Abstract
The growth of new blood vessels after ischemic injury requires endothelial cells (ECs) to divide and proliferate, and the E2F transcription factors are key regulators of the genes responsible for cell-cycle progression; however, the specific roles of individual E2Fs in ECs are largely unknown. To determine the roles of E2F2 and E2F3 in EC proliferation and the angiogenic response to ischemic injury, hind-limb ischemia was surgically induced in E2F2−/− mice, endothelial-specific E2F3-knockout (EndoE2F3Δ/Δ) mice, and their littermates with wild-type E2F2 and E2F3 expression. Two weeks later, laser-Doppler perfusion measurements, capillary density, and endothelial proliferation were significantly greater in E2F2−/− mice and significantly lower in EndoE2F3Δ/Δ mice than in their littermates, and EndoE2F3Δ/Δ mice also developed toe and limb necrosis. The loss of E2F2 expression was associated with increases in the proliferation and G1/S-phase gene expression of isolated ECs, while the loss of E2F3 expression led to declines in these parameters. Thus E2F2 impairs, and endothelial E2F3 promotes, the angiogenic response to peripheral ischemic injury through corresponding changes in EC cell-cycle progression.
Keywords: E2F, Endothelial cells, Proliferation, Angiogenesis, Ischemia
1. Introduction
The growth of new blood vessels after Ischemic injury requires that quiescent endothelial cells (ECs) be stimulated to divide and proliferate, so the E2F transcription factors, which regulate genes involved in cell-cycle control, have been investigated for the design of therapies to manipulate vascular growth [1, 2]. The PREVENT trials investigated the use of a nonspecific E2F decoy oligonucleotide to limit proliferation of vascular smooth muscle cells and the subsequent overgrowth of autologous vein grafts in patients undergoing arterial bypass surgery [3]. The treatments were unsuccessful, however, perhaps because individual members of the E2F family can both promote and prevent cell-cycle progression and, consequently, the nonspecific oligonucleotide likely inhibited both activating and repressive E2F family members [4]. Similarly, the successful development of E2F-targeted angiogenic therapies will likely require a thorough understanding of the specific regulatory role associated with each individual E2F factor, rather than the E2F family as a whole.
Three of the eight known E2F transcription factors, E2F1, E2F2, and E2F3, are considered “activating” E2Fs, because they are believed to stimulate the expression of genes that promote DNA synthesis and the G1/S cell-cycle transition [5]. However, the results presented in a previous report from members of our laboratory indicate that endogenous E2F1 expression represses, rather than activates, vascular growth and EC proliferation after ischemic injury in mice [6]. Here, we continue to characterize the roles of “activating” E2F transcription factors in the angiogenic response to vascular injury by performing experiments with mice and ECs that are deficient in E2F2 or E2F3 expression.
2. Materials and Methods
2.1 Mice
E2F2+/− and E2F3fl/fl mice were provided by Dr. Gustavo Leone (Ohio State University) [7], and Tie2-Cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All breeding, maintenance, and surgical procedures were approved by the Institutional Animal Care and Use Committee of Northwestern University and performed at the University’s Center for Comparative Medicine. Mouse genotypes were determined via polymerase chain reaction (PCR) with tail DNA.
2.2 Hind-limb ischemia (HLI) model and assessments
Hind-limb ischemia was surgically induced in 10- to 12-week-old male mice as previously described [6, 8], and perfusion measurements were performed with a Laser Doppler imaging system (Moor Instruments, Wilmington, DE, USA). Mice received intraperitoneal injections of 5-bromodeoxyuridine (BrdU) on Days 12 and 13 after injury (1 mg administered every 12 hours, for a total of 4 injections) and were euthanized on Day 14. A portion of mice also received an intravenous injection of Lectin (50 μL, Vector Laboratories, Inc., Burlingame, CA, USA) 10 minutes before euthanasia. Tissues were fixed in 4% paraformaldehyde and serially sectioned; then, immunohistochemical staining was performed with fluorescent anti-Lectin (Vector Laboratories, Inc.) and anti-BrdU (Abcam, Cambridge, MA, USA) antibodies as described previously [6]. Capillary densities were assessed in sections of gastrocnemius muscles stained for the expression of lectin, and 3 sections per ischemic limb and 6 fields per section were examined [6, 8].
2.3 Isolation of primary ECs
Primary ECs were isolated from mouse lung tissues as previously described [9]. Briefly, tissues were minced and digested with collagenase and dispase, and then the mixture was passed through a 100-μm cell strainer to obtain single-cell suspensions. Red blood cells, granulocytes, nonvital cells, and cell debris were removed via density centrifugation with Histopaque-1.083 (Sigma-Aldrich Corp., St. Louis, MO, USA); then, the cells were immunostained with CD31-PE, sorted to ≥95% purity via flow cytometry, and cultured in EBM-2 medium (Lonza). The cells were used in subsequent experiments before passage 5.
2.4 In-vitro proliferation and gene expression
The isolated ECs were transduced with adenoviruses coding for GFP alone or both GFP and Cre (Vector BioLabs, Philadelphia, PA, USA), synchronized by incubation in EBM-2 medium containing 0.1% FBS for 24 hours, and then proliferation was stimulated by the addition of EBM-2 medium supplemented with 5% FBS. The incorporation of 5-ethynyl-2′-deoxyuridine (EdU) was measured with a commercially available Click-iT™ EdU flow cytometry assay kit (Invitrogen, Carlsbad, CA, USA) as directed by the manufacturer’s instructions. RNA extraction and real-time reverse transcriptase polymerase chain reaction (RT-PCR) were performed as described previously [6, 9]. Primer sequences are listed in the Online Supplemental Table S1.
2.5 Statistical analysis
All values are expressed as mean ± SEM. Comparisons between two means were evaluated for significance via the unpaired Student’s t test. A P value of less than 0.05 is considered significant.
3. Results
3.1 E2F2 and endothelial specific E2F3 deficient mice
E2F2−/− mice were generated as described previously [9]. Mice that lacked endothelial E2F3 expression were generated by breeding E2F3fl/fl mice with Tie2-Cre (EndoE2F3+/+) mice, and then crossing the female E2F3fl/+ and male Tie2-Cre;E2F3fl/+ offspring. Mice homozygous for the Cre-induced, endothelial-specific E2F3-knockout mutation (EndoE2F3Δ/Δ mice) were born at approximately 8% of the expected frequency, which is similar to the live-birth rate of mice homozygous for the global E2F3-knockout mutation [10] and suggests that the expression of E2F3 by ECs is essential for embryonic development. The surviving EndoE2F3Δ/Δ mice developed normally from birth through adulthood.
3.2 Recovery of ischemic limbs is improved by the loss of global E2F2 expression and impaired by the loss of endothelial E2F3 expression
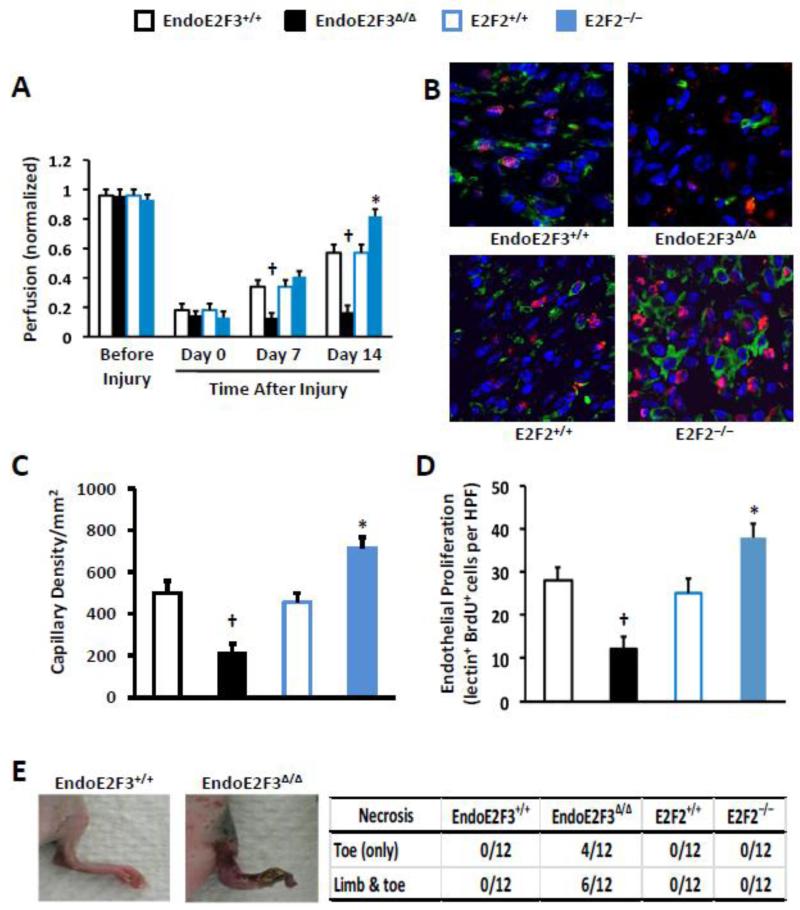
Perfusion was significantly greater in E2F2−/− mice on Day 14 after injury, and significantly lower in EndoE2F3Δ/Δ mice on Days 7 and 14 after injury, than in their littermates with wild-type levels of E2F2 and endothelial E2F3 expression (Figure 1A). The loss of E2F2 expression also led to improvements in capillary density (Figure 1B-C) and endothelial proliferation on Day 14 (Figures 1B and 1D), while the EndoE2F3Δ/Δ mutation was associated with declines in both parameters and with toe and limb necrosis in 83% and 50% of animals, respectively, on Day 7 (Figure 1E).
Figure 1. Vascular recovery is improved in E2F2−/− mice and impaired in EndoE2F3Δ/Δ mice.
HLI was surgically induced in E2F2−/− mice and their wild-type littermates (E2F2+/+) and in EndoE2F3Δ/Δ mice and their littermates with wild-type endothelial E2F3 expression (EndoE2F3+/+). (A) Perfusion was evaluated before injury and for up to 14 days afterward via Laser Doppler imaging; measurements in the ischemic limb were normalized to measurements in the uninjured contralateral limb. (n=6 for EndoE2F3Δ/Δ group at Days 7 and 14; n=12 for the rest groups and time points). (B-D) The animals were injected with BrdU and lectin before sacrifice and then tissue sections were stained with anti-BrdU and anti-lectin antibodies after sacrifice (B). Capillary density was quantified as the number of lectin+ cells (C), and endothelial proliferation was quantified as the number of cells positive for both lectin (red) and BrdU incorporation (green) (D). n=6 randomly selected animals in each group. Six sections per mouse and 3 high power fields (HPFs) per section were evaluated and averaged for each animal. (E) The number of mice with evidence of toe or limb necrosis was evaluated 7 days after the induction of HLI. n=12 mice per group. †P<0.01 versus EndoE2F3+/+; *P<0.01 versus E2F2+/+.
3.3 Endothelial-cell proliferation and G1/S-phase gene expression are enhanced by declines in E2F2 expression and reduced by declines in E2F3 expression
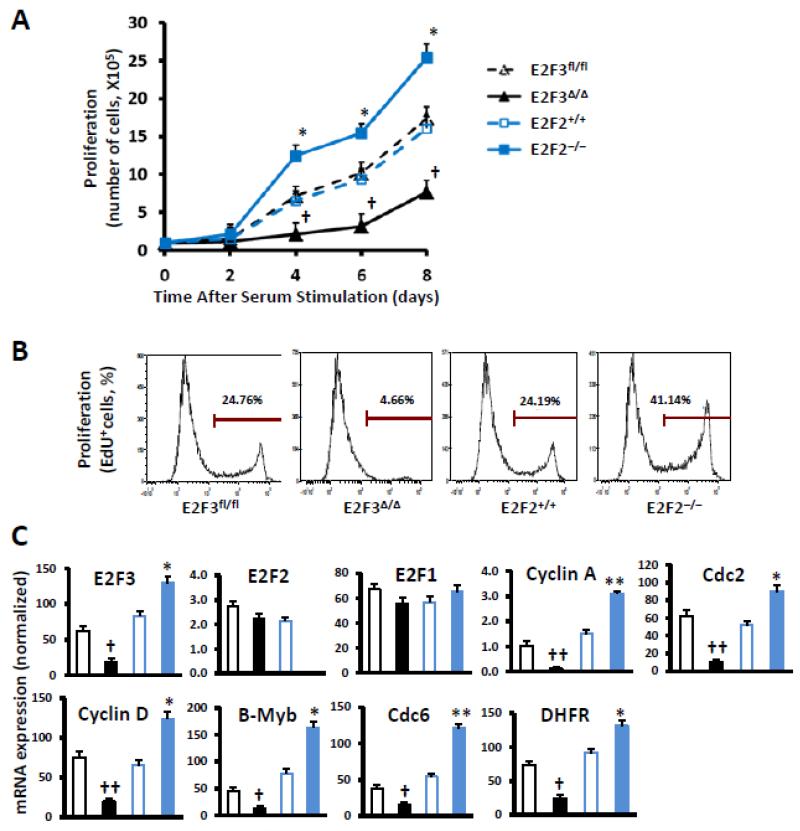
The effect of declines in E2F2 or E2F3 expression on proliferation was also evaluated in ECs isolated from E2F2−/− mice and their wild-type littermates that had been transduced with a vector coding for GFP expression and in E2F3fl/fl ECs that had been transduced with a vector coding for GFP alone (i.e., E2F3fl/fl ECs) or with a vector coding for both GFP and Cre expression (i.e., E2F3Δ/Δ ECs), to knock out the expression of E2F3. Cells were starved to quiescence by serum deprivation and then re-stimulated by the addition of serum; cell counts were significantly higher for E2F2−/− ECs than for E2F2+/+ ECs, and significantly lower for E2F3Δ/Δ ECs than for E2F3fl/fl ECs, on Days 4, 6, and 8 after serum addition (Figure 2A). These results were corroborated by flow-cytometry analyses of EdU incorporation and by real-time PCR analyses of the expression of E2F-targeted genes that are associated with cell-cycle progression. Proliferating (i.e., EdU+) cells were more common in the E2F2−/− population, and less common in the E2F3Δ/Δ population, than in cells with wild-type E2F2 or E2F3 expression (Figure 2B), and the expression of Cyclin A, Cyclin D, Cdc2, Cdc6, B-Myb, and dihydrofolate reductase was enhanced in E2F2−/− cells and impaired in E2F3Δ/Δ cells (Figure 2C); the E2F2-knockout mutation was also associated with a significant increase in E2F3 expression. Notably, similar changes in EdU incorporation and the expression of cell-cycle genes were also observed in wild-type ECs in which E2F2 or E2F3 expression had been knocked down by siRNA technology (Online Supplemental Figure S1), and knockdown of E2F2 or E2F3 expression did not affect the tube-formation and migration activities of ECs nor their expression of well-recognized angiogenic growth factors including vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and basic fibroblast growth factor (bFGF or FGF-2) (Online Supplemental Figures S2-3).
Figure 2. Proliferation and G1/S-phase gene expression are enhanced in E2F2−/− ECs and impaired in EndoE2F3Δ/Δ ECs.
ECs were isolated from the lungs of E2F2−/− mice, their wild-type littermates (E2F2+/+), and E2F3fl/fl mice; then, the E2F2−/− and E2F2+/+ ECs were transduced with an adenovirus coding for GFP expression, and the E2F3fl/fl ECs were transduced with an adenovirus coding for GFP expression (E2F3fl/fl) or with an adenovirus coding for both GFP and Cre (E2F3Δ/Δ) to knock out E2F3 expression. (A) Quiescent cells were stimulated with serum addition, and cell counts were monitored for 8 days (n=4). (B) After serum stimulation for 16 h, cells were treated with EdU and cultured for additional 4 h; then, proliferation was evaluated via flow cytometry and quantified as the proportion of cells stained positively for EdU incorporation (representative results from 2 analyses per experimental group). (C) The expression of E2F2, E2F3, and the G1/S-phase genes Cyclin A, Cyclin D, Cdc2, Cdc6, B-Myb, and dihydrofolate reductase (DHFR) was determined via quantitative RT-PCR and normalized to18S rRNA levels (n=4). †P<0.01, ††P<0.001 versus E2F3fl/fl; *P<0.05, **P<0.01 versus E2F2+/+.
4. Discussion
E2F transcription factors are crucially involved in cell-cycle regulation, and E2F1, E2F2, and E2F3 are often classified as “activating” E2F members because they are believed to promote cell-cycle progression and proliferation [7, 11, 12]. However, previously published experiments from our laboratory demonstrate that E2F1 inhibits angiogenesis and the proliferation of ECs after ischemic injury by suppressing expression of the pro-angiogenic cytokines vascular endothelial growth factor and placental growth factor [6]. Here, we show that perfusion, capillary density, endothelial-cell proliferation, and the expression of G1/S-phase genes in ECs are enhanced by the loss of E2F2 expression and reduced by the endothelial-specific loss of E2F3 expression. In a separate study with a mouse model of myocardial infarction, endothelial E2F3 deletion by VE-cadherin-Cre expression led to a decreased EC growth at ischemic myocardium and an increased infarct size (unpublished observations). Thus, of the three “activating” E2F family members, only E2F3 appears to promote the proliferation, cell-cycle progression, and angiogenic activity of ECs.
The mechanism responsible for the upregulation of G1/S-phase genes in E2F2−/− ECs is unknown; however, the loss of E2F2 expression significantly increased E2F3 levels in cultured ECs, and E2F3 appeared to be a much more prominent activator of cell-cycle progression in our experiments with ECs than in previous studies with other cell types [5, 13-15]. Our ongoing studies also suggest that loss of E2F2 expression could enable E2F3 to bind E2F2-targeted DNA regulatory elements and activate genes that are normally suppressed (or weakly activated) by E2F2 (Online Supplemental Figure S4). Whether E2F2 also suppresses proliferation and cell-cycle progression in other vascular-cell lineages (e.g., vascular smooth muscle cells) will be investigated in subsequent studies. One of the limitations of our study is associated with the use of E2F2−/− mice in which E2F2-deficiency is not limited to endothelial compartment. Whether the loss of E2F2 in other cell types within the ischemic limb also contribute to the accelerated angiogenesis and blood flow recovery remains to be determined in our future investigations.
In conclusion, the results presented here show that E2F2 and E2F3, two members of the “activating” subfamily of E2F transcription factors, have distinctly different roles in the angiogenic response to ischemic injury. Thus, the successful development of E2F-targeted angiogenic therapies will likely require knowledge of the specific regulatory role played by each E2F factor, rather than the E2F family as a whole.
Supplementary Material
Acknowledgments
We thank Dr. Gustavo Leone (The Ohio State University) for providing E2F2 knockout and E2F3 floxed mice. We thank Dr. Douglas W. Losordo (Northwestern University) for insightful suggestions.
This work was supported by National Institute of Health grants (HL093439 and HL113541 to G.Q.) and American Heart Association grants (0430135N to G.Q. and 10POST4360009 to J.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- [1].Qin G, Losordo DW. E2F transcription factors in cardiovascular physiology. In: Yoshida K, editor. Control of Cellular Physiology by E2F Transciption Factors. Research Signpost; Kerala: 2008. pp. 341–66. [Google Scholar]
- [2].Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nature medicine. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- [3].Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr., Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- [4].Giangrande PH, Zhang J, Tanner A, Eckhart AD, Rempel RE, Andrechek ER, et al. Distinct roles of E2F proteins in vascular smooth muscle cell proliferation and intimal hyperplasia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12988–93. doi: 10.1073/pnas.0704754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen H-Z, Tsai S-Y, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–97. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qin G, Kishore R, Dolan CM, Silver M, Wecker A, Luedemann CN, et al. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11015–20. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–62. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- [8].Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. The Journal of experimental medicine. 2006;203:153–63. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou J, Zhu Y, Cheng M, Dinesh D, Thorne T, Poh KK, et al. Regulation of vascular contractility and blood pressure by the E2F2 transcription factor. Circulation. 2009;120:1213–21. doi: 10.1161/CIRCULATIONAHA.109.859207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Humbert PO, Verona R, Trimarchi JM, Rogers C, Dandapani S, Lees JA. E2f3 is critical for normal cellular proliferation. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- [11].DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739–48. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- [12].Ebelt H, Zhang Y, Kampke A, Xu J, Schlitt A, Buerke M, et al. E2F2 expression induces proliferation of terminally differentiated cardiomyocytes in vivo. Cardiovascular research. 2008;80:219–26. doi: 10.1093/cvr/cvn194. [DOI] [PubMed] [Google Scholar]
- [13].Saavedra HI, Wu L, de Bruin A, Timmers C, Rosol TJ, Weinstein M, et al. Specificity of E2F1, E2F2, and E2F3 in mediating phenotypes induced by loss of Rb. Cell Growth Differ. 2002;13:215–25. [PubMed] [Google Scholar]
- [14].Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- [15].Ebelt H, Hufnagel N, Neuhaus P, Neuhaus H, Gajawada P, Simm A, et al. Divergent siblings: E2F2 and E2F4 but not E2F1 and E2F3 induce DNA synthesis in cardiomyocytes without activation of apoptosis. Circulation research. 2005;96:509–17. doi: 10.1161/01.RES.0000159705.17322.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.