in bone marrow. These two niches share structural and functional mediators but exert different functions in modulating HSCs. The endosteum plays a more stimulatory role under conditions of stress to trigger the proliferation of HSCs, however, central vascular regions play a homeostatic role during steady state conditions [2] (Fig. 1B).
Intestinal stem cells (ISCs), which are located at the 4th or 5th position from the bottom of the crypt, the Paneth cell, could give rise to four different lineages of cells: columnar enterocytes, mucin-producing goblet cells, Paneth cells, and entero-endocrine cells [3]. The niche, mesenchymal cells, which can release signals to regulate ISCs is located adjacent to the crypt epithelium by the separation of basal lamina (Fig. 1C).
Neural stem cells (NSCs), the astrocytes (B), are located in the sub-ventricular zone (SVZ), which is separated from the lateral ventricle (LV) by ependymal cells (E). NSCs can differentiate into TA cells (C) and then produce neuroblast cells (A). The extracellular matrix (ECM) rich basal lamina (BL) which directly contacts NSCs serves as the niche [4] (Fig. 1D) for these stem cells.
Spermatogenesis progresses uniformly over the inner surface of the tubules or the seminiferous epithelium, which is composed of the basement membrane, Sertoli and peritubular myoid cells. Specialized sertoli cells, blood vessels, basement membranes, myoid cells and interstitium are thought to function as the niche to maintain the homeostasis of undifferentiated spermatogonia or germ line stem cells (GSCs) [5,6] (Fig. 1E).
In the muscle, satellite cells inhabiting a region under the basal lamina of myofibers are thought to be stem cells due to their self-renewal and myogenic differentiation capabilities (Fig. 1F). Upon injury, progenitor cells will be produced by activated satellite cells and migrate from beneath the basal lamina [7]. Host muscle fibers, basal lamina and the microvasculature are the three major components of the satellite cell niche that modify the function of muscle stem cells [8].
3. Intra-follicle regulation of hair stem cells
The hair follicle is a unique organ that undergoes cyclic bouts of degeneration and regeneration throughout life. A hair follicle cycles through anagen (growth), catagen (involution) and telogen (resting) phases and then re-enters anagen. At the base of this cycle is the ability of hair follicle stem cells to briefly exit their quiescent status to generate transient amplifying progeny which differentiate into different hair components, but maintain a cluster of stem cells. Thus hair follicles can undergo episodic regeneration physiologically or in response to injury. When they regenerate physiologically, hair follicles take the opportunity to generate new hair phenotypes to adapt themselves better to the environment [9]. It is generally believed that a niche microenvironment is important in the control of stem cell homeostasis in various systems [10]. Because of these properties, the hair has become a mainstream model for researches in stem cell biology as it represents rejuvenating power [11].
To keep the hair stem cells under quiescent status, bulge, the microenvironment, expresses BMPs and Wnt inhibitors, including DKK, Wif, and sFRP to suppress cell growth and differentiation. Upon anagen initiation, Wnt signals secreted by the dermal papilla will stabilize the β-catenin which acts on hair stem cells directly within the bulge to reduce the activation threshold. For hair stem cell activation, Wnts are not sufficient on their own. The dermal papilla will produce some other signals, including fibroblast growth factor (FGF) which could coordinate with β-catenin to support hair stem cells to overcome the gate for activation. Interactions between the bulge and dermal papilla form an intrafollicular regulation network to govern the homeostasis of hair stem cells [12–14].
4. Extra-follicle macro-environment and stem cell regulation
The hair growth pattern in rats behaves as a wave composed of periodical anagen spreading through the ventral side of the body to the dorsal side of the body, over the trunk, and progressively decreasing in width with age [15]. Recently, we revisited this classical phenomenon with modern stem cell concepts and molecular bases. We found that in the adult mouse skin (older than 2 month when intra-dermal adipose tissue is mature), in the hair follicle population, regeneration indeed occurs in waves implying the coordination among adjacent follicles and the extra-follicular environment [16]. We also found that periodic expression of dermal bone morphogenic proteins (BMPs) 2/4, which belong to the transforming growth factor beta (TGF-β) superfamily regulates this process. BMPs have an important role during embryonic development on embryonic patterning and early skeletal formation [17]. Our data showed that Bmp cycling in the intra-dermal adipose tissue is out-of-phase with the β-catenin cycling within the hair follicle, thus dividing the conventional telogen into new functional phases: one refractory and the other competent for hair regeneration; characterized by high and low BMP signaling respectively. BMPs also divide the conventional anagen into new functional phases: one propagatory and the other autonomous for propagation of regenerative wave; characterized by low and high BMP signaling respectively. According to these findings, we introduce the concept that the extra-follicular macro-environment could regulate the stem cell activity [18].
We further developed this concept through different animal models and mathematical simulations [19]. We identified some Wnt inhibitors, such as Dkk1 and Sfrp4 located in the dermal region during refractory telogen and autonomous anagen phases, just like the expression pattern of Bmp2 (Fig. 2). These two new macro-environmental modulators served as inhibitors which could block physiological hair wave propagation and inhibit hair stem cell activation (Fig. 3). Interestingly, not only inhibitors, the inter-follicular macro-environment could also induce anagen re-entry through releasing the activator PDGFA by the adipocyte precursor cells located in the inter-follicular dermal region [20]. A lack of adipocyte precursor cells resulted in bulge stem cell activation defects in Ebf1 null mice, this defect is rescued by transplanting of adipocyte precursor cells.. These data also support the idea that a fat/follicle axis is very important to the hair regeneration cycle [21].
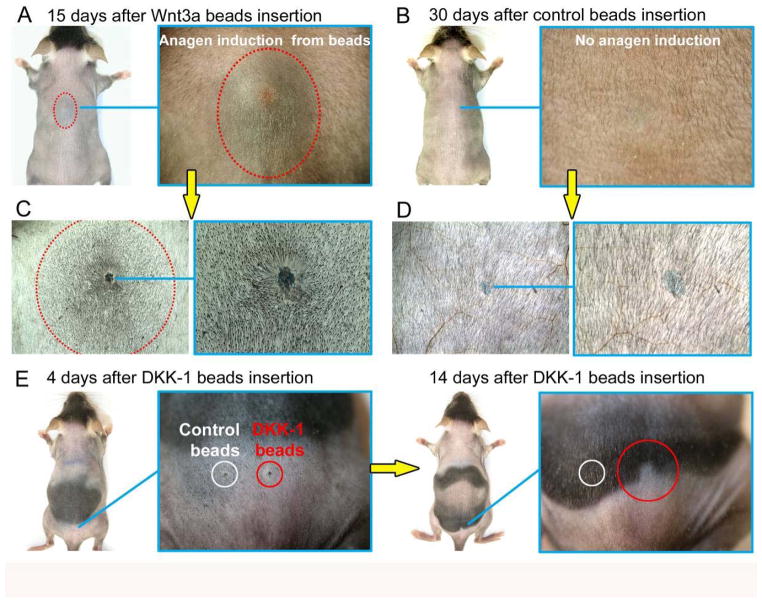
Figure 3. Wnt3a and Dkk1 modulate the propagatory patterns of the regenerative hair wave.
Administration of Wnt3a protein coated beads causes premature anagen initiation surrounding telogen hair follicles (A, C), while administration of Dkk1, a Wnt antagonist, prevents normal progression of the physiological anagen spreading wave (E). Control BSA beads can’t induce anagen re-entry (B, D). Red dotted outlines on A, C mark boundaries between anagen and telogen skin. Adopted from Plikus et al., 2011.
This type of regulatory mechanism is not limited to hair stem cells, and may exist in other organs. For example, bone marrow adipocytes are shown to suppress hematopoiesis and prevent hematopoietic progenitor expansion while preserving the hematopoietic stem cell pool through diminishing production of growth factors such as GM-CSF and G-CSF [22,23] and secreting neutrophilin-1 [24], lipocalin 2 [25,26], adiponectin [27] and TNF-α [28,29], each of which can impair hematopoietic proliferation [30]. As bone marrow adipocytes interfere with the balance between hematopoietic stem cells (HSCs) and their niche, osteoblasts, intradermal adipocytes could influence the interaction between hair stem cells and their niche as well as interactions with the dermal papilla. These two models indicated the fact that the homeostasis of the stem cells are regulated through summing activators as well as inhibitors released from both microenvironments (the niche) and the “extra-niche” macro-environment (Fig. 4A).
5. Systemic factors and stem cell homeostasis
5.1. Sex hormone
Sex hormones, including estrogen and testosterone, are thought to possess the ability to modify stem cells due to the presence of estrogen and androgen receptors on those cells [31–36]. 17β-estradiol (E2) can not only attenuate the self renewal of early osteoblast progenitors [37] but also increase the number of endothelial progenitor cells (EPC) to differentiate into endothelial cells and accelerate healing of injured vessels through a caspase-8–dependent anti-apoptotic effect [38]. In addition, mouse mammary stem cells (MaSCs) are highly responsive to steroid hormone signalling, including estrogen and progesterone. Pregnancy resulted in increasing in MaSC numbers probably mediated through paracrine signalling from RANK ligand [39].
During puberty, androgen is definitely the most important hormone in triggering axillary and pubic hair development in both sexes. However, there exists several biological paradoxes in androgen regulation of hair follicles [40]. First, the response of hair follicles to androgen varies significantly. It can stimulate small, thin and colorless vellus hair follicles to become longer, thicker and pigmented hairs in androgen responsive hair follicles, e.g. beard hair. It also elicits no effect on androgen insensitive hairs, such as eyelashes. Alternatively it can function oppositely to reverse the long, thick and pigmented scalp hair to form tiny miniaturized hair which may lead to alopecia. Second, the hair in two androgen-dependent areas: the beard and the axilla, are stimulated to grow by androgens during puberty. However, while beard growth continues at high levels into old age, axillary hair growth is maximal in the mid-twenties and decreases slowly over time to prepubertal levels [40]. These discrepancies might result from the fact that dermal papilla derived from different areas producing hair follicles contain distinct levels of androgen receptors and 5α-reductase as well as 17β-hydroxysteroid dehydrogenase (17β-HSD) which generate different kinds of testosterone metabolites to affect the performance of hair follicles. [41–44].
While alopecia can be caused by keratin mutations, immunological attacks, etc., the major reason for age related alopecia is more a hair growth problem, which is affected by sex hormones. Recent work suggests that hair stem cells from the balding region of androgenic alopecia patients show similar gene expression profile as those from non-balding regions. However, hair germ cells are deficient, suggesting the failure of proper stem cell activation [45]. It is interesting to note that in the aging related alopecia, baldness in males is shown as a region, suggesting the involvement of a region of extrinsic factors in the skin macro-environment. On the other hand, baldness in females manifests as a reduction of hair fiber density, implying the defect is intrinsic to the follicle. More studies will be required to understand these differences.
Sex hormones also affect stem cell activities of other organs. For example, androgen can stimulate hematopoisis through its direct role in activating TERT gene expression and increasing telomerase activity by aromatization and through estrogen receptor-α (ERα) [46]. Besides, both estrogen and testosterone can affect the potential of chondrogenic progenitor cells (CPCs) and the gene expression of ERα, ERβ, and androgen receptor by regulating the expression of Sox9, Runx2, type II collagen, and type I collagen [47]. All of these evidences support the idea that in addition to the niche and “extra-niche” macro-environment, systemic factors are also crucial in manipulating the function of stem cells in hair follicles and some other organs.
5.2 Immune
The Immune system is important in helping organisms fight against pathogens and tumorigenesis. Other than its protective role, a recent study showed that the inflammatory mediator, TAK1, which results in activation of NF-κB via Tnf-α can regulate hair follicle morphogenesis, and is required for anagen induction and maintenance of mature hair follicles [48]. Wounding of skin can also induce hair follicles to regenerate through apoptotic signals regulating kinase 1 (ASK1) dependent recruitment and activation of macrophages[49]. Recent studies also revealed that Tnf-α accelerates wound healing through paracrine mechanisms [50] and receptor activation of NF-κB (RANK) was important in controlling the hair cycle [51]. In addition, RANKL-stimulated bone resorbing osteoclasts could also affect hematopoietic progenitor mobilization by reducing the stem cell niche components SDF-1 (CXCL12), stem cell factor (SCF) and osteopontin along the endosteum [52].
5.3. Nervous system
The nervous system also plays an important role in defining properties of the hair stem cell niche. Neuronal Sonic Hedgehog (Shh) signals to induce Gli1 in neighboring cells to create a perineural stem cell niche within the telogen bulge. Gli1 positive follicle cells not only function as multipotent stem cells to regenerate hair follicles cyclically but also become epidermal stem cells after wounding under the control of the perineural stem cell niche [53]. The finding that peripheral adrenergic signals are necessary for G-CSF induced mobilization of murine progenitor cells also supports the idea that the nervous system is involved in stem cell regulation [54].
In fact, these two major systems, immune and nervous, can work together to modulate stem cell homeostasis. When stress (physiological or pathological) occurs, enhanced release of catecholamines and upregulation of β-adrenergic receptor expression on hematopoietic stems and progenitor cells (HSPCs) is associated with a reduction in SDF-1 levels in the bone marrow (BM) and an increase in the peripheral blood, as well as increased CXCR4 expression in the BM. This response will trigger expansion and activation of osteoclasts, and the release of various proteolytic enzymes to egress HSPCs from the BM to the bloodstream to participate in host defense and organ repair [55]. These observations inspire in us the concept that all the factors, activators or inhibitors; from the niche, “extra-niche macro-environment” or systemic factors, will be integrated within a complicated network to modulate the activity of stem cells; i.e. the input from different aspects will all be “taken into consideration” by stem cells (Fig. 4B).
5.4. Aging
Once birth happens, each organism moves toward further growth, maturation and aging irreversibly. Aging is a physiological process associated with global deterioration of the tissues and impairment of repair as well as regeneration. It remains an unsolved mystery whether stem cells, their niche or their “extra-niche” macro-environment is responsible for this process. The decline of stem cells, including oxidative stress and DNA damage, is by no means the only factor contributing to age related tissue and organ deterioration. A recent study using parabiotic mouse experiments indicated that the niche and systemic factors also act as a major factor in HSPCs aging. The authors discovered that aging induced functional defects in the HSC regulatory niche cells which triggers aging phenotypes in young HSCs and more interestingly, this phenomenon could be reversed by systemic young circulating factors [56]. In addition, exposure of old muscle stem cells (satellite cells) to young serum increases Notch activation and Notch ligand (Delta) expression. Futhermore, heterochronic parabiosis could also stimulate aged hepatocyte proliferation and restore the cEBP-α complex to levels seen in young animals [57,58]. These observations point out the fact that an age-related decline of progenitor cell activity can be modulated by systemic factors. Our unpublished data also revealed that increased macro-environmental inhibitors are leading factors that result in age related alterations of the hair regenerative cycling.
6. External environment modulation
6.1. Circadian rhythm
Circadian rhythms entrained by a light cycle will affect most physiological processes, including sleep, hormone secretion, cell cycle, and immunity in mammals [59]. Robust circadian fluctuations existed in HSCs and their progenitors, peaking 5 h after the initiation of light and reaching a nadir 5 h after darkness. Circulating HSCs and their progenitors fluctuate in antiphase with the expression of the chemokine CXCL12 in the BM microenvironment. The cyclic release of HSCs and expression of Cxcl12 is regulated by core genes of the molecular clock through circadian noradrenaline secretion by the sympathetic nervous system. These adrenergic signals are locally delivered by nerves in the BM, transmitted to stromal cells by the β3-adrenergic receptor, leading to decreased nuclear content of Sp1 transcription factor and the rapid down regulation of Cxcl12. These data indicate that a circadian, neurally driven release of HSCs during the animal’s resting period may promote the regeneration of the stem cell niche and possibly other tissues [60].
Nocturnin (NOC), a circadian-regulated protein, plays a unique role in regulation of mesenchymal stem-cell lineage allocation by modulating PPAR-γ activity through nuclear translocation. These data illustrate a unique mechanism whereby a nutrient responsive gene influences BMSC differentiation, adipogenesis, and ultimately body composition [61].
Clock and Bmal1 genes, which control circadian rhythms, could also regulate the hair stem cell regeneration cycle by delaying anagen progression, by decreasing levels of phosphorylated Rb and by causing a lack of mitotic cells in secondary hair germ cells. The delay in anagen progression is likely due to a block at the G1 phase of the cell cycle in the secondary hair germ cells, as these cells lack phosphorylated Rb and Cdkn1a (p21) is upregulated in Bmal1−/− mice [62]. In addition, the circadian molecular clock could establish heterogeneous populations of epidermal stem cells which have a differential predisposing response to activation or dormancy stimulations. The clock machinery plays an important role in fine-tuning the homeostasis in tissue coexisting with both dormant and active populations of stem cells. Neoplastic transformation will ensue if the balance of the epidermal stem cell clock is altered [63].
6.2. Seasonal influences
Different animals exhibit different levels of robustness in their ability to regenerate hairs depending on their habits (Fig. 5). Animals in cold regions grow warm downy hairs in winter, but lose those in summer. This occurs in horses, cows, dogs, etc. In these animals, secondary hairs are responsible for keeping warmth and their growth is stimulated by change of seasons. How the season affects the initiation of the regenerative hair cycling and the duration of anagen is not completely understood. Earlier studies have suggested that photo-period and / or temperature play an important role in triggering the change. This change could be mediated systemically by changes of neuro-endocrine hormone secretion which may involve prolactin, FSH, and beta-endorphins [64,65]. How the systemic changes affect hair stem cell activity is an issue raised in this review, and the molecular mechanism is yet to be worked out.
The other question is whether the circadian rhythms described in the last section can also have direct local effect on the initiation and duration of regenerative cycling in this normal physiological seasonal change. This remains to be solved.
Although human hair growth is much less robust compared to animals which have to live in the wild, humans also experience seasonal hair shedding [66]. By observing the scalp hair condition from healthy women for 6 years, it was found that the telogen rate is low in winter and high in summer along with a shedding peak by autumn which manifests the fact that these phenomena might exist for the protection of our scalp against cold in winter and midday sun in summer from an evolutionary point of view [67].
7. Conclusion
One fundamental issue of stem cell biology is how stem cells are regulated by both the micro-environment immediately adjacent to stem cells, and by the general macro-environment that reflects the body’s physiological status. While the stem cell niche plays an important role, work from different approaches imply that multiple hierarchical layers control stem cell activity. These multi-layer controls ensure all intrinsic and extrinsic clues are taken into consideration and allow hair stem cells to sum the total regulators (activators / inhibitors) in making the decision to either activate or inhibit stem cells (Fig. 4). There is evidence that similar multi-layer controls also exist that regulate other adult stem cells. For example, the release of HSCs can also be affected by adipose tissue [30] and circadian rhythms [68]. Thus the principles discussed here are likely to be shared commonly by adult stem cell in different organs.
Stem cells bring great hope for tissue regeneration, treating degenerative disorders and even for slowing or reversing the aging process. In order to fulfill this dream, scientists have tried their best to identify and harvest stem cells in various tissues. The wish is to obtain therapeutic applications by manipulating the functions and abilities of stem cells. However, the number of stem cells in tissues is very small and it is difficult to definitively identify them or isolate them or expand their population for applicational usage. This hurdle makes using stem cells in a treatment strategy tenuous at present. We have learned that the niche, extra-niche macro-environment, and systemic factors as well as external environment are all key regulators for the homeostasis of stem cells. The more we know about how the homeostasis of stem cells is regulated, the closer we will be to overcome diseases associated with tissue degeneration or wounding. Developing a better understanding of the mechanisms through which multiple layers of the environment can modulate stem cell activities will enable us to target some or multiple layers of these environments as a new direction to regulate stem cells for therapeutic applications (Fig. 4B).
Acknowledgments
CCC is supported by NSC100-2314-B-075-044, Taipei Veterans General Hospital (Industry-Government-Academic Cooperation projects, grant R11004), YEN TJING LING MEDICAL FUNDATION. CMC is supported by grants from NIAMS RO1-AR42177, AR60306, AR47364. We thank Dr. R.B. Widelitz and de la Cruz D for help in manuscript preparation.
Biographies
 Chih-Chiang Chen got his M.D. degree from National Yang Ming University, Taipei, Taiwan in 1998. He received his residence training at Taipei Veterans General Hospital and become an attending physician in 2006. He did basic research about aging process of hair follicle stem cell under the supervision of Prof. Cheng-Ming Chuong in the Department of Pathology, University of Southern California, USA, from 2008 to 2010. His research specialty is focusing on macro-environmental regulation of stem cells under physiological and pathological conditions, and its application.
Chih-Chiang Chen got his M.D. degree from National Yang Ming University, Taipei, Taiwan in 1998. He received his residence training at Taipei Veterans General Hospital and become an attending physician in 2006. He did basic research about aging process of hair follicle stem cell under the supervision of Prof. Cheng-Ming Chuong in the Department of Pathology, University of Southern California, USA, from 2008 to 2010. His research specialty is focusing on macro-environmental regulation of stem cells under physiological and pathological conditions, and its application.
 Dr. Cheng-Ming Chuong received his M.D. from National Taiwan University in 1978. He then obtained his Ph.D. from The Rockefeller University in 1983. Later he moved to the University of Southern California in 1987 where he works on the development and regeneration of feather, teeth, and hairs. He is currently a professor of pathology and also serves as the Chairman of the Graduate Committee in the Department of Pathology. In 2008, he was elected to the Academia Sinica, the National Academy equivalent of Taiwan. Dr. Chuong directs the Laboratory of Tissue Development and Engineering (http://www-hsc.usc.edu/~cmchuong/) in the Department of Pathology, USC. Using the ectoderm as a Rosetta stone, his laboratory has learned from nature how to mold stem cells into different ectodermal organs during development, evolution and stem cell engineering.
Dr. Cheng-Ming Chuong received his M.D. from National Taiwan University in 1978. He then obtained his Ph.D. from The Rockefeller University in 1983. Later he moved to the University of Southern California in 1987 where he works on the development and regeneration of feather, teeth, and hairs. He is currently a professor of pathology and also serves as the Chairman of the Graduate Committee in the Department of Pathology. In 2008, he was elected to the Academia Sinica, the National Academy equivalent of Taiwan. Dr. Chuong directs the Laboratory of Tissue Development and Engineering (http://www-hsc.usc.edu/~cmchuong/) in the Department of Pathology, USC. Using the ectoderm as a Rosetta stone, his laboratory has learned from nature how to mold stem cells into different ectodermal organs during development, evolution and stem cell engineering.
Footnotes
The authors have no conflict of interest to declare
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the hamatopopietic stem cell. A hypothesis Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Oh IH, Kwon KR. Concise Review: Multiple Niches for Hematopoietic Stem Cell Regulations. Stem Cells. 2010;28(7):1243–9. doi: 10.1002/stem.453. [DOI] [PubMed] [Google Scholar]
- 3.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–99. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Xie T. Stem Cell Niche: Structure and Function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida S. Stem cells in mammalian spermatogenesis. Dev Growth Differ. 2010;52(3):311–7. doi: 10.1111/j.1440-169X.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 7.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem Cell Function, Self-Renewal, and Behavioral Heterogeneity of Cells from the Adult Muscle Satellite Cell Niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Kuang S, Gillespie MA, Rudnicki MA. Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell Stem Cell. 2008;2(1):22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Chuong CM, Randall VA, Widelitz RB, Wu P, Jiang TX. Physiology. Physiological regeneration of skin appendages. (Minor revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 11.Hughes MW, Wu P, Jiang T-X, Lin S-J, Dong C-Y, Li A, et al. In search of the Golden Fleece: Unraveling principles of morphogenesis by studying the integrative biology of skin appendages. Integrative Biology. 2011;3:388–407. doi: 10.1039/c0ib00108b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huelsken J, Vogel R, Erdmann B, et al. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 13.Reddy S, Andl T, Bagasra A, Lu MM, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 14.Lowry WE, et al. Defining the impact of b-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher EO. Hair growth on skin transplants in the immature albino rat. Anat Rec. 1936;64:161–171. [Google Scholar]
- 16.Plikus MV, Chuong CM. Complex Hair Cycle Domain Patterns and Regenerative Hair Waves in Living Rodents. J Invest Dermatol. 2008;128:1071–80. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340–4. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plikus MV, Widelitz RB, Maxson R, Chuong CM. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol. 2009;53(5–6):857–68. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332(6029):586–9. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte Lineage Cells Contribute to the Skin Stem Cell Niche to Drive Hair Cycling. Cell. 2011;146(5):761–71. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahoda CA, Christiano AM. Niche Crosstalk: Intercellular Signals at the Hair Follicle. Cell. 2011;146(5):678–81. doi: 10.1016/j.cell.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa M, Ozawa K, Tojo A, Yoshikubo T, Okano A, et al. Changes in hematopoiesis-supporting ability of C3H10T1/2 mouse embryo fibroblasts during differentiation. Blood. 1993;81:1184–1192. [PubMed] [Google Scholar]
- 23.Corre J, Barreau C, Cousin B, et al. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol. 2006;208:282–288. doi: 10.1002/jcp.20655. [DOI] [PubMed] [Google Scholar]
- 24.Belaid-Choucair Z, Lepelletier Y, Poncin G, Thiry A, Humblet C, et al. Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells. 2008;26:1556–1564. doi: 10.1634/stemcells.2008-0068. [DOI] [PubMed] [Google Scholar]
- 25.Miharada K, Hiroyama T, Sudo K, Danjo I, Nagasawa T, Nakamura Y. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol. 2008;215:526–537. doi: 10.1002/jcp.21334. [DOI] [PubMed] [Google Scholar]
- 26.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 27.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 28.Zhang Y, Harada A, Bluethmann H, Wang JB, Nakao S, et al. Tumor necrosis factor (TNF) is a physiologic regulator of hematopoietic progenitor cells: increase of early hematopoietic progenitor cells in TNF receptor p55-deficient mice in vivo and potent inhibition of progenitor cell proliferation by TNF alpha in vitro. Blood. 1995;86:2930–2937. [PubMed] [Google Scholar]
- 29.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 30.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone marrow adipocytes as negative regulators of the hematopoietic microenvironment. Nature. 2009;460(7252):259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O’Driscoll J, et al. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J Investig Dermatol Symp Proc. 2003;8:100–3. doi: 10.1046/j.1523-1747.2003.12181.x. [DOI] [PubMed] [Google Scholar]
- 32.Thornton MJ, Nelson LD, Taylor AH, Birch MP, Laing I, Messenger AG. The modulation of aromatase and estrogen receptor alpha in cultured human dermal papilla cells by dexamethasone: a novel mechanism for selective action of estrogen via estrogen receptor beta? J Invest Dermatol. 2006;126:2010–8. doi: 10.1038/sj.jid.5700344. [DOI] [PubMed] [Google Scholar]
- 33.Chang CY, Hsuuw YD, Huang FJ, Shyr CR, Chang SY, Huang CK, Kang HY, Huang KE. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertil Steril. 2006;85 (Suppl 1):1195–203. doi: 10.1016/j.fertnstert.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Han HJ, Heo JS, Lee YJ. Estradiol-17beta stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol. 2006;290(4):C1067–75. doi: 10.1152/ajpcell.00222.2005. [DOI] [PubMed] [Google Scholar]
- 35.Hong SH, Nah HY, Lee YJ, Lee JW, Park JH, Kim SJ, et al. Expression of estrogen receptor- alpha and -beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells. 2004;18(3):320–5. [PubMed] [Google Scholar]
- 36.Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex Steroids and Stem Cell Function. Mol Med. 2008;14(7–8):493–501. doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, et al. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17 beta-estradiol. J Clin Invest. 2001;107(7):803–12. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107(24):3059–65. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 39.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signaling. Nature. 2010;465(7299):798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 40.Randall VA. Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol. 2007;18(2):274–85. doi: 10.1016/j.semcdb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g beard) contain more androgen receptors than those from non-balding areas of scalp. J Endocrinol. 1992;3:141–7. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- 42.Hamada K, Thornton MJ, Laing I, Messenger AG, Randall VA. The metabolism of testosterone by dermal papilla cells cultured from human pubic and axillary hair follicles concurs with hair growth in 5 alpha-reductase deficiency. J Invest Dermatol. 1996;106(5):1017–22. doi: 10.1111/1523-1747.ep12338582. [DOI] [PubMed] [Google Scholar]
- 43.Hibberts NA, Howell AE, Randall VA. Dermal papilla cells from human balding scalp hair follicles contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 44.Ando Y, Yamaguchi Y, Hamada K, Yoshikawa K, Itami S. Expression of mRNA for androgen receptor, 5α-reductase and 17β-hydroxysteroid dehydrogenase in human dermal papilla cells. Br J Dermatol. 1999;141:840–5. doi: 10.1046/j.1365-2133.1999.03156.x. [DOI] [PubMed] [Google Scholar]
- 45.Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121(2):613–22. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236–43. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koelling S, Miosge N. Sex Differences of Chondrogenic Progenitor Cells in Late Stages of Osteoarthritis. Arthritis Rheum. 2010;62(4):1077–87. doi: 10.1002/art.27311. [DOI] [PubMed] [Google Scholar]
- 48.Sayama K, Kajiya K, Sugawara K, Sato S, Hirakawa S, Shirakata Y, et al. Inflammatory mediator TAK1 regulates hair follicle morphogenesis and anagen induction shown by using keratinocyte-specific TAK1-deficient mice. PLoS One. 2010;5(6):e11275. doi: 10.1371/journal.pone.0011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osaka N, Takahashi T, Murakami S, Matsuzawa A, et al. ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J Cell Biol. 2007;176(7):903–9. doi: 10.1083/jcb.200611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131(7):1559–67. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 51.Duheron V, Hess E, Duval M, Decossas M, Castaneda B, Klöpper JE, et al. Receptor activator of NF-kappaB (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc Natl Acad Sci U S A. 2011;108(13):5342–7. doi: 10.1073/pnas.1013054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006 Jun;12(6):657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 53.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8(5):552–65. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress from Bone Marrow. Cell. 2006;124(2):407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 55.Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem Cell Regulation via Dynamic Interactions of the Nervous and Immune Systems with the Microenvironment. Cell Stem Cell. 2008;3(5):484–92. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463(7280):495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- 57.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 58.Gopinath SD, Rando TA. Stem Cell Review Series: Aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7(4):590–8. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 59.Méndez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16(4):235–42. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 61.Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-γ nuclear translocation. Proc Natl Acad Sci U S A. 2010;107(23):10508–13. doi: 10.1073/pnas.1000788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, et al. Circadian Clock Genes Contribute to the Regulation of Hair Follicle Cycling. PLoS Genet. 2009;5(7):e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480(7376):209–14. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 64.Dicks P, Russel AJ, Lincoln GA. The role of prolactin in the reactivation of hair follicles in relation to moulting in cashmere goats. J Endocrinol. 1994;143(3):441–8. doi: 10.1677/joe.0.1430441. [DOI] [PubMed] [Google Scholar]
- 65.Lincoln GA, Baker BI. Seasonal and photoperiod-induced changes in the secretion of alpha-melanocyte-stimulating hormone in Soay sheep: temporal relationships with changes in beta-endorphin, prolactin, follicle-stimulating hormone, activity of the gonads and growth of wool and horns. J Endocrinol. 1995;144(3):471–81. doi: 10.1677/joe.0.1440471. [DOI] [PubMed] [Google Scholar]
- 66.Randall VA, Ebling FJ. Seasonal changes in human hair growth. Br J Dermatol. 1991;124(2):146–51. doi: 10.1111/j.1365-2133.1991.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 67.Kunz M, Seifert B, Trüeb RM. Seasonality of hair shedding in healthy women complaining of hair loss. Dermatology. 2009;219(2):105–10. doi: 10.1159/000216832. [DOI] [PubMed] [Google Scholar]
- 68.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3(4):364–6. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]