Abstract
Objective
The genetic contributions to the multifactorial disorder osteoarthritis (OA) have been increasingly recognized. Our goal was to use OA-related biomarkers of severity and disease burden as quantitative traits to identify genetic susceptibility loci for OA.
Methods
In a large multigenerational extended family (CARRIAGE family, n=350), we measured five OA-related biomarkers: HA (hyaluronan), COMP (cartilage oligomeric matrix protein), PIIANP (type IIA collagen N-propeptide), CPII (type II procollagen carboxy-propeptide), and C2C (type II collagen cleavage neoepitope). SNP markers (6,090) covering the whole genome were genotyped using the Illumina HumanLinkage-12 BeadChip. Variance components analysis as implemented in SOLAR was used to estimate heritabilities of the quantitative traits, and to calculate two-point and multi-point LOD scores using a polygenic model.
Results
Four of the five biomarkers showed significant heritability (p<0.01 age and sex adjusted h2r: PIIANP 0.57, HA 0.49, COMP 0.43, C2C 0.30). Fourteen of the 19 loci with multi-point LOD scores >1.5 were near or overlapped previously reported OA susceptibility loci. Four of these loci were identified by more than one biomarker. The maximum multi-point LOD scores for the heritable quantitative biomarker traits were LOD 4.3 for PIIANP (chromosome 8p23.2); LOD 3.2 for COMP (chromosome 8q11.1); LOD 2.0 for HA (chromosome 6q16.3); LOD 2.0 for C2C (chromosome 5q31.2).
Conclusions
We report the first evidence of genetic susceptibility loci identified by OA-related biomarkers in an extended family. Serum concentrations of PIIANP, HA, COMP and C2C have substantial heritable components and identified several genetic loci potentially contributing to the genetic diversity of OA.
Keywords: whole-genome scan, osteoarthritis, biomarkers, quantitative trait, linkage, heritability
Introduction
Osteoarthritis (OA) is the most common joint disorder worldwide and the most common cause of disability in Western countries with significant socioeconomic consequences (1). Although many studies have shown that OA has a strong genetic component (2–4), with an estimated heritability ranging from 39 to 74% based on the pattern of joint involvement (5), the genetic and phenotypic heterogeneity of OA present challenges in the ongoing attempt to identify the genetic contributions to this complex disease (6). Over the last decade the whole-genome linkage scan approach has led to mapping of a number of susceptibility loci for OA. These findings were all based on phenotyping by radiograph, clinical examination, or clinical history (total joint replacement) (7–15). However, the hallmark of OA is cartilage loss; reflected on radiographs as the joint-space width, it is a fairly late stage manifestation of disease with poor sensitivity for OA initiation (16). An alternative approach, the use of intermediate biomarker traits, has been used successfully for genetic analyses of other diseases [anti-cyclic citrullinated peptide in rheumatoid arthritis (17) and YKL-40 in asthma (18)], but never in OA. Existing OArelated biomarkers have the potential to detect disease earlier than is possible by radiograph (19), and to reflect not only OA severity but also total body burden of disease (20). Moreover, OA is clearly not only a cartilage disorder (21, 22) but rather a disease of the whole joint organ consisting of cartilage, bone, synovium, meniscus and tendon.
We hypothesized that we could replicate known OA susceptibility genes, and identify additional OA-related genes and shared genetic determinants through monitoring of the turnover of the whole joint organ through a biomarker approach, thereby potentially providing data that could augment existing knowledge of OA etiologic pathways and progression. Based on the strength of previous validation evidence (23), we chose to analyze five serum OA-related biomarkers in this study: hyaluronan (HA), cartilage oligomeric matrix protein (COMP), type IIA collagen N-propeptide (PIIANP), type II procollagen carboxy-propeptide (CPII), and type II collagen neoepitope (C2C). Each of these markers has data to support their classification (24, 25) in at least two categories of the BIPED (26) biomarker classification scheme (Burden of disease, Investigational, Prognostic, Efficacy of therapy, and Diagnostic markers): HA – categories B, P; COMP – categories D, B, P; PIIANP – categories B, P, D; CPII – categories P, E, D; and C2C – categories P, E, D.
For these analyses, we studied a unique extended family, the CARRIAGE (CARolinas Region Interaction of Aging, Genes and Environment) family. The CARRIAGE family is one of the most extensively pedigreed existing families in the United States comprising 10 generations with 3327 pedigreed members, and originating from one founder born in the 1700s. The ethnic origin of this family is primarily African and Native American. Linkage analysis in this single founder lineage provides many advantages for mapping complex traits due to the reduction of genetic heterogeneity and confounding by population stratification (27). This family was selected for study because of its size and strong genealogical records and was not selected for a particular condition or disease, including OA. Nevertheless, as we have previously documented, this cohort had a prevalence of clinical hand OA of 17% and clinical knee OA of 30% based on American College of Rheumatology criteria (28). This prevalence of knee OA is modestly elevated over that of a Dutch population but the hand OA prevalence is consistent with estimates for individuals of mean age 55 years (the mean age of the ascertained CARRIAGE family members) (29). We have also observed an association of hand OA phenotypes in this cohort with serum OA-related biomarkers (30). Here, we report the first evidence for genetic linkage in OA using these biomarker traits in this large extended family.
Methods
Family Cohort
Pedigree data for the CARRIAGE family were obtained from three sources: 1) a book detailing the genealogy of the descendents of this forefather; 2) family history questionnaires distributed by mail and completed during three family reunions over 4 years (2002, 2004 and 2006); and 3) genealogy data collected by a family member. These data were combined using Progeny© software (www.progenygenetics.com) for genetic database and pedigree management. We were able to successfully document 3327 family members from the nine generations, with 2795 family members completely connected to the original founder. This family came to be studied in the context of health fairs conducted at several large family reunions. Detailed ascertainment of 350 family members (mean age 54 years) was accomplished during the three family reunions. Further details are provided in previous reports (28, 30). Written informed consent was obtained from each participant, and the study was approved by the Duke Institutional Review Board. All information and work was conducted under a Federal Certificate of Confidentiality to ensure the privacy of each participating member’s clinical and genetic data.
Analyses of Serum Biomarkers Related to OA
Serum was isolated, aliquoted and stored within 4 hours of blood collection at −80°C until biomarker analyses were performed. Serum biomarker analyses were repeated as necessary for samples with a >15% coefficient of variation (CV). We measured five OA-related serum biomarkers: two type II collagen biomarkers indicative of collagen synthesis (PIIANP, CPII); a type II collagen biomarker indicative of collagen degradation (C2C); a glycoprotein biomarker (COMP) originating from cartilage, synovium and tendon, which is associated with spine and knee OA (31) and impacted by synovitis (31, 32); and a high molecular weight polysaccharide (HA), an excellent indicator of total body burden of OA including osteophyte and cartilage loss (20). When serum for a given individual was available from more than one reunion, the most recent sample was used.
PIIANP (type IIA collagen N-propeptide), a marker of a fetal form of collagen II recapitulated in OA, was measured by competitive ELISA (LINCO Research, St. Charles, MO, USA). The minimum detection limit is 17.2 ng/ml. Intra-assay and inter-assay CVs were < 6.6% and < 7.8%, respectively. CPII (type II procollagen carboxy-propeptide), a marker of the adult form of collagen II synthesis, was measured by competitive ELISA (IBEX, Montreal, Quebec, Canada). The minimum detection limit is estimated to be 35.1 ng/ml. Intra-assay and inter-assay CVs were < 3.7% and < 9.1%, respectively. C2C (type II collagen cleavage neoepitope), a competitive ELISA (IBEX) was used to measure the neoepitope produced by the cleavage of type II collagen (C2C). The minimum detection limit is reported to be 7.3 ng/ml. Intra-assay and inter-assay CVs are < 2.4% and < 9.5%, respectively. COMP (cartilage oligomeric matrix protein) was measured with an in-house sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (33, 34), using monoclonal antibodies 17C10 (epitope in the epidermal growth factor–like domain) and 16F12 (epitope in the NH2-terminal domain) against human COMP (35). The minimum detection limit is 120 ng/ml. Intra-assay and inter-assay CVs were < 5.8% and < 8.7%, respectively. HA (hyaluronan) was measured by enzyme-linked binding protein assay (Corgenix Inc. Westminster, Colorado, USA). The assay uses enzymeconjugated hyaluronic acid binding protein (HABP) from bovine cartilage to specifically capture HA from human serum. The minimum detection limit is established at 10 ng/ml. Intra-assay and inter-assay CVs were < 4.7% and < 7.0%, respectively.
DNA isolation and quality control
DNA was isolated from buffy coat (derived from 5 ml fresh EDTA blood) (n= 347) or 4 ml saliva (n=3). Saliva samples were obtained by mail when available blood was insufficient for DNA isolation but sufficient for serum biomarker analyses. DNA was extracted from blood and saliva using the PUREGENE DNA Purification Kit (Gentra Systems Inc, Minneapolis, Minn) per the manufacturer's instructions. DNA concentration was quantified by Thermo Scientific NanoDrop™ spectrophotometry (Wilmington,DE). DNA quality was verified on 0.8% agarose gels (0.8 g Seakem, 5 µl Ethidium Bromide in 100ml 1x Tris-acetate-EDTA buffer), run at 90 V for one hour) using 0.5 µl aliquots of each sample. A HindIII digest of lambda DNA (New England Biolabs, N3012S) was used as a reference ladder. DNA was scored 0 – 5 with score ≥4 indicating that a single high molecular weight DNA band was clearly visible, and score <4 indicating that DNA degradation had occurred and the single high molecular band was accompanied by a visible smear of smaller fragments. Samples with a score ≥4 were used for whole-genome genetic mapping assays (n=349).
Whole-genome genotyping
The Infinium Human Linkage-12 Genotyping BeadChip (Illumina, San Diego, CA) was used for whole-genome genotyping by fluorescence-based methods. The BeadChip included 6,090 SNP markers with an average spacing of 0.58 cM across the genome. Two blinded samplings of CEPH controls were genotyped for each plate as quality controls to insure accuracy for these assays. The genotype assignments were determined by Illumina® Beadstudio Genotyping (GT) module software. A total of 98.8% (6,015 SNPs) of the 6,090 SNPs met quality control benchmarks for the accuracy of genotype assignments based on the duplicated genotypes and for genotyping efficiency based on proportion of samples with high quality genotypes. Two blood-derived DNA samples were dropped from the analysis because of low call rate (0.961–0.971). There was no difference in the success of genotyping DNA derived from saliva and blood. The 3 saliva-derived DNA samples had high call rates (average call rate 0.998), which did not differ from the 345 blood-DNA derived samples (average call rate 0.999). The distance from the telomere was estimated using the deCODE map.
Statistical analysis
Variance components analysis implemented in the Sequential Oligogenic Linkage Analysis Routines (SOLAR, SFBR/NIH, San Antonio, TX)(36) was used for linkage analysis. Heritability (H2r) was estimated by fitting a polygenic variance components model as implemented in SOLAR. Both twp-point and multi-point genome-wide linkage scans using five OA-related biomarkers as quantitative traits were performed. Linkage between each of the biomarker traits and marker loci was tested by maximum-likelihood methods, as advocated for multigenerational pedigrees, adjusted by age and gender, and according to the concepts of the variance components approach (37).
The variance component method partitions each biomarker trait into unobserved QTL, residual additive genetic, and residual non-genetic components. The phenotypic variancecovariance matrix consists of parameters of the kinship coefficient and the identity-by-descent (IBD) probability at a given marker locus between each pair of individuals (38). Due to the complexity of the CARRIAGE pedigree, the IBD probabilities were computed using the Markov chain Monte Carlo algorithm as implemented in Loki (39). The Loki IBD files were converted into SOLAR format for subsequent linkage analysis of the full pedigree. The whole-genome linkage used IBD values calculated for 6,015 SNP markers. Biomarker data for all family members (including unaffected members) were included in the QTL analysis with the exception of seven participants: two with known rheumatoid arthritis to avoid confounding by other forms of arthritis; and five individuals younger than 25 years of age to avoid confounding by high cartilage biomarker concentrations due to cartilage growth plate turnover from skeletal immaturity. OA-biomarker concentrations were logarithmically transformed to achieve a normal distribution for SOLAR analyses. For those biomarkers (CPII, HA) with residual kurtosis (RK) >1, outliers were removed that exceeded 3 standard deviations from the mean. The resulting residual kurtosis (RK) for each biomarker was <0.7. Due to low trait standard deviations, the values for CPII, C2C and COMP were multiplied by a scaling factor (2.6, 3.8 and 2.4 respectively) to increase the standard deviation above 0.5. The polygenic model that the QTL was built upon was adjusted for age and sex. For all biomarkers, linkage was considered significant if the LOD score exceeded 3.0. Results of LOD > 1.5, suggestive of linkage, are also reported (12).
Results
Heritability of biomarkers
The ascertainment was available to any one attending one of the reunions and serum biomarker analyses and genotyping were performed on all individuals for whom we had biological samples; therefore there was no selection for individuals with OA. Heritability estimates, which reflect effect sizes, were performed with age- and sex-adjustment for each OA-related biomarker. After removing the outliers, biomarker and genetic marker data were available on 333 family members for COMP, PIIANP and C2C; 330 family members for CPII; and 327 family members for HA. The highest residual heritability (after the removal of age and gender effects) was observed for PIIANP (57%), followed by HA (49%), COMP (43%), and C2C (30%), all p<0.01; CPII was not significantly heritable (3%). The four significantly heritable biomarker traits (Table 1), were used subsequently as quantitative traits in genome-wide linkage analyses using two-point and multiple-point models.
Table 1.
Heritability (h2) of osteoarthritis-related serum biomarker quantitative traits.
| OA endophenotypes |
Number of samples analyzed |
Mean + SD of Ln biomarker§ |
h2* |
p value for heritability |
|---|---|---|---|---|
| COMP | 333 | 7.39 ± 0.45 | 0.43 | 0.001 |
| HA | 327 | 3.60 ± 0.86 | 0.49 | 0.001 |
| PIIANP | 333 | 7.17 ± 0.52 | 0.57 | <0.001 |
| CPII | 330 | 7.06 ± 0.37 | 0.03 | 0.4 |
| C2C | 333 | 5.35 ± 0.27 | 0.30 | 0.01 |
Adjusted for age and sex
COMP=cartilage oligomeric matrix protein, HA=hyaluronan, PIIANP=type IIA collagen N-propeptide, CPII=type II procollagen carboxy-propeptide, C2C=neoepitope from cleavage of CII SD= standard deviation. Heritability and p value were calculated using SOLAR.
Biomarker concentrations were reported in ng/ml prior to natural log transformation.
Genome-wide linkage analyses
Two-point linkage analysis
A total of 39 markers with LOD >1.5 were identified by two-point linkage analysis (Table 2). One marker exceeded a LOD score of 3. The maximum LOD (3.1) was obtained at rs2780701 (chromosome 9q22.2) for PIIANP. The next highest LOD score (2.7) was found at rs1563796 (chromosome 4q13.1) for PIIANP. For COMP and HA, the maximum LOD scores were 2.23 at rs221924 (chromosome 14q24.2) and 1.79 at rs1020782 (chromosome 1q25.3), respectively. No LOD scores >1.5 were achieved by two-point linkage with C2C.
Table 2.
SNPs showing evidence for linkage (two-point LOD >1.5) in the CARRIAGE Family.
| OA endo phenotypes |
Chromosome | Location (cM) |
Genetic Marker |
Peak LOD |
Previously reported OA candidate genes near these regions* |
|---|---|---|---|---|---|
| PIIANP | 1 | 133.27 | rs1246194 | 1.79 | COL11A1 |
| 2 | 167.91 | rs964176 | 1.58 | TNFAIP6, FAP | |
| 4 | 34.5 | rs1325107 | 1.73 | SOD3 | |
| 53.76 | rs10023150 | 1.71 | SOD3 | ||
| 76.25 | rs1563796 | 2.65 | IGFBP7, ADAMTS3 | ||
| 7 | 98.06 | rs473880 | 1.56 | CD36 | |
| 179.01 | rs6953751 | 1.88 | |||
| 8 | 1.69 | rs763869 | 1.70 | ||
| 2.4 | rs4242539 | 1.66 | |||
| 7.79 | rs3849827 | 1.82 | |||
| 9 | 88.03 | rs729958 | 2.52 | CTSL, ASPN, OGN | |
| 94.51 | rs2780701 | 3.09 | CTSL, ASPN, OGN | ||
| 95.55 | rs1316268 | 1.72 | CTSL, ASPN, OGN | ||
| 100.39 | rs6478437 | 1.67 | CTSL, ASPN, OGN | ||
| 127.84 | rs1013324 | 1.70 | EDG2 | ||
| 128.7 | rs4679 | 1.93 | |||
| 128.74 | rs1571586 | 2.23 | |||
| 136.55 | rs913275 | 1.86 | |||
| 138.31 | rs1220789 | 1.65 | |||
| 154.29 | rs2989726 | 1.86 | |||
| 14 | 52.37 | rs1950209 | 1.55 | ESR2 | |
| 15 | 132.59 | rs2949 | 1.78 | AGC1 | |
| 16 | 38.48 | rs1389504 | 1.58 | IL4R | |
| 41.19 | rs724307 | 1.62 | IL4R | ||
| 57.05 | rs1843609 | 1.75 | IL4R | ||
| 57.1 | rs11647994 | 1.59 | IL4R | ||
| 57.85 | rs17734120 | 1.63 | |||
| 17 | 10.04 | rs149245 | 1.76 | ||
| 15.04 | rs7221818 | 1.76 | |||
| 33.94 | rs2240519 | 1.56 | |||
| COMP | 14 | 69.55 | rs221924 | 2.23 | ESR2, DIO2 |
| 16 | 14.53 | rs1035564 | 1.56 | ||
| 18 | 40.13 | rs1893495 | 2.22 | ||
| HA | 1 | 181.52 | rs1020782 | 1.79 | PTGS2, PLA2G4A |
| 6 | 64.36 | rs722269 | 1.76 | IL-17A, IL-17F, COL11A2, HLA | |
| 101.6 | rs1133503 | 1.63 | |||
| 146.82 | rs583341 | 1.54 | ESR1 | ||
| 8 | 132.31 | rs7814955 | 1.61 | TNFRSF11B | |
| 19 | 63.42 | rs4805201 | 1.56 | TGFB1 |
ADAMTS3=ADAM metallopeptidase with thrombospondin type 1 motif, 3; AGC1=aggrecan 1; ASPN=aspirin; CD36=platelet glycoprotein 4; COL11A1=collagen, type XI, alpha 1; COL11A2=collagen, type XI, alpha 2; CTSL=cathepsin L; DIO2=Type II iodothyronine deiodinase; EDG2=endothelial differentiation, lysophosphatidic acid (LPA) GPCR, 2; ESR1=estrogen receptor 1; ESR2=estrogen receptor 2; FAP=fibroblast activation protein, alpha; IGFBP7=insulin-like growth factor binding protein 7; IL4R=interleukin 4 receptor; IL17A=interleukin 17A; IL17F=interleukin 17F; OGN=osteoglycin; PLA2G4A=phospholipase A2, group IVA; PTGS2=prostaglandin-endoperoxide synthase 2; SOD3=superoxide dismutase 3, extracellular; TGFB1=transforming growth factor, beta 1; TNFAIP6=tumor necrosis factor, alpha-induced protein 6; TNFRSF11B=tumor necrosis factor receptor superfamily, member 11b; LOD=Logarithm of Odd;
Previously reported OA candidate genes which are less than 10cM from the SNP (48–51); We defined significant linkage as LOD≥3 and depict these results in bold lettering.
Multi-point linkage analysis
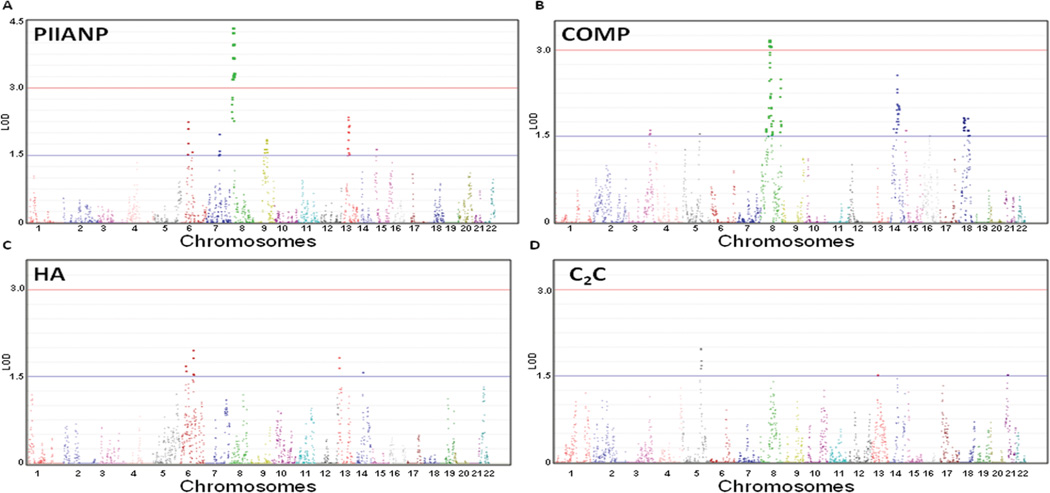
Results of multipoint analysis of the genome-wide linkage scan are plotted separately for the four highly heritable OA-related biomarkers (Figure 1). A total of 23 loci (from 19 separate non-overlapping regions) with LOD >1.5 were identified (Table 3). Two significant linkage peaks (LOD>3) were obtained for chromosome 8, with PIIANP and COMP as quantitative traits (Figure 2A). The highest LOD score (4.33) was obtained using PIIANP, yielding linkage to chromosome 8p23.2 (near marker rs3849827). The next most significant LOD score (3.18) was obtained using COMP, yielding linkage to chromosome 8q11.1 (near marker rs7826304). Another high LOD score (2.5) was obtained using COMP yielding linkage to chromosome 8q24.2 at 149cM (near marker rs2282).
Figure 1. Quantitative trait loci (QTL) mapping for the 4 highly heritable OA-related biomarkers in 22 chromosomes (multipoint LOD scores).
A. PIIANP (type IIA collagen N-propeptide); B. COMP (cartilage oligomeric matrix protein); C. HA (hyaluronan); D. C2C (neoepitope from cleavage of CII); significant linkage if LOD >3.0 and suggestive of linkage if LOD >1.5. All figure panels were generated with HaploView software.
Table 3.
Regions of linkage (multipoint LOD >1.5) to quantitative traits in CARRIAGE Family with citation of studies reporting OA associations that overlap these regions.
| OA endophenotype |
Chromo- some |
Multipoint LOD |
Location of peak LOD score in cM |
1-LOD interval (cM) |
Overlap ping biomarkers |
Previously reported OA candidate genes in these region*(cm from peak) |
Previous reported OA linkages overlapping these regions |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Peak LOD |
Distance (cM) |
Population | Ref | |||||||
| PIIANP | 6 | 2.25 | 50 | 47–52 | HA |
HFE(0.1), HLA DR-2/4, TNF(1.3), COL11A2(2.4) |
Female Hip | 4.8 | 53–56 | UK | (40, 48) |
| 7 | 1.97 | 98 | 96–102 | CD36(5.4) | |||||||
| 8 | 4.33 | 8 | 5–15 | Hand JSN | 1.57 |
8.3– 21.3 |
UK | (13) | |||
| 9 | 1.85 | 98 | 72–108 |
CTSL(9.9), ASPN(2.1) ,OGN(2.2) ,LPAR1(17) |
Hand JSN-sum | 2.3 | 76 | USA | (11,48,51) | ||
| 13 | 2.35 | 50 | 45–57 | C2C | LRCH1(0.3) | ||||||
| 15 | 1.63 | 9 | 0–14 | COMP | |||||||
| COMP | 3 | 1.61 | 179 | 173–183 | |||||||
| 5 | 1.55 | 127 | 121–129 | ||||||||
| 8 | 3.18 | 62 | 60–70 | Hand U-Dip | 2.56 | 41.5–79.4 | UK | (13) | |||
| 8 | 2.50 | 149 | 145–155 | WISP1(4.1) | (43) | ||||||
| 14 | 1.64 | 40 | 29–52 | Hand U-Dip | 1.44 | 40.5–47.5 | UK | (13) | |||
| 14 | 2.57 | 66 | 59–82 | HA |
ESR2(2.2), DIO2(14.7) ,FLRT2(17.7), GPX2(1.7), CALM1(26.5) |
Hand U-JSN | 2.64 | 48–57 | UK | (13,49,52) | |
| 15 | 1.60 | 6 | 2–20 | PIIANP | |||||||
| 16 | 1.51 | 50 | 45–68 | IL4R(3.5) | Early-onset Hip |
2.6 | 28–47 | Iceland | (53) | ||
| Female Hip | 1.7 | 46 | UK | (14) | |||||||
| Hand U-JSN | 2.64 | 48.5–57.8 | UK | (13) | |||||||
| 18 | 1.82 | 39 | 36–49 | ||||||||
| 18 | 1.81 | 72 | 65–91 | Hand OST | 1.34 | 71–85 | UK | (13) | |||
| Knee OA | 2.41 | 60.1–86.1 | US/UK | (54) | |||||||
| C2C | 5 | 1.98 | 139 | 133–144 | SLC26A2(14.8) | (55) | |||||
| 13 | 1.51 | 45 | 41–51 | PIIANP |
LRCH1(5.3), KL(13.6) |
Hand OST | 1.28 | 17.2–25.1 | UK | (13) | |
| Hand K/L sum |
1.6 | 36 | USA | (11) | |||||||
| 21 | 1.52 | 15 | 6–22 | ||||||||
| HA | 6 | 1.69 | 44 | 38–48 | PIIANP |
HLA DR-2/4, HFE(5.9), COL11A2(8.4), TNXB(7.6) |
Female Hip | 4.8 | 53–56 | UK | (40) |
| 6 | 1.96 | 104 | 100–108 | COL10A1(14.2) | Hand U-OST | 1.11 | 82.6–109.9 | Netherlands | (10) | ||
| 13 | 1.83 | 7 | 5–11 | KL(24.4) | |||||||
| 14 | 1.57 | 63 | 60–67 | COMP |
ESR2(0.8), GPX2(1.2) |
Hand U- JSN |
2.64 | 48–57 | UK | (13) | |
ASPN=asporin;CALM1=calmodulin 1;CD36=platelet glycoprotein 4;COL10A1=collagen, type X, alpha 1;COL11A2=collagen, type XI, alpha ;2CTSL=cathepsin L;DIO2= type II iodothyronine deiodinase;, GPCR,2;ESR2=estrogen receptor 2;FLRT2= fibronectin leucine rich transmembrane protein 2;GPX2=glutathione peroxidase 2;HLA-DR=human leukocyte antigen of the major histocompatibility complex, MHC class II;HFE=hemochromatosis;IL4R=interleukin 4 receptor; KL=KLOTHO;LPAR1=lysophosphatidic acid receptor 1;LRCH1=leucine-rich repeats and calponin homology domain-containing 1;OGN=osteoglycin;SLC26A2=solute carrier family 26;TNF=tumor necrosis factor;TNXB=tenascin XB;WISP1=wnt-1-induced secreted protein-1. JSN=joint-space narrowing;OST=osteophyte;JSN-sum=sum of joint space narrowing scores;K/L sum=sum of Kellgren-Lawrence scores;U-DIP=unaffected distal interphalangeal;U-JSN=unaffected joint-space narrowing;U-OST=unaffected osteophyte; LOD=Logarithm of odds; h2r=heritability of nearest marker. All candidate genes are less than 10cM from the border of the 1-LOD drop support interval with one exception: the KLOTHO locus is ~31.4 cM from the HA chromosome 13 peak while the 1-LOD interval is 5–11 cM. But this candidate gene has been retained in the gene list because it is <10cM from the 1-LOD drop interval for the C2C chromosome 13 peak (41–51 cM). We defined significant linkage as LOD≥3 and depict these results in bold lettering.
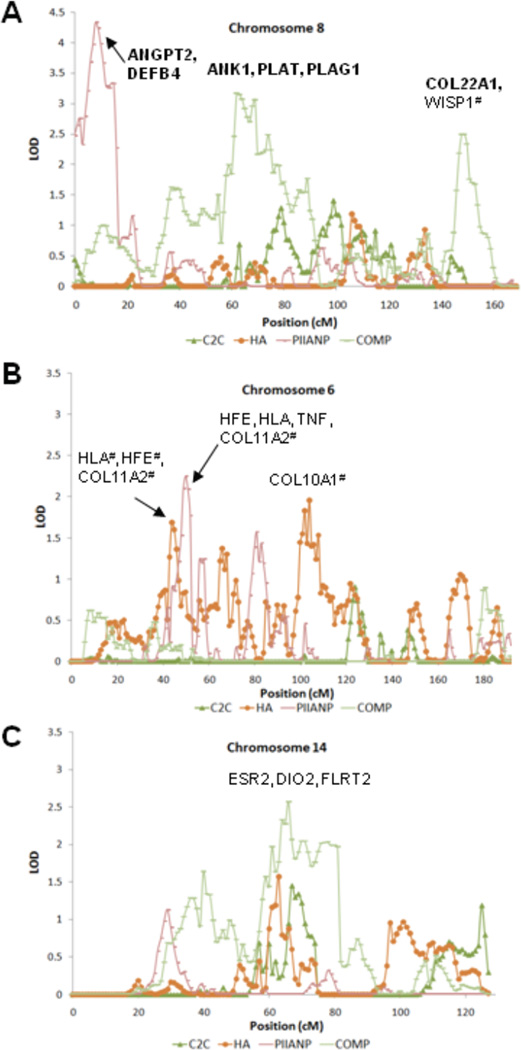
Figure 2. Potential OA candidate genes on chromosome 6, 8, and 14.
Genes listed in bold over the peaks represent potential novel candidate genes associated with biomarkers of OA in this study that have potential biological relevance for OA based on the literature. Genes listed without bold type over the peaks represent candidate genes linked previously to OA. The # sign indicates that the genes are less than 10 cM from the border of the 1-LOD drop support interval. ANGPT2=angiopoietin 2; DEFB4=beta-defensin 4; ANK1=ankyrin 1; PLAT=plasminogen activator, tissue; PLAG1= pleomorphic adenoma gene 1; COL22A1=collagen, type XXII, alpha 1; WISP1=wnt-1-induced secreted protein 1; HFE=hemochromatosis; COL11A2=collagen, type XI, alpha 2; TNF=tumor necrosis factor; COL10A1=collagen, type X, alpha 1; ESR2=Estrogen receptor 2; DIO2=Type II iodothyronine deiodinase; FLRT2=Fibronectin leucine-rich transmembrane protein 2.
Chromosome 6 was notable for overlapping regions of linkage (LOD 1.69–2.25) in the interval 38–52 cM identified by PIIANP and HA (Figure 2B). This region corresponds to a report of linkage in a female UK cohort with hip OA (40). Chromosome 14 was notable for overlapping regions of linkage (LOD 1.57–2.57) in the interval 59-82 cM identified by COMP and HA (Figure 2C). This region corresponds to a report of linkage in a UK cohort with hand OA (13). Overall, PIIANP yielded three overlapping regions with HA, C2C, and COMP on chromosomes 6, 13, and 15 respectively. For HA and C2C, the highest LOD scores were obtained for additional regions on chromosomes 6q16.3 (LOD 1.96 for HA) and 5q31.2 (LOD 1.98 for C2C). In addition to identifying previous reported OA candidate genes within or near our linkage peaks on chromosome 6, 8 and 14 (Figure 2A–C), we also list potential candidates based on their potential biological relevance.
Discussion
Our study represents the first linkage study to identify genetic loci associated with OA using biological markers. In the previous five genome-wide linkage studies, OA phenotypes were uniformly defined by X-ray evidence, physical examination, or joint replacement, which detect late stages of OA (7–13). There have been few studies investigating the heritability of OA biomarkers. In previous UK twin and GARP sibling-pair studies, the heritability of COMP and PIIANP were significant, 40–70% and 62% respectively (41, 42). To our knowledge, the heritability of serum HA, C2C, and CPII has not been assessed previously. The genetic components were in agreement with our findings, despite different race and study designs (41, 42). The genetic influence on OA-related biomarker levels may operate through allelic variation or factors regulating expression of the gene encoding the biomarker protein, or through effects on biologic pathways influencing cartilage metabolism and degradation (41). The latter appears most likely given that the significant linkage regions did not contain the genes encoding the biomarker used for the linkage.
The validity of our overall strategy was borne out by our replication of several previously reported genetic associations with OA identified by other means of phenotyping (Table 3). Overall, we identified 14 regions of linkage to OA-related quantitative traits that overlap or are near (within 10 cM) regions with reported genetic association with OA in the current literature. By two-point linkage, the maximum LOD (3.1) obtained for PIIANP, is within 2 Mb of the asporin (ASPN) gene and within 3 Mb of the cathepsin L (CTSL) and Osteoglycin (OGN) genes. The next highest LOD score (2.7) for PIIANP is close to the insulin-like growth factor binding protein 7 (IGFBP7) and ADAMTS3 genes. For COMP and HA, the maximum LOD scores of 2.23 and 1.79 are close to the type II deiodinase iodothyronine (DIO2) gene (less than 10 Mb away), and the prostaglandin-endoperoxide synthase 2 (PTGS2) gene (less than 5 Mb away), respectively.
In addition, this study provides evidence for two novel OA loci on chromosome 8 based on PIIANP and COMP quantitative traits (PIIANP on chromosome 8p23.2; COMP on chromosome 8q11.1). Suggestive linkage (LOD 1.57 and LOD 2.56) overlapping two of these regions has been reported by Grieg based on hand radiographic phenotypes (13), but candidate genes have not yet to be identified for these regions. The COMP linkage to chromosome 8q24.2 at 149cM overlaps a region of linkage previously reported to Wnt-1-induced secreted protein 1 (WISP1) based on a spinal OA radiographic phenotype in postmenopausal Japanese women (43). The signals observed for the top genomic loci by multipoint analysis (the PIIANP chromosome 8p23.2 QTL; the COMP chromosome 8q11.1 QTL; and the HA chromosome 6q16.3 QTL), were also observed in the two-point analysis.
Several of the OA-associated loci identified in this study were detected by more than one biomarker trait. This supports our hypothesis that a panel of biomarkers could identify shared genetic determinants. Loci identified by more than one OA-related biomarker may be of particular interest for further study as they are less likely to represent false positives and more likely to represent genes regulating the whole joint organ. We are aware that linkage disequilibrium (LD) can inflate multipoint LOD scores. However, the genotyping platform used was optimized for minimal LD. Inflation of LOD scores due to LD occurs only when there are missing parental genotypes (44). In our own study, given the multigenerational nature of the family pedigree, we had a large number of included parental genotypes (22 children had both parents genotyped, 73 children had 1 parent genotyped). Using our own study data (examining LD between available married-in unrelated individuals), there was no significant LD between SNPs in the top QTLs (defining LD by r2>0.4). Taken together, these data suggest the LD between markers will have minimum impact on the LOD scores reported here.
Of note, we did not detect significant or suggestive linkage peaks covering several genes with known OA association including frizzled-related protein β (FRZB), growth differentiation factor 5 (GDF5), and von Willebrand factor A domains (DVWA); this may be due to the lack of SNP markers covering these genes in the Infinium Human Linkage-12 BeadChip. All three of these proteins are related to skeletal morphogenesis and bone morphogenetic cell signaling (15, 45, 46). Our biomarker panel did not include a primary bone marker and so may have failed to account for the metabolic pathways impacted by these genes. These seminal studies have been performed in Caucasian or Asian populations while our study was performed in individuals of mixed African American and Native American heritage; thus, ethnic variation in genetic etiologies of OA may in part account for the failure to detect these loci in our cohort. Statistical power may also be an issue, as these three studies included between 1696 and 4361 individuals. Finally, our study was conducted in one large extended family, and it would not be reasonable to expect that every possible genetic etiology of OA would be reflected in this one family.
A strength of this study is that it is based on data from a large extended family with a pedigree spanning 300 years and 10 generations from a single founder. Statistical power can be increased by the use of biomarkers as quantitative traits (47). Increased statistical power may also come from minimizing genetic variability through study of a cohort from a single founder. This family was not ascertained on having a large number of OA cases and thus was also not ascertained for OA biomarkers, providing an opportunity to perform an unbiased linkage analysis of the biomarker levels. Thus the strengths of this study stem from the detailed biomarker analysis in a very large family, randomly selected with respect to OA cases. A limitation of the study, however, was the inability to perform radiographic phenotyping due to the health fair setting in which individuals were ascertained. Nevertheless, our biomarker traits led to replication of several loci reported in previous OA genetic studies that used radiographic phenotyping. Also, we have previously shown that several of the OA biomarkers used here were associated with clinical OA phenotypes in this large multigenerational family (30). In addition, all the biological markers (PIIANP, COMP, C2C, HA and CPII) have been associated with OA in other studies (26).
In summary, we report the first evidence for OA linkage using quantitative biomarker traits in a large extended family. We not only replicated several loci reported in previous OA genetic studies, but also identified two significant novel loci on chromosome 8. Several of the loci were identified by more than one OA-related biomarker. Further studies of the candidate genes at these loci may provide new insights into the mechanisms of joint metabolism, and OA initiation and progression.
Acknowledgements
We would like to thank the CARRIAGE family members for their participation in this study, Dr Vladimir Vilim for the kind gift of the 16F12/17C10 anti-COMP monoclonal antibodies, Norine Hall, and Milton Campbell for helping organize the collection of samples from family members, and everyone who made these family reunions possible.
Funding: NIH/NIA Claude D. Pepper OAIC 2P60 AG11268, the Mary Duke Biddle Foundation, the Trent Foundation, and a student grant from the Taiwanese government.
Footnotes
Author contributions
Dr. V. Kraus had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Chen, Shah, Hauser, W. Kraus, V. Kraus
Acquisition of data. Chen, Shah, Nelson, Haynes, Johnson, Stabler, W. Kraus, V. Kraus
Analysis and interpretation of data. Chen, Shah, Nelson, Haynes, Hauser, Gregory, W. Kraus, V. Kraus
Manuscript preparation. Chen, Shah, Li, Hauser, Gregory, W. Kraus, V. Kraus
Statistical analysis. Chen, Shah, Li, Haynes, V. Kraus
References
- 1.Brooks PM. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. 2002;14(5):573–577. doi: 10.1097/00002281-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Peach CA, Carr AJ, Loughlin J. Recent advances in the genetic investigation of osteoarthritis. Trends Mol Med. 2005;11(4):186–191. doi: 10.1016/j.molmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Jordan JM, Kraus VB, Hochberg MC. Genetics of osteoarthritis. Curr Rheumatol Rep. 2004;6(1):7–13. doi: 10.1007/s11926-004-0078-0. [DOI] [PubMed] [Google Scholar]
- 4.Ikegawa S. New gene associations in osteoarthritis: what do they provide, and where are we going? Curr Opin Rheumatol. 2007;19(5):429–434. doi: 10.1097/BOR.0b013e32825b079d. [DOI] [PubMed] [Google Scholar]
- 5.Valdes AM, Spector TD. The contribution of genes to osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):581–603. doi: 10.1016/j.rdc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, et al. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 7.Chapman K, Mustafa Z, Irven C, Carr AJ, Clipsham K, Smith A, et al. Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet. 1999;65(1):167–174. doi: 10.1086/302465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loughlin J, Mustafa Z, Irven C, Smith A, Carr AJ, Sykes B, et al. Stratification analysis of an osteoarthritis genome screen-suggestive linkage to chromosomes 4, 6, and 16. Am J Hum Genet. 1999;65(6):1795–1798. doi: 10.1086/302685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppavuori J, Kujala U, Kinnunen J, Kaprio J, Nissila M, Heliovaara M, et al. Genome scan for predisposing loci for distal interphalangeal joint osteoarthritis: evidence for a locus on 2q. Am J Hum Genet. 1999;65(4):1060–1067. doi: 10.1086/302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefansson SE, Jonsson H, Ingvarsson T, Manolescu I, Jonsson HH, Olafsdottir G, et al. Genomewide scan for hand osteoarthritis: a novel mutation in matrilin-3. Am J Hum Genet. 2003;72(6):1448–1459. doi: 10.1086/375556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demissie S, Cupples LA, Myers R, Aliabadi P, Levy D, Felson DT. Genome scan for quantity of hand osteoarthritis: the Framingham Study. Arthritis Rheum. 2002;46(4):946–952. doi: 10.1002/art.10149. [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, Demissie S, Cupples LA, Aliabadi P, Felson DT. A genome scan for joint-specific hand osteoarthritis susceptibility: The Framingham Study. Arthritis Rheum. 2004;50(8):2489–2496. doi: 10.1002/art.20445. [DOI] [PubMed] [Google Scholar]
- 13.Greig C, Spreckley K, Aspinwall R, Gillaspy E, Grant M, Ollier W, et al. Linkage to nodal osteoarthritis: quantitative and qualitative analyses of data from a whole-genome screen identify trait-dependent susceptibility loci. Ann Rheum Dis. 2006;65(9):1131–1138. doi: 10.1136/ard.2005.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster T, Chapman K, Marcelline L, Mustafa Z, Southam L, Loughlin J. Finer linkage mapping of primary osteoarthritis susceptibility loci on chromosomes 4 and 16 in families with affected women. Arthritis Rheum. 2004;50(1):98–102. doi: 10.1002/art.11427. [DOI] [PubMed] [Google Scholar]
- 15.Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101(26):9757–9762. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnero P. Use of biochemical markers to study and follow patients with osteoarthritis. Curr Rheumatol Rep. 2006;8(1):37–44. doi: 10.1007/s11926-006-0023-5. [DOI] [PubMed] [Google Scholar]
- 17.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis--a genomewide study. N Engl J Med. 2007;357(12):1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleasel JF, Poole AR, Heinegard D, Saxne T, Holderbaum D, Ionescu M, et al. Changes in serum cartilage marker levels indicate altered cartilage metabolism in families with the osteoarthritisrelated type II collagen gene COL2A1 mutation. Arthritis Rheum. 1999;42(1):39–45. doi: 10.1002/1529-0131(199901)42:1<39::AID-ANR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Kraus V, Kepler T, Stabler T, Renner J, JM J. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. 2009 doi: 10.1371/journal.pone.0009739. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansell JP, Collins C, Bailey AJ. Bone, not cartilage, should be the major focus in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(6):306–307. doi: 10.1038/ncprheum0505. [DOI] [PubMed] [Google Scholar]
- 22.Quasnichka HL, Anderson-MacKenzie JM, Bailey AJ. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology. 2006;43(3–4):389–397. [PubMed] [Google Scholar]
- 23.J Cibere HZ, Garnero P, Poole AR, Lobanok T, Saxne T, Kraus VB, Way A, Thorne A, Wong H, Singer J, Kopec J, Guermazi A, Peterfy C, Nicolaou S, Munk P, Esdaile JM. Differences in biomarker associations in pre-radiographic and radiographic knee osteoarthritis in a populationbased study. Arthritis Rheum. 2009 doi: 10.1002/art.24473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(6):346–356. doi: 10.1038/ncprheum0508. [DOI] [PubMed] [Google Scholar]
- 25.Charni-Ben Tabassi N, Garnero P. Monitoring cartilage turnover. Curr Rheumatol Rep. 2007;9(1):16–24. doi: 10.1007/s11926-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 26.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14(8):723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Ober C, Abney M, McPeek MS. The genetic dissection of complex traits in a founder population. Am J Hum Genet. 2001;69(5):1068–1079. doi: 10.1086/324025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HC, Shah SH, Li YJ, Stabler TV, Jordan JM, Kraus VB. Inverse association of general joint hypermobility with hand and knee osteoarthritis and serum cartilage oligomeric matrix protein levels. Arthritis Rheum. 2008;58(12):3854–3864. doi: 10.1002/art.24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Chen HC, Shah S, Stabler TV, Li YJ, Kraus VB. Biomarkers associated with clinical phenotypes of hand osteoarthritis in a large multigenerational family: the CARRIAGE family study. Osteoarthritis Cartilage. 2008;16(9):1054–1059. doi: 10.1016/j.joca.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Addison S, Edward R, Feng S, McDaniel G, Kraus V. Whole body bone scintigraphy provides a measure of total body burden of osteoarthritis for the purpose of systemic biomarker validation. Arthritis Rheum. 2009 doi: 10.1002/art.24856. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilim V, Vytasek R, Olejarova M, Machacek S, Gatterova J, Prochazka B, et al. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2001;9(7):612–618. doi: 10.1053/joca.2001.0434. [DOI] [PubMed] [Google Scholar]
- 33.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42(11):2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48(3):675–681. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 35.Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM, Kraus VB. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum. 2006;54(8):2496–2504. doi: 10.1002/art.21977. [DOI] [PubMed] [Google Scholar]
- 36.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amos CI. Robust variance-components approach for assessing genetic linkage in pedigrees. Am J Hum Genet. 1994;54(3):535–543. [PMC free article] [PubMed] [Google Scholar]
- 38.Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus coincident linkages. Genet Epidemiol. 1997;14(6):953–958. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Heath SC. Markov chain Monte Carlo methods for radiation hybrid mapping. J Comput Biol. 1997;4(4):505–515. doi: 10.1089/cmb.1997.4.505. [DOI] [PubMed] [Google Scholar]
- 40.Southam L, Dowling B, Ferreira A, Marcelline L, Mustafa Z, Chapman K, et al. Microsatellite association mapping of a primary osteoarthritis susceptibility locus on chromosome 6p12.3-q13. Arthritis Rheum. 2004;50(12):3910–3914. doi: 10.1002/art.20634. [DOI] [PubMed] [Google Scholar]
- 41.Williams FM, Andrew T, Saxne T, Heinegard D, Spector TD, MacGregor AJ. The heritable determinants of cartilage oligomeric matrix protein. Arthritis Rheum. 2006;54(7):2147–2151. doi: 10.1002/art.21931. [DOI] [PubMed] [Google Scholar]
- 42.Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio-Le Graverand MP, et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage. 2007;15(4):379–385. doi: 10.1016/j.joca.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Urano T, Narusawa K, Shiraki M, Usui T, Sasaki N, Hosoi T, et al. Association of a single nucleotide polymorphism in the WISP1 gene with spinal osteoarthritis in postmenopausal Japanese women. J Bone Miner Metab. 2007;25(4):253–258. doi: 10.1007/s00774-007-0757-9. [DOI] [PubMed] [Google Scholar]
- 44.Boyles AL, Scott WK, Martin ER, Schmidt S, Li YJ, Ashley-Koch A, et al. Linkage disequilibrium inflates type I error rates in multipoint linkage analysis when parental genotypes are missing. Hum Hered. 2005;59(4):220–227. doi: 10.1159/000087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S, et al. A functional polymorphism in the 5' UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39(4):529–533. doi: 10.1038/2005. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto Y, Shi D, Nakajima M, Ozaki K, Sudo A, Kotani A, et al. Common variants in DVWA on chromosome 3p24.3 are associated with susceptibility to knee osteoarthritis. Nat Genet. 2008;40(8):994–998. doi: 10.1038/ng.176. [DOI] [PubMed] [Google Scholar]
- 47.Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82(1):139–149. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryder JJ, Garrison K, Song F, Hooper L, Skinner J, Loke Y, et al. Genetic associations in peripheral joint osteoarthritis and spinal degenerative disease: a systematic review. Ann Rheum Dis. 2008;67(5):584–591. doi: 10.1136/ard.2007.073874. [DOI] [PubMed] [Google Scholar]
- 49.Meulenbelt I, Min JL, Bos S, Riyazi N, Houwing-Duistermaat JJ, van der Wijk HJ, et al. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet. 2008;17(12):1867–1875. doi: 10.1093/hmg/ddn082. [DOI] [PubMed] [Google Scholar]
- 50.Valdes AM, Loughlin J, Timms KM, van Meurs JJ, Southam L, Wilson SG, et al. Genome-wide association scan identifies a prostaglandin-endoperoxide synthase 2 variant involved in risk of knee osteoarthritis. Am J Hum Genet. 2008;82(6):1231–1240. doi: 10.1016/j.ajhg.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mototani H, Iida A, Nakajima M, Furuichi T, Miyamoto Y, Tsunoda T, et al. A functional SNP in EDG2 increases susceptibility to knee osteoarthritis in Japanese. Hum Mol Genet. 2008;17(12):1790–1797. doi: 10.1093/hmg/ddn069. [DOI] [PubMed] [Google Scholar]
- 52.Mototani H, Mabuchi A, Saito S, Fujioka M, Iida A, Takatori Y, et al. A functional single nucleotide polymorphism in the core promoter region of CALM1 is associated with hip osteoarthritis in Japanese. Hum Mol Genet. 2005;14(8):1009–1017. doi: 10.1093/hmg/ddi093. [DOI] [PubMed] [Google Scholar]
- 53.Ingvarsson T, Stefansson SE, Gulcher JR, Jonsson HH, Jonsson H, Frigge ML, et al. A large Icelandic family with early osteoarthritis of the hip associated with a susceptibility locus on chromosome 16p. Arthritis Rheum. 2001;44(11):2548–2555. doi: 10.1002/1529-0131(200111)44:11<2548::aid-art435>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 54.Jordan J, Atif U, Chiano M, Reck B, Doherty M, Hochberg M, et al. Genome-wide linkage scan and high-density associations studies implicate chromosome 18q21with generalized osteoarthritis. Osteoarthritis Cartilage. 2008;16(Supplement 4):S33–S34. [Google Scholar]
- 55.Ikeda T, Mabuchi A, Fukuda A, Hiraoka H, Kawakami A, Yamamoto S, et al. Identification of sequence polymorphisms in two sulfation-related genes, PAPSS2 and SLC26A2, and an association analysis with knee osteoarthritis. J Hum Genet. 2001;46(9):538–543. doi: 10.1007/s100380170036. [DOI] [PubMed] [Google Scholar]