Abstract
Recent reports indicate an increase in intranasal use of prescription oral stimulant medication. However, there do not appear to be any published clinical studies that have characterized the behavioral and cardiovascular effects of intranasally administered d-amphetamine, which is commonly prescribed for ADHD. In this study, a range of d-amphetamine doses (0, 16, 24 and 32 mg/70 kg) was administered as an intranasal solution delivered using a mucosal atomization device. Equal oral doses were included for comparison. Assessments were conducted before, and at regular intervals for three hours following drug administration, and included self-reported drug-effect questionnaires, cardiovascular indices, a performance task, and two measures of impulsivity. d-Amphetamine produced prototypical stimulant effects (e.g., increased subject ratings of Stimulated and Like Drug, elevated heart rate and blood pressure, and improved rate and accuracy on the DSST) irrespective of dose, but the onset of these effects was generally earlier following intranasal administration, with significant effects emerging at 15–30 minutes after intranasal dosing and 45–60 minutes after oral dosing. These results demonstrate that intranasal administration of d-amphetamine results in a more rapid onset compared to oral dosing, which could be associated with the popularity of intranasal prescription stimulant use and an enhanced potential for abuse.
Keywords: d-Amphetamine, Intranasal, Subjective, Digit Symbol Substitution Task, Balloon Analog Risk Task, Delay Discounting
Introduction
Compared to the oral route, intranasal drug administration produces a faster onset of action with higher bioavailability, and is associated with enhanced abuse potential.1 Intranasally administered drug is absorbed directly into the bloodstream from the vasculature in the turbinates on the medial wall of the nasal cavity by passive diffusion across cell membranes or passage through the tight-cell junctions between cells, which bypasses first-pass gastrointestinal metabolism. Some drugs may also enter the central nervous system more directly via transportation through the olfactory nerve.1 Laboratory studies indicate that this faster rate of drug delivery can increase the effectiveness of a drug as a reinforcer.2–4 Likewise, increasing a drug’s bioavailability results in the delivery of a larger dose, and larger drug doses are typically preferred in self-administration studies using choice procedures.5–6 The nonmedical use of drugs via the intranasal route of administration is prevalent, with cocaine being the most well known example, but many others, including illicit drugs such as heroin, legal drugs like snuff tobacco, and diverted prescription drugs such as benzodiazepine sedative-hypnotics, opioid analgesics and psychomotor stimulants, are also administered intranasally.
Prescription stimulant drugs such as methylphenidate and amphetamines, which include mixed-salts and the d-enantiomer, are most commonly prescribed for Attention Deficit Hyperactivity Disorder (ADHD). These medications are recognized as being effective for managing ADHD symptoms, and longitudinal data indicate that prescription stimulant use in children with ADHD does not increase the risk for developing substance use disorders compared to untreated patients, but instead appears to be protective.7 However, the rapid rise in ADHD diagnoses and prescriptions for stimulant medications beginning in the early 1990’s coupled with the abuse potential of psychomotor stimulants has focused attention on the nonmedical use of these drugs.8–11 Reports from a variety of sources (e.g., lay press, internet message boards, epidemiological studies, case reports) have documented the nonmedical use of prescription stimulants, and have also described the popularity of crushing and snorting these medications.
Despite the occurrence of nonmedical intranasal use of prescription stimulants, there has been little clinical research on their effects when administered by this route. We are aware of one published study that evaluated intranasal methylphenidate in healthy individuals under controlled laboratory conditions,12 but none that have tested intranasal d-amphetamine. Therefore, the purpose of the present study was to characterize the time course of the behavioral and cardiovascular effects of intranasal d-amphetamine to determine if this route was associated with a more rapid onset of action and/or increased bioavailability. A range of d-amphetamine doses (0, 16, 24 and 32 mg/70 kg) was administered as an intranasal solution delivered using a mucosal atomization device. Equal oral doses were included for comparison. Assessments were conducted before, and at regularly scheduled intervals for three hours following drug administration, and included self-reported drug-effect questionnaires, cardiovascular indices, a psychomotor performance task, and two measures of impulsivity/reward seeking. d-Amphetamine produced prototypical stimulant effects (e.g., increased subject ratings of Stimulated and Like Drug, elevated heart rate and blood pressure, and improved psychomotor performance), but the onset of these effects was generally more rapid following intranasal administration.
PARTICIPANTS AND METHODS
Participants
Healthy adult subjects were recruited from the local community. All potential subjects completed a brief telephone interview or an internet-based questionnaire addressing general medical and legal status. Respondents who reported good health and previous stimulant use (e.g., caffeine) were contacted by telephone and invited to participate in the study.
During an orientation and medical screening day, subjects completed a battery of medical and psychological questionnaires, as well as blood and urine chemistry tests. Subjects were excluded if they had a history of, or current, medical conditions that would contraindicate participation (e.g., cardiovascular disease, Axis I psychiatric disorders) or if there was any indication of elevated medical risk associated with administration of the study drug. All subjects provided written informed consent, and the confidentiality of their personal information was maintained throughout.
Subjects were informed that during each experimental session they would receive capsules and an intranasal solution and that either could contain placebo or d-amphetamine; however, subjects were blind to the dose and order of administration. They were told that the purpose of the study was to see how different drugs affect mood and behavior.
Six subjects (5 Caucasian males, 1 Caucasian female) completed a 10-session experiment. They ranged in age from 20 to 28 years (median = 22 years), in education from 14 to 20 years (median = 16) and in weight from 56 to 94 kg (median = 75 kg). Subjects reported consuming 0 to 10 standard alcohol-containing beverages per week (median = 7) and 40–140 mg caffeine per day (median = 40). One subject reported daily use of 5 tobacco cigarettes. No other amphetamine or other substance use history was reported or identified via urinalysis throughout the study.
Subjects earned approximately $800, including per diem and task earnings, as well as a bonus for completing all scheduled sessions and abstaining from drug use for the duration of the study.
Study Design
A double-blind, double-dummy, placebo-controlled, randomized design was used to compare the effects of intranasal and oral d-amphetamine (0, 16, 24 and 32 mg). This study was approved by the Institutional Review Board of the University of Kentucky Medical Center and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Initially, subjects completed two practice sessions to become familiarized with the behavioral and cardiovascular measures and daily laboratory routine. During these sessions, the subjects also practiced administration of an intranasal drug solution (saline), but no active doses of d-amphetamine were administered. Subjects then completed eight experimental sessions, conducted Monday through Friday. Session start times were fixed for each subject. Subjects were instructed to abstain from drug use for the duration of the study and to abstain from eating for 4 hours prior to all sessions.
At the beginning of each session, subjects answered open-ended questions regarding sleep, medication use, eating behavior and health status during the preceding 24 hours, and completed field-sobriety, breath (Alco-Sensor III, Intoximeters, Inc., St. Louis MO) and urine tests (Integrated E-Z Split Cut, Acon Laboratories, San Diego, CA) to assess recent drug use and pregnancy. All samples were negative for alcohol and illicit drugs and female urine samples were negative for hCG. Subjects then consumed a low-fat snack prior to completion of the assessments, which were presented at fixed times for the next 3.5 hours.
Pharmacodynamic Evaluations
Measurements were generally completed 15 min before and 15, 30, 45, 60, 90, 120 and 180 min after dose administration, with three exceptions; the BART and the DSST were not included at the 30 and 45 min time points because of the short interval between the early assessments, the Delayed Discounting Procedure was only conducted at the 120-min time point, and the Intranasal Dose Questionnaire was only administered immediately after subjects ingested the intranasal solution. Data were collected on an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA), with the exception of the Delay Discounting Procedure and the Intranasal Dose Questionnaire, which were collected using paper and pen.
Intranasal Dose Questionnaire
For this locally developed instrument, subjects rated five items (How much does the intranasal solution sting, burn and hurt right now; How much irritation is the intranasal solution producing right now; and How uncomfortable does the intranasal drug solution make you feel right now) on a VAS (see below) to evaluate the effectiveness of the active placebo. Subjects were also asked if they thought they received placebo or active drug in the intranasal solution.
Visual-Analog Scales (VAS)
Subjects rated eight individually presented items (I like the drug effect; I feel stimulated, sedated, hungry, thirsty, anxious, high and a drug effect) on the computer by marking a 100-unit line anchored on the extremes by Not At All and Extremely.
Addiction Research Center Inventory (ARCI)
The computerized version of the 49-item short form of the true-false inventory13 yielded information on five scales: Lysergic Acid Diethylamide (LSD), Amphetamine (A), Benzedrine-Group (BG), Morphine-Benzedrine Group (MBG) and Pentobarbital, Chlorpromazine, Alcohol Group (PCAG).
Adjective Rating Scale (ARS)
This scale consists of 32 items and contains two subscales: Sedative and Stimulant.14 In the present study, only the 16 items from the Stimulant subscale were presented. Subjects rated each item using a numeric keypad to select among one of five response options: Not at All, A Little Bit, Moderately, Quite a Bit, and Extremely (scored numerically from 0 to 4, respectively; maximum score = 64).
Vital Signs
Oscillometric heart rate (HR) and systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured (Sentry II, NBS Medical, New Brunswick, NJ).
Digit Symbol Substitution Task (DSST)
Subjects completed a 1.5 min computerized version of the DSST adapted from the original version.15 Subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns displayed on a video screen identified on a given trial. The dependent variables for this task were trial completion rate and accuracy.
Balloon Analog Risk Task (BART)
This task simulated a balloon being inflated in small increments controlled by clicking on a computer mouse.16 On each trial the subject decided to inflate the balloon or move to another balloon. A successful inflation added money to a temporary bank and an increase in the probability of the balloon popping on the next inflation. If a subject chose to move to another balloon, the temporary bank was placed in a permanent bank; if a subject chose to inflate the balloon and it popped, money in the temporary bank was lost. The task consisted of 20 trials (i.e., balloon inflations) and was presented six times each session. Earnings from each task presentation were recorded, and at the end of the session subjects were given the earnings from one of the six task presentations, selected at random. The dependent variables for this task were the number of popped balloons, the number of responses from trials in which the balloon did not pop, and task earnings.
Delay Discounting Procedure
A series of choices between an immediate, smaller amount of money and a larger, delayed amount of money was presented. The delayed money option was fixed ($20) and was presented at 10 delay values (1 day, 3 days, 5 days, 1 week, 10 days, 2 weeks, 3 weeks, 25 days, 1 month, and 2 months). The immediate amount ($20, $18, $15, $12, $10, $8, $6, $4, $2, and $1) was increased until preference between the immediate amount and the delayed amount ($20) reached indifference. This indifference point served as the dependent measure. Subjects were paid for one randomly selected choice (e.g., $15 in 2 weeks) of the 1000 total choices made across the study (10 sessions X 100 choices per session).
Statistical Analysis
All results were considered significant at p<0.05. Data from the active d-amphetamine doses were analyzed as raw scores. Placebo data were averaged across both placebo conditions.
Data from the VAS, ARCI, ARS, DSST, BART and cardiovascular assessments were analyzed using a repeated-measures ANCOVA model (SAS, SAS Institute Inc., Cary, NC) with d-amphetamine dose (0, 16, 24 and 32 mg), route of administration (intranasal and oral) and time post-drug (15, 30, 45, 60, 90, 120 and 180 min for VAS, ARCI, ARS, HR, SBP, DBP; or 15, 60, 90, 120 and 180 min for DSST and BART) as factors. Pre-drug values were included as a covariate. Planned comparisons of each active drug dose with placebo, and comparisons of active doses of oral versus intranasal d-amphetamine were conducted for each post-drug time point using Tukey’s test.
VAS data from the Intranasal Dose Questionnaire were analyzed using repeated-measures ANOVA with intranasal dose (0, 16, 24 and 32 mg) as the factor. The average of the three sessions during which subjects received active doses of oral d-amphetamine and the two sessions in which both the capsules and intranasal solution contained placebo (i.e., sessions in which the intranasal solution did not contain active drug) served as the intranasal placebo value in this analysis.
Data from the Delay Discounting Procedure were analyzed with non-linear regression (Prism 4.0, GraphPad Software, Inc. La Jolla, CA), using Mazur’s equation: V =1/(A +kD), where V represents the subjective value of the delayed reward (e.g., the indifference point), A is the value of the delayed reward (e.g., $20), D is the delay and k is a free parameter that is related to the rate of discounting.17 Comparison of k values was conducted using repeated-measures ANOVA with d-amphetamine dose (0, 16, 24 and 32 mg) and route of administration as factors.
Drug
Oral and intranasal doses of d-amphetamine (0, 16, 24 and 32 mg) were prepared by the University of Kentucky Investigational Pharmacy and administered in a double-blind, double-dummy fashion. Doses were administered randomly with the exception that the intranasal dose of 32 mg/70 kg d-amphetamine was not administered before the two lowest doses of intranasal d-amphetamine. Each session, subjects ingested capsules and intranasal drug solution, but only one route (or neither for the placebo condition) contained active d-amphetamine.
d-Amphetamine capsules were prepared by over-encapsulating commercially available d-amphetamine sulfate powder admixed with corn starch in opaque size 0 gelatin capsules. Intranasal d-amphetamine is not commercially available. The investigational pharmacist prepared intranasal solutions as needed using sterile injectible water and d-amphetamine sulfate powder. Placebo doses contained 100 mg/mL magnesium sulfate to produce a mild stinging sensation and bitter taste to mask the cues associated with active drug administration. Solutions were filtered using a 0.22-micron sterile filter for antimicrobial management and stored frozen until the day of an experimental session. Subjects received two plastic 1.0 mL injectors capped with a mucosal atomization device (MAD100, Wolfe Tory Medical, Inc. Salt Lake City, UT) and were instructed to spray the solution from the syringes into each nostril cavity. The total volume of solution contained in each syringe was 0.29 mL, which delivered 0.2 mL per naris (assuming a 0.09 mL “dead space” in each syringe) for a total of 0.4 mL.
Doses of d-amphetamine were chosen for the present study based on preliminary unpublished data from our laboratory on the effects of 16 mg/70 kg intranasal d-amphetamine in 10 healthy subjects. The magnitude of the response to that dose of intranasal d-amphetamine was modest and not greater than what was observed following administration of an approximately equivalent oral dose (16 mg) to healthy subjects in a separate study,18 which suggested that the potency of d-amphetamine might not differ as a function of route of administration. Because few prior studies in healthy subjects had tested doses above 32 mg/70 kg, higher doses were not examined due to the absence of safety information.
RESULTS
Intranasal Dose Questionnaire
100 mg/mL magnesium sulfate (i.e., placebo intranasal solution) functioned as an effective blind. Subjects identified this intranasal solution as drug 50% of the time, and no significant main effect of dose was found for any of the VAS items on this questionnaire.
VAS
A significant interaction of dose and route (F’s3,330 = 3.7–8.8; p’s ≤ 0.01) and main effect of time (F’s3,330 = 3.8–12.0; p’s ≤ 0.001) was observed for subject ratings of High, Stimulated, Thirsty, Feel Drug and Like Drug. Planned comparisons indicated that intranasal d-amphetamine increased subject ratings on more of the VAS items compared to oral dosing and that the onset of the subject-rated effects of d-amphetamine was generally earlier with the intranasal route of administration.
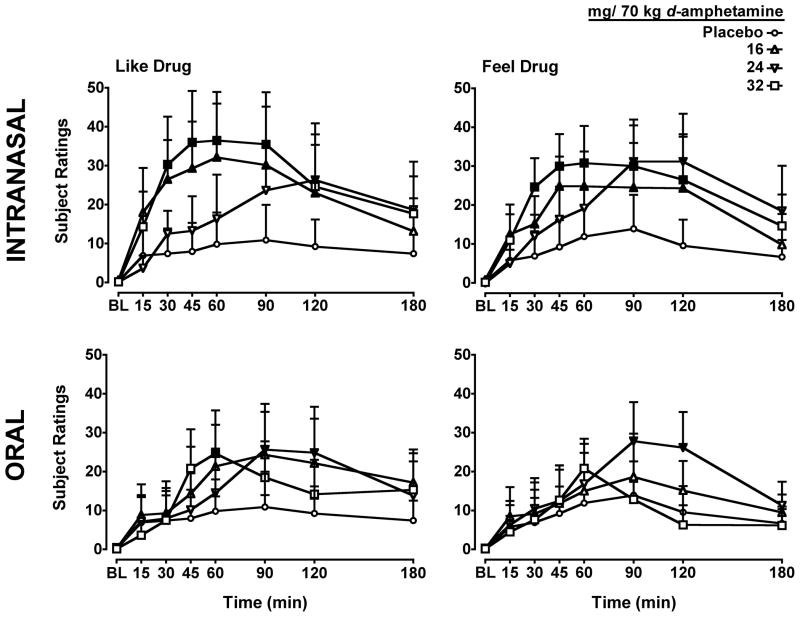
The differences in the onset of action of the self-reported effects for the two routes of administration of d-amphetamine were most apparent for the VAS items Like Drug and Feel Drug (Figure 1). Subject ratings of Like Drug and Feel drug were significantly increased compared to placebo for all intranasal doses, with the earliest significant self-reported effects occurring 30-min after administration of 16 and 32 mg/70 kg d-amphetamine. Following oral dosing, however, the earliest time point at which significant differences were found between d-amphetamine and placebo for subject ratings of Like Drug was 60 min after administration of the 32 mg/70 kg dose and 90 minutes after the 24 mg/70 kg dose. For the item Feel Drug, only the 24mg/70 kg oral dose increased ratings starting 90 min after drug administration.
Figure 1.
Dose- and time-response function for d-amphetamine administered by the intranasal (Top Panels) and oral (Bottom Panels) routes of administration for the items Like Drug (Left Panels) and Feel Drug (Right Panels) on a Visual Analog Scale. X-axis: Time after dose administration in minutes and baseline (BL). Filled symbols indicate values that are significantly different from placebo at each time point. Data points show means of 6 subjects. Uni-directional brackets indicate 1 SEM.
Relative to placebo, d-amphetamine increased subject ratings of High and Thirsty only after administration of intranasal doses. All intranasal doses increased ratings of High, with the earliest time point at which significant differences were found being 30 min after administration of the 32 mg/70 kg dose. For the item Thirsty, the intranasal 24 and 32 mg/70 kg doses significantly increased subject ratings only at the 120-min time point.
For the VAS item Stimulated, all intranasal doses significantly increased ratings compared to placebo, with the earliest difference noted at 30 min after administration of the 32 mg/70 kg dose. In contrast, only the 24 mg/70 kg dose of oral d-amphetamine increased subject ratings of Stimulated relative to placebo and only at the 90-min post-dose time point.
Significant main effects of dose and route were also observed for subject ratings of Anxious and Sedated and an interaction of dose and route was also found for the item Hungry; however, planned comparisons did not reveal any active doses that were significantly different from placebo at any time point.
ARCI
A significant interaction of d-amphetamine dose and route (F’s3,330 = 4.5–8.1; p’s ≤ 0.01) and main effect of time (F’s3,330 = 3.3–8.1; p’s ≤ 0.01) was observed for scores on the PCAG, MBG and A scales. The 24 and 32 mg doses of d-amphetamine administered via the intranasal route significantly decreased scores on the PCAG scale relative to placebo at the 30 and 45 min time points, whereas only the oral dose of 32 mg d-amphetamine reduced scores on this scale beginning at the 45 min time point. Both oral (16 mg/70 kg) and intranasal (16, 24 and 32 mg/70 kg) d-amphetamine increased scores on the MBG scale relative to placebo, and the earliest time point at which significant differences occurred was 60 min for both routes of administration. Likewise, both oral (24 and 30 mg/70 kg) and intranasal (16 mg/70 kg) d-amphetamine increased scores on the A scale relative to placebo, and the earliest time point at which significant differences occurred was 90 min for both routes of administration.
Significant main effects of dose (F’s3,330 = 2.7, 3.7; p’s ≤ 0.05) and time (F’s3,330 = 2.9, 7.3; p’s ≤ 0.01) were found for scores on the BG and LSD scales. For the BG scale, oral (16 mg/70 kg) and intranasal (24 mg/70 kg) doses of d-amphetamine significantly increased scores compared to placebo, with the earliest difference noted at 45 min and 60 min, respectively. Only the 24 mg/70 kg dose of intranasal d-amphetamine increased subject scores on the LSD scale relative to placebo beginning at the 90-min post-dose time point.
ARS Stimulant
A significant interaction of dose and route (F3,330 = 3.4; p ≤ 0.05) and a main effect of time (F3,330 = 6.8; p ≤ 0.001) was observed for scores on the Stimulant subscale of the ARS. Intranasal administration of all active d-amphetamine doses increased scores relative to placebo beginning at 30 min (16 and 32 mg/70 kg) or 45 min (24 mg/70 kg) post dose. In contrast, only the 16 and 24 mg/70 kg doses of orally administered d-amphetamine significantly increased scores at the 90 and 120 min time points, respectively.
Vital Signs
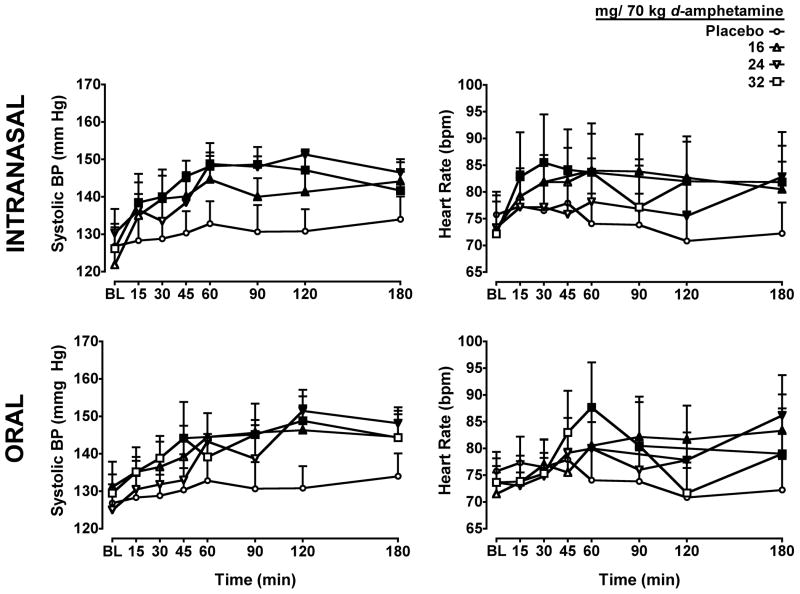
A significant interaction of dose and route (F3,330 = 2.7; p ≤ 0.05) and dose and time (F3,330 = 2.5; p ≤ 0.001) was observed for heart rate (Figure 2, right panel). Heart rate was significantly increased by all doses of intranasal d-amphetamine. The earliest time point at which a significant difference from placebo occurred was 15 min after administration of the 32 mg/70 kg dose. Orally administered d-amphetamine also significantly increased heart rate compared to placebo; however, the earliest time point for this effect was 60 min for all doses.
Figure 2.
Dose- and time-response function for d-amphetamine for diastolic blood pressure (Left Panels) and heart rate (Right Panels). Other details are as in Figure 1.
A significant interaction of dose and time (F3,330 = 2.3; p ≤ 0.01) was found for diastolic blood pressure (Figure 2, left panel), and significant main effects of dose (F3,330 = 24.2; p ≤ 0.001) and time (F3,330 = 7.0; p ≤ 0.001) were detected for systolic blood pressure. All active doses of both oral and intranasal d-amphetamine significantly increased diastolic blood pressure compared to placebo, with the earliest time point at which significant differences were observed occurring 45 min following oral and intranasal administration of the 32 mg/70 kg dose. Similarly, all active doses of both oral and intranasal d-amphetamine significantly increased systolic blood pressure compared to placebo, with the earliest time point at which significant differences were observed occurring 30 min following administration of the 16 and 32 mg/70 kg dose of intranasal d-amphetamine and 45 min after the 32 mg/70 kg dose of oral d-amphetamine.
DSST
Significant interactions of dose and route (F3,330 = 8.9; p ≤ 0.001) and dose and time (F3,330 = 2.0; p ≤ 0.05) were observed for trial completion rate. Both intranasal (all doses) and oral (16 and 32 mg/70 kg) d-amphetamine significantly increased the number of trials completed, with the earliest time point at which significant differences between active drug and placebo were detected occurring 60 min after drug administration. A significant interaction of dose and route (F3,330 = 7.1; p ≤ 0.001) was observed for trial accuracy. All doses of d-amphetamine administered via both routes significantly improved trial accuracy, with the earliest significant time point occurring at 60 min. Trial completion rate and accuracy were significantly higher than placebo at a greater number of time points following intranasal administration of the 24 mg/70 kg dose compared to oral administration.
BART
A significant interaction of dose and route was revealed for the number of responses from trials in which the balloon did not pop; however, planned comparisons did not reveal any active doses that were significantly different from placebo at any time point. No significant effects of d-amphetamine were detected for the other dependent variables from the BART.
Delay Discounting Procedure
Prototypical discounting curves were observed; that is, the subjective value of the fixed reward decreased as the delay increased. However, k values did not differ significantly as a function of d-amphetamine dose.
DISCUSSION
The aim of the present study was to characterize the behavioral and cardiovascular effects of intranasal d-amphetamine and to compare the results to those obtained with the same doses administered orally. The behavioral and cardiovascular effects of d-amphetamine were consistent with previous studies,19–23 with increased stimulant-type ratings on several items such as VAS Like Drug and Stimulated, elevated heart rate and blood pressure, and improved rate and accuracy on the DSST, regardless of route of administration. Both routes of d-amphetamine administration were well tolerated by all subjects.
The time course for the behavioral and cardiovascular effects of intranasal d-amphetamine, which has not been published previously, exhibited statistically significant differences from placebo as early as 15–30 min. Following oral administration, initial significant effects of d-amphetamine were not detected until 45–60 min, consistent with the onset for therapeutic effects.11 The peak response to intranasal and oral d-amphetamine administration occurred at comparable times however. Peak self-reported effects and heart rate occurred an average of 60 and 75 minutes, respectively, after intranasal administration. Similarly, the peak response to oral administration occurred at approximately 60 minutes for self-reported effects and 90 minutes for heart rate. For comparison, the peak positive subject-rated and cardiovascular response to intravenous d-amphetamine administration occurred within 2–18 min after injection in healthy individuals and stimulant users in previous studies.24–25 Prior research demonstrated that peak self-reported effects following oral dosing typically occurs 1.5–2 hours after oral dosing, with peak heart rate effects having been reported as late as 5–8 hours post dose.22,26–28 The reason for the discrepancy in the time at which peak effects occurred across studies is unknown, but could be related to the subjects’ experience with the more rapid onset following intranasal administration. The similar time course for peak effects following the two routes of administration in the present study can be explained by gastrointestinal absorption. Nasal drug absorption typically occurs within 15–20 minutes, with any remaining drug eventually being swallowed and absorbed in the gastrointestinal tract.29 If the peak response to intranasal d-amphetamine was attributable only to drug absorption occurring only in the nasal mucosa, the predicted time at which the peak response occurred should have been earlier than following oral administration. That the peak effects for both routes were observed at approximately the same times suggests that the maximum response to intranasal d-amphetamine may reflect cumulative drug absorption from both the nasal and GI mucosa, albeit at different time points. This “nas-oral” phenomenon has been documented previously.30
Although there was overlap in the self-reported effects of d-amphetamine administered via the intranasal and oral route, VAS ratings of High and Thirsty, and scores on the LSD scale of the ARCI, were limited to the intranasal route only. In addition, for those measures sensitive to both routes of administration, oral dosing typically produced effects that were significantly different than placebo at fewer time points (i.e., smaller area-under-the curve) compared to intranasal dosing. Planned comparisons at time points for which both routes of d-amphetamine administration were significantly different from placebo did not reveal significant differences between the routes of administration for any dose on any measure. In addition, peak effects analysis also did not reveal differences by route for any measure (data not shown). Together, these data demonstrate that, although the maximum effect produced by the two routes of administration did not differ, the total effect across the 3-h session was greater following intranasal administration of d-amphetamine, which can be accounted for by the earlier onset of the effects observed for intranasal drug delivery. These results support the notion that intranasal drug delivery increases bioavailability, which could also be associated with the “nas-oral” phenomenon, although a more complete time course assessment and determination of blood levels of d-amphetamine would be required to more fully address this possibility.
The reinforcing effects of drugs are influenced by the rate of drug onset. Comer and colleagues,2 for example, found that drug onset between 15 min compared to 60 min enhanced the reinforcing strength of oxycodone. A difference in drug onset between 15 and 60 minutes mirrors the difference in onset of the intranasal vs. oral administration of d-amphetamine in the present study, suggesting that the reinforcing effects of prescription amphetamines may be greater via the intranasal than the oral route of administration. As noted above, survey data31–33 and case reports34–36 have described nonmedical use of prescription stimulants via the intranasal route. Further evidence for the prevalence of intranasal use of stimulant medications comes from the steady stream of lay press coverage of this issue as well as from the existence of internet-based drug discussion forums. These sites provide user-posted recommendations for intranasal use as well as recommendations about mixing with other drugs or other formulations of stimulant medications (e.g., oral administration of an extended release stimulant combined with intranasal use of a crushed immediate release preparation). As might be expected, there is evidence that individuals engaging in intranasal use of prescription stimulants are more likely than oral only users to exhibit maladaptive behaviors suggestive of more problematic drug use. For example, intranasal prescription stimulant users score higher on the Drug Abuse Screening Test (DAST-10).33
This experiment had some limitations, which should be addressed. First, only six subjects were enrolled, which might have limited the ability to detect differences earlier in the experimental session. Several measures appeared to have values that were increased beyond baseline levels following intranasal administration at the 15-min time point, but were not significantly different from placebo. As an example, effect sizes for the ARS Stimulant subscale and VAS items Like Drug and Feel Drug were 0.3 at the 15-min time point, but ranged from 1.1–1.3 at the 30-min time point. A considerably larger number of subjects would have been required to detect differences between intranasal d-amphetamine and placebo 15-min after drug administration with sufficient power (i.e., 0.80) given these effect sizes. Another limitation is that blood samples needed to determine the concentrations of d-amphetamine over time across the two routes of administration were not obtained. However, part of the intention of developing intravenous d-amphetamine delivery procedures was to avoid subject risk and distress from intravenous needle sticks, which might have otherwise interfered with study recruitment and retention. Moreover, a direct relationship between drug concentration and behavioral and physiological response is not always observed,27,37 and in this case the behavioral and physiological measures were of primary interest. Finally, a complete time course for the DSST, BART and delay-discounting task, which would have been informative, was not feasible, given the length of time needed to complete these tasks.
This study characterized the subject-rated, performance and cardiovascular effects of intranasal d-amphetamine, which does not appear to have been reported previously. These results show that the onset of the self-reported and cardiovascular effects of intranasal d-amphetamine occurs earlier and the duration of effects occur longer than for oral administration, and that the peak effects are observed within 1 hr of drug administration. These findings contribute to the clinical literature regarding the diversion and misuse of prescription drugs by directly comparing the effects of a commonly prescribed ADHD medication administered via the oral and intranasal route. The faster onset d-amphetamine’s effects following intranasal administration observed in the laboratory coupled with the reports of intranasal amphetamine use in the natural environment support a conclusion that more rapid onset of action is associated with increased abuse potential in human subjects.
Acknowledgments
The authors wish to thank Stephanie Douglas, Glenn Robbins, Beth Eaves, Jillian O’Rourke, Caroline Kimathi and Allen Mayberry for help in executing the study and preparing the manuscript. The authors also appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service and biostatistical consultation with Dr. Heather Bush. Finally, we would like to thank the Reviewers for their helpful comments on a previous version of this manuscript.
Funding
This research and the preparation of this manuscript were supported by a National Center for Research Resources Center for Biomedical Research Excellence grant (P20 RR015592) awarded to Dr. Thomas Curry and National Institute on Drug Abuse grants (P50 DA05312, K01 DA018772) awarded to Drs. Michael Bardo and Joshua Lile, respectively.
References
- 1.Wermeling D. Intranasal delivery of antiepileptic medications for treatment of seizures. Neurotherapeutics. 2009;6:352–358. doi: 10.1016/j.nurt.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comer SD, Ashworth JB, Sullivan MA, Vosburg SK, Saccone PA, Foltin RW. Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manag. 2009;5:203–212. doi: 10.5055/jom.2009.0022. [DOI] [PubMed] [Google Scholar]
- 3.Wee S, Carroll FI, Woolverton WL. A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants. Neuropsychopharmacology. 2006;31:351–362. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- 4.Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KG, Woolverton WL. Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology. 2003;167:424–430. doi: 10.1007/s00213-003-1435-9. [DOI] [PubMed] [Google Scholar]
- 6.Kelly TH, Foltin RW, Emurian CS, Fischman MW. Effects of delta 9-THC on marijuana smoking, dose choice, and verbal report of drug liking. J Exp Anal Behav. 1994;61:203–211. doi: 10.1901/jeab.1994.61-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilens TE, Adamson J, Monuteaux MC, Faraone SV, Schillinger M, Westerberg D, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162:916–921. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98:1084–1088. [PubMed] [Google Scholar]
- 10.Setlik J, Bond GR, Ho M. Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics. doi: 10.1542/peds.2008-0931. Published online Aug 24, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 12.Stoops WW, Glaser PE, Rush CR. Reinforcing, subject-rated, and physiological effects of intranasal methylphenidate in humans: a dose-response analysis. Drug Alcohol Depend. 2003;71:179–186. doi: 10.1016/s0376-8716(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 13.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 14.Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- 15.McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instr. 1982;14:463–466. [Google Scholar]
- 16.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 17.Mazur JE. An adjusting amount procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analyses of behavior: the effects of delay and of intervening events on reinforcement value. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- 18.Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly TH, Foltin RW, Fischman MW. The effects of repeated amphetamine exposure on multiple measures of human behavior. Pharmacol Biochem Behav. 1991;38:417–426. doi: 10.1016/0091-3057(91)90301-h. [DOI] [PubMed] [Google Scholar]
- 20.Lile JA, Stoops WW, Durell TM, Glaser PE, Rush CR. Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2006;14:136–147. doi: 10.1037/1064-1297.14.2.136. [DOI] [PubMed] [Google Scholar]
- 21.Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacol Biochem Behav. 2007;87:258–266. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoops WW, Lile JA, Glaser PE, Rush CR. Discriminative stimulus and self-reported effects of methylphenidate, d-amphetamine, and triazolam in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2005;13:56–64. doi: 10.1037/1064-1297.13.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Jasinski DR, Krishnan S. Human pharmacology of intravenous lisdexamfetamine dimesylate: abuse liability in adult stimulant abusers. J Psychopharmacology. 2009;23:410–418. doi: 10.1177/0269881108093841. [DOI] [PubMed] [Google Scholar]
- 25.Kahn DA, Prohovnik I, Lucas LR, Sackeim HA. Dissociated effects of amphetamine on arousal and cortical blood flow in humans. Biol Psychiatry. 1989;25:755–767. doi: 10.1016/0006-3223(89)90247-3. [DOI] [PubMed] [Google Scholar]
- 26.Angrist B, Corwin J, Bartlik B, Cooper T. Early pharmacokinetics and clinical effects of oral d-amphetamine in normal subjects. Biol Psychiatry. 1987;22:1357–1368. doi: 10.1016/0006-3223(87)90070-9. [DOI] [PubMed] [Google Scholar]
- 27.Asghar SJ, Tanay VA, Baker GB, Greenshaw A, Silverstone PH. Relationship of plasma amphetamine levels to physiological, subjective, cognitive and biochemical measures in healthy volunteers. Hum Psychopharmacol. 2003;18:291–299. doi: 10.1002/hup.480. [DOI] [PubMed] [Google Scholar]
- 28.Brauer LH, Ambre J, De Wit H. Acute tolerance to subjective but not cardiovascular effects of d-amphetamine in normal, healthy men. J Clin Psychopharmacol. 1996;16:72–76. doi: 10.1097/00004714-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41:1225–1231. doi: 10.1177/00912700122012779. [DOI] [PubMed] [Google Scholar]
- 31.Arria AM, Caldeira KM, O’Grady KE, Vincent KB, Johnson EP, Wish ED. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28:156–169. doi: 10.1592/phco.28.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupont RL, Coleman JJ, Bucher RH, Wilford BB. Characteristics and motives of college students who engage in nonmedical use of methylphenidate. Am J Addict. 2008;17:167–171. doi: 10.1080/10550490802019642. [DOI] [PubMed] [Google Scholar]
- 33.McCabe SE, Teter CJ. Drug use related problems among nonmedical users of prescription stimulants: a web-based survey of college students from a Midwestern university. Drug Alcohol Depend. 2007;91:69–76. doi: 10.1016/j.drugalcdep.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coetzee M, Kaminer Y, Morales A. Megadose intranasal methylphenidate (ritalin) abuse in adult attention deficit hyperactivity disorder. Subst Abus. 2002;23:165–169. doi: 10.1080/08897070209511486. [DOI] [PubMed] [Google Scholar]
- 35.Garland EJ. Intranasal abuse of prescribed methylphenidate. J Am Acad Child Adolesc Psychiatry. 1998;37:1242–1243. doi: 10.1097/00004583-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe SL. Intranasal abuse of prescribed methylphenidate by an alcohol and drug abusing adolescent with ADHD. J Am Acad Child Adolesc Psychiatry. 1991;30:773–775. [PubMed] [Google Scholar]
- 37.Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, et al. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]