Abstract
Purpose
Cranberry proanthocyanidins have been identified as possible inhibitors of Escherichia coli adherence to uroepithelial cells. However, little is known about the dose range of this effect. Furthermore, it has not been studied directly in the urogenital system. To address these issues we tested the effect of a cranberry powder and proanthocyanidin extract on adherence of a P-fimbriated uropathogenic E. coli isolate to 2 new urogenital model systems, namely primary cultured bladder epithelial cells and vaginal epithelial cells.
Materials and Methods
E. coli IA2 was pre-incubated with a commercially available cranberry powder (9 mg proanthocyanidin per gm) or with increasing concentrations of proanthocyanidin extract. Adherence of E. coli IA2 to primary cultured bladder epithelial cells or vaginal epithelial cells was measured before and after exposure to these products.
Results
Cranberry powder decreased mean adherence of E. coli IA2 to vaginal epithelial cells from 18.6 to 1.8 bacteria per cell (p <0.001). Mean adherence of E. coli to primary cultured bladder epithelial cells was decreased by exposure to 50 μg/ml proanthocyanidin extract from 6.9 to 1.6 bacteria per cell (p <0.001). Inhibition of adherence of E. coli by proanthocyanidin extract occurred in linear, dose dependent fashion over a proanthocyanidin concentration range of 75 to 5 μg/ml.
Conclusions
Cranberry products can inhibit E. coli adherence to biologically relevant model systems of primary cultured bladder and vaginal epithelial cells. This effect occurs in a dose dependent relationship. These findings provide further mechanistic evidence and biological plausibility for the role of cranberry products for preventing urinary tract infection.
Keywords: bladder, vagina, cranberry, Escherichia coli, urinary tract infections
Urinary tract infection is a common bacterial infection in women of all ages, resulting in considerable morbidity and health care costs. A recent population based study showed that the risk of UTI during a lifetime is at least 60% and the cost of diagnosing and treating these infections during a 20-year period exceeded $25 billion.1 Antimicrobial resistance has become a prevalent and clinically significant problem in the last decade in community acquired UTI, in which it was previously absent or not clinically relevant.2 Thus, new methods to prevent and treat UTIs are increasingly important.
Cranberry (Vaccinium macrocarpon), a native North American fruit, has traditionally been used to prevent acute UTIs. Although scientific evidence for the efficacy of cranberry for preventing acute UTIs is mounting, additional studies demonstrating biologically plausible mechanisms for the actions of cranberry are needed.3
Early research suggested that cranberry juice was effective due to its ability to increase urinary acidity through the excretion of hippuric acid. However, further studies showed only transient increases in urine acidity after cranberry juice consumption of up to 4 l daily.4 A more likely mechanism by which cranberry might prevent UTI is by inhibiting the adherence of Escherichia coli to the bladder epithelium. Early in vitro studies suggested that the fructose contained in cranberry juice as well as in orange and pineapple fruit juices was responsible for inhibiting the adherence of type 1 fimbriated E. coli to various cell types.5 In contrast, a non-dialyzable compound present only in cranberry juice inhibited the adherence of P-fimbriated E. coli.6 Although fructose decreased the adherence of E. coli to epithelial cells in vitro, there is no clinical evidence to correlate the ingestion of dietary fructose with the risk of UTI. Thus, further investigation has focused on identifying the nondialyzable compound, which was subsequently shown to be A-type PAC.7 PACs are condensed tannins found in various plants that function as defense compounds against microbes and predatory animals. Unlike most other fruits, cranberry contains a relatively high proportion of A-type PACs.8,9
Most in vitro and clinical studies published to date are strongly supportive of a role for cranberry products for preventing UTI but some limitations remain to be addressed. Preliminary studies demonstrated that pre-incubation of uropathogenic E. coli with cranberry juice or isolated cranberry PAC extract decreased adherence of the organisms to exfoliated epithelial cells collected from urine.6,10 However, epithelial cells collected in this manner are 90% dead and composed of a mixed population of squamous epithelial cells derived from the distal urethra and vagina with only a minority of transitional cells from the bladder.11 More recently a T24 cell line was used to demonstrate inhibition of adherence of uropathogenic E. coli isolates that were grown in urine collected after the ingestion of cranberry juice cocktail.12 A bioassay was also used to demonstrate anti-adherence activity in urine after the ingestion of sweetened, dried cranberries.13 However, there is still a paucity of data on the dose-response characteristics of cranberry products in laboratory or clinical studies. Cranberry solids in particular have not been well studied for the direct inhibition of E. coli adherence. Demonstration of this activity would further support the findings reported in clinical studies of decreased UTI rates with cranberry solids.3,14
To address these issues we tested the effects of 2 cranberry products, including a commercially available capsule containing 300 mg Vaccinium macrocarpon without other additives and the putative active component of cranberry, that is purified cranberry PAC extract. The efficacy and dose range of cranberry PAC extract on adherence of a representative isolate of uropathogenic E. coli was studied using a new model system, primary cultured bladder transitional epithelial cells. The effect of the cranberry capsule was studied using a second model system of primary cultured VECs. We also used a previously described MRHA assay to evaluate the dose-response of PAC extract and compare it to the BEC adherence assay.6
MATERIALS AND METHODS
Bacteria
We performed standard bacterial adherence assays using E. coli IA2, a wild type uropathogenic isolate expressing class 2 P-fimbriae that adheres to exfoliated VECs, and primary cultured VECs and BECs reproducibly as a representative isolate.11,15 E. coli HB101, an isolate lacking fimbrial adhesins that does not adhere to epithelial cells, served as a negative control.16
Cranberry Compounds
We used 2 cranberry products in adherence inhibition assays, including a commercially available, whole cranberry product and a purified cranberry based extract. To choose a commercially available, whole cranberry based product that was representative of preparations available to consumers we visited a local health food cooperative. We investigated all cranberry products available on the shelves and chose a freeze-dried whole cranberry powder prepared in a capsule without further additives or alterations, containing 300 mg organic cranberry (Vaccinium macrocarpon). PACs were isolated from the cranberry powder using a previously described method and the concentration was determined to be 9 mg PAC per gm powder or approximately 2.7 mg PAC per capsule.8
The second cranberry preparation we tested was a PAC extract from cranberries. Purified PAC extract was prepared from fresh cranberry fruit by reverse phase and adsorption chromatography, as previously described,6,7 and stored under nitrogen at 4C to prevent oxidation.
The cranberry capsules were maintained at room temperature in the original packaging and were protected from heat and light until ready for use. For adherence assays the cranberry capsule contents (powder) were removed and re-suspended in DPBS at a concentration of 20 mg/ml, filtered at 0.22 and 2.5 μm, and adjusted to pH 7.0 with 1 M NaOH before use. Similarly a stock solution of PAC extract was prepared, serially diluted in DPBS to pH 6.7 and filtered at 0.22 μm.
Epithelial Cells
Primary cultures of human BECs and VECs were established from discarded surgical specimens obtained in accordance with human subject regulations at University of Washington using standard tissue culture techniques.17 For bacterial adherence assays early passage cells were seeded to 4-chambered Lab-Tek™ glass slides and grown to 90% confluence.
Adherence Assays
E. coli IA2 was grown overnight on sheep blood agar plates at 37C, scraped off and collected in PBS, washed and resuspended to an optical density600 of 0.5 (corresponding to 4 × 108 cfu/ml) in 1.0 ml of various concentrations of the cranberry solutions and pre-incubated while rocking at room temperature for 30 minutes. Primary cultured BECs or VECs were washed 3 times with DPBS. E. coli suspended in the cranberry test solutions were then added and incubated for 1 hour at 37C or at room temperature. Cells treated with DPBS alone served as additional controls. Assays were then washed 6 times with DPBS, and fixed and stained using a modified Wright-Giemsa stain (Diff-Quik®). Adherence was measured as the average number of bacteria attached to the first 50 BECs or VECs counted using light microscopy.
Hemagglutination
A 30 μl drop of each cranberry test solution (25, 50 or 100 μg/ml PACs) was incubated with 10 μl bacterial suspension on a 24-well polystyrene plate for 10 minutes at room temperature on a rotary shaker, as previously described. Freshly drawn human RBCs (A1, Rh+) and sheep RBCs, used to detect P-fimbriation, were suspended (3%) in PBS and added separately (10 μl drops) to test solutions with 2% mannose solution serving as a control. Solutions were incubated for 20 minutes on a rotary shaker at room temperature and evaluated microscopically for the ability of the cranberry solutions to prevent MRHA.
Statistical Methods
Data were analyzed using SAS® software. E. coli adherence to BECs were plotted and regressed against PAC concentrations and their log transformed values to analyze the correlation between these 2 variables. The mean, 95% CI, t test and linear regression methods were used to summarize and analyze data.
RESULTS
Inhibition of E. Coli Adherence to BECs
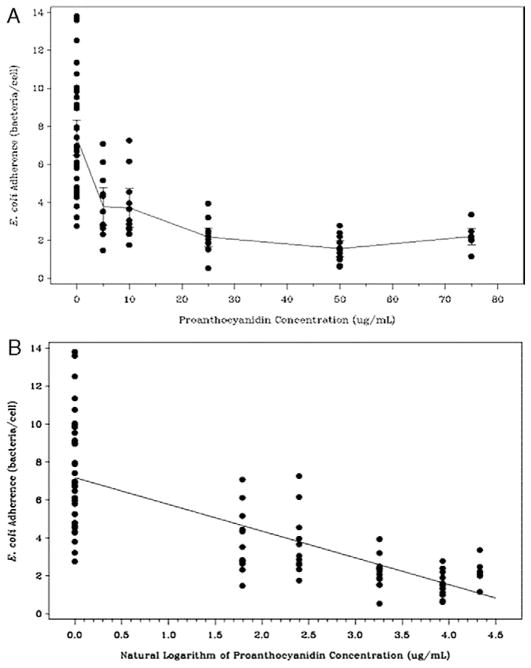
The adherence of E. coli IA2 to BECs was decreased from a mean of 6.9 bacteria per cell at baseline (no exposure to PAC extract) to 2.2 bacteria per cell following pre-incubation with 25 μg/ml purified cranberry PAC extract (t test p <0.001). Figure 1 shows a photograph of a representative example of a bacterial adherence assay using BECs and E. coli IA2 with and without pre-incubation in PAC solution at 25 μg/ml. At 50 μg/ml PAC extract mean adherence was further decreased to 1.6 bacteria per cell. Inhibition of adherence of E. coli by PACs occurred in linear, dose dependent fashion over a concentration range of PACs of 5 to 75 μg/ml. We noted apparent damage to bacterial and mammalian cells at PAC concentrations above 75 μg/ml. Mammalian cells appeared slightly crenated at 100 μg/ml PACs and markedly damaged at higher concentrations in pilot experiments that were not pursued further. Bacteria developed a flocculated appearance after exposure to concentrations of PACs above 75 μg/ml, possibly due to autoagglutination of the bacteria.
Fig. 1.
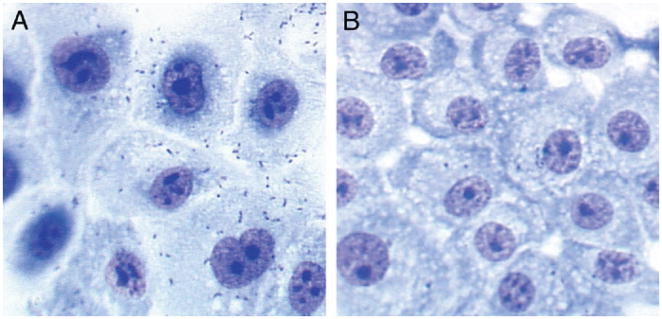
Primary BECs incubated at 37C with E. coli IA2 alone (A) or with E. coli IA2 pre-incubated with 25 μg/ml cranberry PAC extract (B) show decreased bacterial adherence from 6.9 to 2.2 bacteria per cell (t test p <0.001).
A scatterplot of the results of the adherence inhibition assays (0 to 75 μg/ml) demonstrated statistically significant inhibition of E. coli adherence mediated by exposure to PACs (p <0.0001, fig. 2, A). Linear regression modeling of log transformed PAC concentrations showed that the inhibition of E. coli adherence by PACs was dose dependent and highly statistically significant (p <0.0001, fig. 2, B).
Fig. 2.
Scatterplots of E. coli adherence by PAC concentration overlaid with mean and 95% CI of E. coli adherence (A), and log transformed PAC concentrations overlaid with linear regression line (p <0.001) (B). ug, μg.
Inhibition of E. Coli Adherence to VECs
The commercially available cranberry powder also inhibited E. coli adherence to primary cultured urogenital epithelial cells in vitro. Pre-incubation of E. coli with the cranberry powder (3 mg/ml in DPBS, 9 mg PACs per gm) significantly decreased mean adherence of E. coli IA2 to VECs from 18.6 to 1.8 bacteria per cell (independent samples t test p <0.001).
Inhibition of MRHA Mediated by E. Coli IA2
We tested the effect on MRHA of human and sheep RBCs by pre-incubation of E. coli IA2 in PACs for the concentration range of 25 to 100 μg/ml. MRHA of human or sheep RBCs was 100% inhibited by pre-incubation of E. coli IA2 in PAC concentrations of 50 μg/ml and 50% inhibited at 25 μg/ml.
DISCUSSION
We investigated the ability of 2 cranberry derived products, including a commercially available cranberry capsule and a cranberry PAC extract, to inhibit the adherence of uropathogenic E. coli using new model systems of primary cultured BECs and VECs. Recent studies identified PAC as a component of cranberry that specifically inhibits E. coli adherence, as measured by the proxy of inhibiting P-fimbrial mediated MRHA.7 Our findings demonstrated that a PAC extract purified from cranberry specifically inhibited E. coli adherence to primary cultured BECs in dose dependent fashion. These results expand previous studies of the anti-adherence properties of cranberry PACs since living normal epithelial cells were used rather than exfoliated squamous cells collected from urine or a T24 continuous cell line. This allowed a more physiologically accurate examination of the effects of cranberry PACs. It also appears that this assay may be more sensitive for detecting inhibitory activity than the MRHA method since 50% inhibition of bacterial adherence was seen at a PAC concentration of approximately 5 μg/ml using the direct adherence assay compared to 25 μg/ml with the MRHA method.
To our knowledge no previous published studies evaluated the effects of cranberry products on adherence to VECS. Adherence of E. coli to VECs is important in the early stages of UTI initiation and preventing bacterial adhesion to VECs may aid in circumventing infection in the bladder. Although to our knowledge it is unknown whether cranberry compounds are excreted into vaginal secretions, our results show that there is the potential for cranberry compounds to prevent adherence of E. coli to vaginal as well as to bladder epithelium, potentially preventing UTI by interrupting colonization before the organisms ascend to the bladder.
The anti-adherence activity observed in these in vitro experiments provides mechanistic support for clinical studies demonstrating an effect of cranberry products for preventing UTI in women.14,18–20 Little is known about the PAC content of the capsules used in the published clinical studies to date and, thus, direct comparison with our in vitro findings is not possible. However, even if only 1% of the 2.7 mg of PACs found in 1 of the 300 mg capsules that we tested were active in the bladder, our data suggest that an in vivo inhibitory effect could be achieved. Likewise since it is probable that only a small proportion of PAC is ultimately excreted in urine, it is encouraging that concentrations as low as 5 to 75 μg PAC extract demonstrated an in vitro inhibitory effect on E. coli adherence. It is likely that these concentrations of PAC are easily achieved with 1 commercially available 240 ml glass of cranberry juice cocktail containing 35 to 83 mg PAC depending on the brand and percent of cranberry in the juice.8 Direct correlation between the in vitro and clinical effects of increasing doses of cranberry products remains an important research goal.4
CONCLUSIONS
This in vitro method for demonstrating the anti-adherence activity of cranberry or PACs on E. coli adherence to specific primary cultured urogenital epithelial cells provides a useful and clinically relevant model for further clinical and laboratory studies of preventing UTI with cranberry products. As antibiotic resistance becomes an increasing concern, alternative strategies such as cranberry consumption become more important for preventing UTIs. We tested a P-fimbriated E. coli isolate in these studies. Future studies will address the ability of PACs to inhibit the adherence of E. coli isolates with other fimbriae and virulence characteristics as well as potential inhibitory effects on the adherence of other uropathogenic bacteria to further elucidate the mechanisms and effects of cranberry. Further studies of the pharmaco-kinetics, dosing, metabolism and excretion of cranberry derived compounds, especially PACs, are warranted. Ultimately the in vitro effects of PACs and other cranberry derived products must be correlated with the clinical efficacy of these products in vivo to further our understanding of how cranberry may act in preventing acute UTIs.
Acknowledgments
Purified cranberry based extract was prepared and PACs were isolated at the laboratory of Dr. A. Howell. Pacita Roberts assisted with statistical analysis and data interpretation.
Supported by the University of Washington Medical Student Research Training Program (MC), HD39365 (AS), DK53369 (AS) and DK02660 (KG).
Abbreviations and Acronyms
- DPBS
Dulbecco’s PBS
- MRHA
mannose resistant hemagglutination
- PAC
proanthocyanidin
- PBS
phosphate buffered saline
- RBC
red blood cell
- UTI
urinary tract infection
- VEC
vaginal epithelial cell
Footnotes
Study received University of Washington institutional review board approval.
References
- 1.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113:5S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K. Emerging antibiotic resistance in urinary tract pathogens. Infect Dis Clin North Am. 2003;17:243. doi: 10.1016/s0891-5520(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 3.Jepson RG, Mihaljevic L, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2004:CD001321. doi: 10.1002/14651858.CD001321.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Raz R, Chazan B, Dan M. Cranberry juice and urinary tract infection. Clin Infect Dis. 2004;38:1413. doi: 10.1086/386328. [DOI] [PubMed] [Google Scholar]
- 5.Zafriri D, Ofek I, Adar R, Pocino M, Sharon N. Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eucaryotic cells. Antimicrob Agents Chemother. 1989;33:92. doi: 10.1128/aac.33.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell AB, Vorsa N, Der Marderosian A, Foo L. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med. 1998;339:1085. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- 7.Foo L, Lu Y, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prods. 2000;63:1225. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 8.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 10.Sobota AE. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol. 1984;131:1013. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton AE, Fennell CL, Coder DM, Wobbe CL, Roberts PL, Stamm WE. Precise and rapid assessment of Escherichia coli adherence to vaginal epithelial cells by flow cytometry. Cytometry. 2002;50:31. doi: 10.1002/cyto.10046. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino P, Agniel R, David K, Templer C, Gaillard JL, Denys P, et al. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: a double-blind randomized placebo-controlled crossover trial. World J Urol. 2006;24:21. doi: 10.1007/s00345-005-0045-z. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg JA, Newmann SJ, Howell AB. Consumption of sweetened dried cranberries versus unsweetened raisins for inhibition of uropathogenic Escherichia coli adhesion in human urine: a pilot study. J Altern Complement Med. 2005;11:875. doi: 10.1089/acm.2005.11.875. [DOI] [PubMed] [Google Scholar]
- 14.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558. [PubMed] [Google Scholar]
- 15.Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982;38:739. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhen M, Tenhunen J, Vaisanen-Rhen V, Pere A, Baga M, Korhonen T. Fimbriation and P-antigen recognition of Escherichia coli strains harbouring mutated recombinant plasmids encoding fimbrial adhesins of the uropathogenic E. coli strain KS71. J Gen Microbiol. 1986;132:71. doi: 10.1099/00221287-132-1-71. [DOI] [PubMed] [Google Scholar]
- 17.Cilento BG, Freeman MR, Schneck FX, Retik AB, Atala A. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J Urol. 1994;152:665. doi: 10.1016/s0022-5347(17)32676-9. [DOI] [PubMed] [Google Scholar]
- 18.Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 19.Haverkorn M, Mandigers J. Reduction of bacteriuria and pyuria using cranberry juice. JAMA. 1994;272:590. doi: 10.1001/jama.272.8.590a. [DOI] [PubMed] [Google Scholar]
- 20.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]