Abstract
Human cytomegalovirus (HCMV) is the most genetically and structurally complex human herpesvirus and is composed of an envelope, a tegument, and a dsDNA-containing capsid. HCMV tegument plays essential roles in HCMV infection and assembly. Using cryo-electron tomography (cryoET), here we show that HCMV tegument compartment can be divided into two sub-compartments: an inner and an outer tegument. The inner tegument consists of densely-packed proteins surrounding the capsid. The outer tegument contains those components that are loosely packed in the space between the inner tegument and the pleomorphic glycoprotein-containing envelope. To systematically characterize the inner tegument proteins interacting with the capsid, we used chemical treatment to strip off the entire envelope and most tegument proteins to obtain a tegumented capsid with inner tegument proteins. SDS-polyacrylamide gel electrophoresis analyses show that only two tegument proteins, UL32-encoded pp150 and UL48-encoded high molecular weight protein (HMWP), remains unchanged in their abundance in the tegumented capsids as compared to their abundance in the intact particles. 3D reconstructions by single particle cryo-electron microscopy (cryoEM) reveal that the net-like layer of icosahedrally-ordered tegument densities are also the same in the tegumented capsid and in the intact particles. CryoET reconstruction of the tegumented capsid labeled with an anti-pp150 antibody is consistent with the biochemical and cryoEM data in localizing pp150 within the ordered tegument. Taken together, these results suggest that pp150, a betaherpesvirus-specific tegument protein, is a constituent of the net-like layer of icosahedrally-ordered capsid-bound tegument densities, a structure lacking similarities in alpha and gammaherpesviruses.
Keywords: human cytomegalovirus, tegument proteins, single-particle analysis, cryo-electron microscopy, cryo-electron tomography, chemical treatment, pp150
Introduction
Human cytomegalovirus (HCMV), a member of the betaherpesvirus subfamily of the Herpesviridae, is the leading viral cause of birth defects and life-threatening complications in immuno-compromised individuals (Britt and Alford 1996; Mocarski et al. 2007). HCMV is the most genetically and structurally complex human herpesvirus, with a dsDNA genome of 240 kilo-basepairs encoding over 220 open reading frames (ORFs) and at least145 unique genes (Chee et al. 1990; Dunn et al. 2003; Murphy et al. 2003; Dolan et al. 2004; Mocarski et al. 2007; Cunningham et al. 2010). Like other herpesviruses, the virion of cytomegalovirus has a multilayer structure composed of an envelope, a tegument layer, and an icosahedral capsid enclosing a dsDNA genome. The capsid shares a characteristic architecture with other herpesviruses, composed of pentons, hexons and triplexes arranged on a T=16 icosahedral lattice (Butcher et al. 1998; Chen et al. 1999; Trus et al. 1999; Bhella et al. 2000).
About 30 proteins of the ~145 HCMV gene products with molecular masses ranging from 8.5 to over 200 kDa can be readily detected from purified HCMV virions as major constituents of the capsid, tegument and envelope layers by electrophoresis. About 20 of these are components of the tegument compartment, which accounts for ~40% of the total HCMV protein mass. As in all other herpesviruses, tegument proteins play important roles in initiating infection, modulating host cell metabolism, regulating viral gene expression, assisting transport of newly synthesized viral proteins across the nuclear membrane, controlling viral DNA packaging, and coordinating the maturation and egress of progeny virions (Zhu et al. 1997; Newcomb et al. 2001; Mocarski et al. 2007). Despite their important functional and structural roles in HCMV infection, the tegument proteins remain poorly characterized both biochemically and structurally (see below). The major HCMV tegument proteins include in their order of abundance as detected on SDS gels; the UL83-encoded lower matrix protein (LM/ppUL83/pp65), the UL32-encoded basic phosphorylated protein ( BPP/ppUL32/pp150), the UL48-encoded high molecular weight protein (HMWP), the UL47-encoded HMWP-binding protein (HMWBP/pUL47), the UL82-encoded upper matrix protein (UM/ppUL82/pp71), and ppUL99 (pp28). Most of these proteins are phosphorylated (as designated by the ‘pp’ prefix) (Baldick and Shenk 1996; Gibson 1996; Varnum et al. 2004).
Among the three structurally distinct compartments of the HCMV, the innermost compartment, the icosahedral capsid, is structurally and biochemically well characterized. The three-dimensional (3D) capsid structures of the HCMV (Butcher et al. 1998; Chen et al. 1999) and the simian CMV (Trus et al. 1999) from cryo-electron microscopy (cryoEM) have revealed features similar to those of herpes simplex virus type-1 (HSV-1) (Schrag et al. 1989; Booy et al. 1991; Trus et al. 1992; Zhou et al. 1994; Steven and Spear 1997; Zhou et al. 2000) and Kaposi’s sarcoma-associated herpesvirus (Wu et al. 2000; Nealon et al. 2001; Trus et al. 2001; Lo et al. 2003), members of the alpha and gammaherpesvirus subfamilies, respectively. The capsid shell of all these herpesviruses is composed of four major proteins: the major capsid protein (MCP; encoded by UL86) (Chee et al. 1989), the minor capsid protein (mCP; encoded by UL85), the mCP binding protein (mC-BP; encoded by UL46) (Gibson et al. 1996a), and the smallest capsid protein (SCP; encoded by UL48.5) (Baldick and Shenk 1996; Gibson et al. 1996b; Borst et al. 2001; Yu et al. 2005).
Beyond this conserved capsid shell, however, little is known about the 3D structural organization of HCMV tegument and membrane layers. CryoEM reconstruction, which smeared pleomorphic structural features due to averaging across different particles, also revealed some icosahedrally ordered tegument densities beyond this conserved capsid shell. However, these tegument densities bear no similarities not only between alphaherpesvirus (such as HSV-1) and betaherpesvirueses (such as HCMV) (Zhou et al. 1999; Chen et al. 2001), but also between different members of betaherpesvirus subfamily, such as human cytomegalovirus (Chen et al. 2001) and simian cytomegalovirus (Trus et al. 1999). In HCMV, a cluster of three filamentous tegument densities emanates from each triplex and binds to three MCP subunits at their upper domains; thus 320 such clusters in total form a net enclosing each entire capsid (Chen et al. 1999). 3D structures of cytoplasmic capsids of simian cytomegalovirus revealed two kinds of protein densities, one type bound only to the hexons/pentons (960 copies/capsid) and the other only to triplexes (640 copies/capsid), respectively (Trus et al. 1999). In HSV-1, a different set of tegument density is observed and they have an extended ribbon shape and bind only pentons and triplexes surrounding pentons (Zhou et al. 1999; Chen et al. 2001) and a heterodimer of UL25/UL17 proteins is shown to be the constituent of this ribbon-shaped density (Trus et al. 2007). However, the identities of the capsid-bound tegument densities remain unknown.
In the present study, we use cryo-electron tomography (cryoET) to reveal the pleomorphic structural compartments of the intact HCMV particle, including the glycoprotein-containing envelope, and the tegument compartment. We then systematically characterized the capsid-bound, ordered tegument proteins and their interactions with the capsid proteins using biochemical and structural methods. These results are further correlated with cryoET studies of tegumented capsid generated by chemical treatment and subsequently labeled with anti-pp150 antibodies. Our results indicate that pp150, an essential tegument protein abundantly presents in betaherpesviruses but not in alpha and gammaherpesviruses, is a constituent of the HCMV inner tegument layer directly interacting with the conserved capsid.
Results
Architecture of HCMV revealed by cryo-electron tomography
Previous structural studies have been limited to HCMV capsids and ordered tegument proteins by single-particle cryoEM; but the envelope and the bulk of the tegument compartment are pleomorphic thus cannot be resolved by such technique. Here, we use cryoET to resolve the pleomorphic structures of HCMV (Fig. 1). Serial slices extracted from the 3D tomograms of the HCMV particles (Fig. 1b, d) clearly reveal mass densities of the capsid, the viral envelop, and the tegument compartment. Densities of glycoproteins (shown by arrows in Fig. 1b-e) radiate out from the viral envelope. Some of these glycoprotein densities appear as clusters, suggesting possible glycoprotein complex formation (arrow in Fig. 1c, e). Viral glycoproteins are responsible for the early steps of infection, such as recognition and binding host cell receptors. For HCMV, at least 8 glycoproteins have been experimentally identified, among which gB (UL55), gM/gN, and gH/gL, have been shown to be essential for viral replication (Britt and Boppana 2004). The limited resolution of cryoET structure has precluded assigning densities to any of these individual glycoproteins.
Fig.1. Cryo-electron tomography of intact HCMV particles.
(a) Representative images in a cryoET tilt series of ice-embedded intact HCMV particles. (b, d) Serial slices were extracted from 3D tomograms of two representative intact HCMV particles, one in which the capsid is almost centrally located (b) and another in which the capsid is located near some part of the membrane (d). (c, e) Shaded surface representations of slabs from the virus particles in b, d. Both of them show some tegument proteins (green) tightly bind and surround the whole capsid shell (yellow). Except those tightly bind tegument proteins, the other tegument proteins (blue) seem to randomly float between the membrane (purple) and the capsid. The outmost density protrusions from the membrane should be the glycoprotein spikes.
Our cryoET reconstructions also reveal the structural organization of the HCMV tegument compartment and the enclosed capsid. The tegument densities are organized into two distinctive parts: an inner tegument (green) and an outer (blue) tegument (Fig. 1c, e). The inner tegument appears to be ordered and forms a densely packed density layer (shown in green in Fig. 1c, e) completely enclosing the entire icosahedral capsid shell (shown in yellow in Fig. 1c, e). The outer tegument includes densities located in the space between the envelope and the inner tegument, which appear to be “free-floating” and thus pleomorphic (shown in blue in Fig. 1c, e).
Biochemical characterization of tegumented capsids from chemically treated HCMV
While much is known about the protein composition of the viral envelope and capsid of HCMV (Baldick and Shenk 1996; Gibson 1996; Varnum et al. 2004), the identity and arrangement of proteins in the tegument compartment remain elusive. Here, we carried out stepwise treatment of intact HCMV particles with increasing concentrations of chemicals to remove the envelope and those tegument proteins that are unbound or loosely bound to the capsid (Table 1), producing tegumented capsids. Negative stain transmission electron microscopy and cryoEM reconstruction, are used to monitor the structural organization and integrity of the tegumented capsids after each treatment. We followed two criteria to obtain an optimized treatment protocol for generating tegumented capsids: (1) The capsid within the resulting tegumented capsid should be intact and its 3D structure should remain the same as that of nuclear capsid; (2) The relative chemical abundance of capsid shell proteins MCP and mCP in the resulting tegumented capsids should remain unchanged upon treatment (capsid protein mCP-BP is heat-aggregable (Gibson et al. 1996a) and not consistent in SDS-PAGE).
TABLE I.
Selective removal of envelope and tegument proteins
| Reagents Used in the Treatments |
Effects on Virion Particles | |
|---|---|---|
| Assessed by negative stain electron microscopy |
Assessed by cryoEM and 3D reconstruction |
|
| 0.5% NP-40 or 0.5% Triton X-100 |
Envelope stripped completely; however, chunks of tegument proteins remained irregularly attached to the capsid, masking the visibility of capsomers |
Not performed |
| 0.5% NP-40 or 0.5% Triton X-100, 10 mM DTT |
Envelope stripped completely; fewer chunks of irregularly attached tegument proteins were visible. |
A thin layer of tegument proteins can be seen attached to the capsid in the cryoEM micrographs. |
| 1% NP-40 or 1% Triton X- 100, 0.01% SDS, 10 mM DTT |
Envelope stripped completely; most tegument proteins appeared to be removed, resulting in capsid-like particles |
Particles in the cryoEM micrographs appeared very similar to those of purified capsids. 3D structures showed the presence of tegument densities attached to the capsid, similar to those in the virion. |
| 1% Triton X-100, 0.02% SDS, 10 mM DTT for 8 h |
Same as above | Same as above |
| 1% Triton X-100, 0.02% SDS, 10 mM DTT, 1M NaCl for 8 h |
Same as above | Very few capsid-like particles were visible in cryoEM micrographs due to the high salt concentration. |
| 2% Triton X-100, 0.02% SDS, 10 mM DTT |
Disintegrated capsids begin to appear |
Not performed |
We have developed a treatment protocol that reproducibly generates tegumented capsids (Fig. 2a) from intact HCMV particles (Fig. 2b). In our protocol, intact HCMV particles were incubated in 1% Triton X4100, 10 mM DTT and 0.02% SDS for 8 h at 4°C. The resulting tegumented capsids were isolated and analyzed by SDS-PAGE (Fig. 2c). The density profiles of the sample lanes of the gel were background subtracted, scaled (Fig. 3), and used to quantify changes in the protein composition of the treated sample, as summarized in Table 2. The results are summarized in Figs. 2c, 3 and Table 2 and include the following observations. First, no glycoproteins were found in our tegumented capsid preparation. Second, the relative chemical abundance of capsid shell proteins MCP and mCP in the resulting tegumented capsids were essentially unchanged following the treatment. (The slight decrease in the SCP abundance is as expected. SCP binds the outermost region of the capsid loosely and is known to readily fall off from the capsid during purification and storage). Third, the treatment did not significantly change the abundance of two tegument proteins (pp150 and HMWP), but reduced the relative abundance of all other tegument proteins.
Fig. 2. Cryo-electron microscopy and SDS PAGE of intact HCMV particles and tegumented capsids.
(a) Electron micrograph of tegumented capsids embedded in vitreous ice, recorded at 100 kV. The tegumented capsids were obtained by treatment of intact HCMV particles with 1% Triton X-100, 0.02% SDS and 10 mM DTT for 8 hours at 4°C, and purified through a double sucrose cushion. Some tegument proteins can be seen tethered to the capsids (indicated by arrow). The underfocus value was determined to be 2.7 μm. The inset shows a cryoEM picture of two nuclear capsids. (b) Electron micrographs of ice-embedded intact HCMV particles were recorded at 400 kV. The underfocus value of this image was determined to be 3.8 μm. (c) 10–20% gradient SDS-PAGE showing protein compositions of intact HCMV particles and tegumented capsids. The gel was stained with silver nitrate.
Fig. 3. Comparison of protein densities on SDS-PAGE gel.
The silver-stained SDS-PAGE shown in Fig. 2 was digitized using a densitometer and each lane was horizontally averaged to produce a one-dimensional representation of the protein bands. The profile of the tegumented capsid lane was normalized against that of the intact HCMV using the lane profile of the intact HCMV.
TABLE II.
Differences in the structural protein composition of treated and untreated HCMV particles
| Compartment or Location |
ORF | Common Protein Name | Size (kDa) |
% Decrease in Chemically Treated Particles |
|---|---|---|---|---|
|
Capsid
Proteins |
UL86 | MCP (major capsid protein) | 154 | no change |
| UL85 | mCP (minor capsid protein) | 35 | no change | |
| UL46 | mC-BP (mCP binding protein) | 30 | ||
| UL48.5 | SCP (smallest capsid protein) | 8.5 | 40 | |
| UL80a | AP (assembly protein) | 38 | 15 | |
|
Tegument
Layer |
UL83 | LM (lower matrix protein), pp65 | 65 | 90 |
| UL82 | UM (upper matrix protein), pp71 | 71 | 100 | |
| UL32 | BPP (basic phosphoprotein or pp150) | 150 | 5 | |
| UL99 | pp28 | 28 | 100 | |
| UL48 | HMWP (high molecular weight protein) | 212 | no change | |
| UL47 | HMWBP (HMWP binding protein) | 108 | 55 | |
|
Lipid
Envelope |
UL55 | gB | 58 | 100 |
| UL115 | gL | 34 | 100 |
3D structural comparisons of nuclear capsid, tegumented capsid and intact HCMV particle
CryoEM imaging of the tegumented capsids obtained under our optimal treatment conditions clearly showed that the treatment produced non-enveloped particles (Fig. 2a) with dimensions similar to those of the nuclear capsids (inset of Fig. 2a). The tegumented capsids are approximately half the diameter of the intact particles and lacked the prominent lipid-bilayer envelope and the amorphous tegument layer (cf. Fig. 2a and b). This capsid-like appearance of the treated particles indicated that the tegument proteins remaining associated with the particles were in close proximity to the capsid.
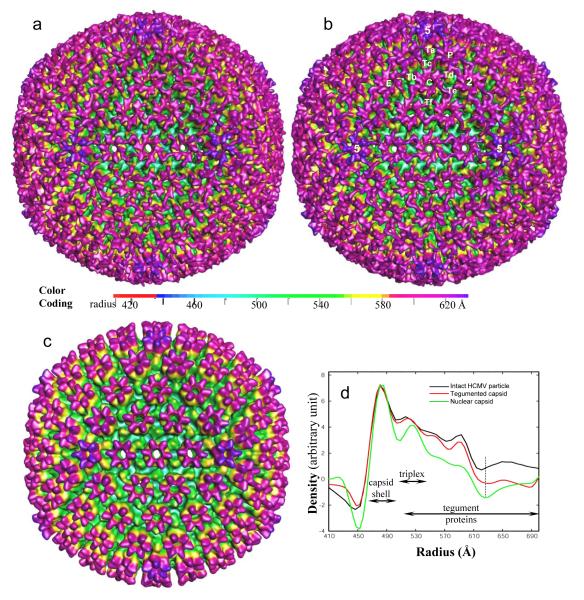
The shaded surface representation of the 3D reconstruction of the tegumented capsids determined at 25-Å resolution (Fig. 4a) is different from that of the nuclear capsids (Fig. 4c), but almost indistinguishable from that of the intact particle (Fig. 4b). Except for a slightly larger internal diameter, the HCMV capsid is similar to the capsids of other herpesviruses (Baker et al. 1990; Trus et al. 1992; Zhou et al. 1994; Butcher et al. 1998; Trus et al. 1999; Wu et al. 2000; Trus et al. 2001), having a characteristic T=16 icosahedral capsid with 12 pentons, 150 hexons, and 320 triplexes (Fig. 4c). The outer tegument layer and the envelope of the intact HCMV particle are organized in a non-icosahedrally symmetric manner, and thus were smeared by icosahedral averaging and became invisible in the surface representation of the 3D reconstruction (Fig. 4b) (Chen et al. 1999). Unlike other herpesviruses, the reconstructions of the tegumented capsids and intact HCMV particles both contain additional clusters of filamentous tegument densities forming a net enclosing the capsid (Fig. 4a, b). Radial density distributions of the reconstructions of the nuclear capsid, the tegumented capsids and the intact particles show that the averaged density in the tegumented capsid extends only about 40 Å before it reaches the level of background density in the capsid, but averaged density in the intact particle remains obviously above the background level beyond this point (Fig. 4d). This extended density level is due to the existence of other non-icosahedrally ordered tegument proteins beyond the inner tegument. The small difference in both the radius and density levels between the tegumented capsid and the nuclear capsid further confirms that most, if not all, disordered or non-specifically packed tegument proteins in the intact particle have been completely or largely removed by the treatment, as is also apparent in Fig. 2a. The nearly identical icosahedral 3D reconstructions of the tegumented capsid and the intact particle reconstructions indicate that the treatment did not disrupt the icosahedrally ordered capsid-tegument interactions while it stripped off the components of the outer tegument and envelope.
Fig. 4. Comparisons of the 3D reconstructions by single particle cryoEM.
(a–c) Shaded surface representations of the 3D maps of the tegumented capsids (a), intact HCMV particles (b), and nuclear capsids (c), as viewed along an icosahedral 2-fold symmetry axis. The labels in (b) include 5-fold (5) and 2-fold (2) axes and pentons (5), three hexons (P, E, C) and six triplexes (Ta, Tb, Tc, Td, Te, and Tf). The icosahedral 3-fold axis passes through the middle of Tf. The maps are displayed at a threshold of one standard deviation above the mean density and color-coded according to the particle radius (see color bar), such that the outermost densities (the tegument densities and the upper domain of pentons and hexons) are in purple, the triplexes are in green, the bulk of the pentons and hexons in are yellow, and the capsid floor is in aquamarine. (d) Comparison of the radial density distributions of the 3D reconstructions of the nuclear capsid, the tegumented capsid and the intact particle. The dotted line at around 625 Å denotes the exterior boundary of the capsid. The approximate radial locations of the capsid shell, triplexes and tegument proteins are indicated by the double-headed arrows.
Each particle contains a total of 960 ‘L’-shaped densities sitting over a triplex. The longer side of the ‘L’ anchors to the upper domain of a triplex and the shorter side to the outermost region of either a penton or hexon subunit (Fig. 5a, c). Except for triplex Tf along the 3-fold axis, there are some noticeable differences among the three L-shaped densities interacting with each triplex, suggesting conformational flexibilities (Fig. 5a).
Fig. 5. Interactions of the tegument densities with the triplex and the top domains of MCP.
(a) Zoom-in surface representation of a region from the 3D reconstruction of the tegumented capsids showing the hexon along a 2-fold axis (labeled with 2), triplex Te and its associated tegument proteins. The densities are color-coded according to the particle radius as in Fig. 4, such that the triplex (Te here) is in green and the upper domain of hexon and the bulk of the tegument densities are in purple. Three densities, two thin (solid arrows) and one thick (open arrow), are resolved interacting with each triplex. Each of these densities has the shape of a flipped “L”. (b) Shaded surface representation of the nuclear capsid reconstruction viewed similarly to that shown in (a), except that the map is now only shown in light blue. (c) Superposition of the structures shown in (a) and (b). The same color coding as in (a) is used for the densities in the tegumented capsid reconstruction; semi-transparent light blue is used for the densities of the nuclear capsid reconstruction.
We further confirmed the location of pp150 through cryoET reconstruction of tegumented capsids labeled with antibody specific to pp150. In this experiment, purified HCMV tegumented capsids were labeled by over-saturation with an anti-pp150 antibody, and then subjected to cryoET analyses. Fig. 6a shows representative raw images in the tomography tilting series. A central slice from its 3D tomography reconstruction revealed that the antibodies caused cross-linking of tegumented capsids (Fig. 6b), secondary to the bivalent binding property of antibodies. The surface representation of a segmented particle reveals additional external densities decorating the tegumented capsid. The size and shape of these densities are roughly consistent with those expected for individual IgG molecules (Fig. 6c-e). This labeling result and the results from the above biochemical and structural analyses all support the interpretation of pp150 being a component of the ordered tegument densities bound to the capsid.
Fig. 6. Confirmation of pp150 identification by cryoET of tegumented capsid labeled by anti-pp150 antibody.
Tegumented capsids were labeled with over-saturated rabbit anti-pp150 antibody (Sanchez et al. 1998) and then imaged by cryoET. (a) Representative raw images in a cryoET tilt series. (b) Central slice of the 3D cryoET reconstruction. The tegumented capsids aggregate due to antibody cross-linking. (c) Surface representation of particles segmented out from the tomogram. Brown: tegumented capsid. Red: additional densities attached to the tegumented capsid. (d) Ribbon diagram of the crystal structure of an IgG molecule (PDB code: 1HZH). (e) Zoom-in view of the boxed region of (c) showing a placement of the IgG crystal structure (cyan ribbon) into the semitransparent red density attached to the surface of the tegumented capsids. The size and shape of the attached densities match those of the IgG model.
Discussion
The HCMV tegument proteins have been shown to have multiple roles in the replicative cycle of HCMV. Our 3D tomographic reconstructions of HCMV particles show that the tegument proteins are localized into two sub-compartments within the tegument: outer tegument proteins that are detached from the capsid and inner tegument proteins that are attached to the capsid. Some tegument proteins, such as the most abundant LM, have trans-acting function roles (e.g., immune evasion and regulating viral gene expression) as detached molecules inside newly infected cells, thus need to be released into the host cell cytoplasm upon viral entry. Such proteins can be thought of as “cargo” proteins packed into intact particles in a detached form, so as to facilitate their eventual release as individual molecules into host cells to help establish infection. In contrast to outer tegument proteins, inner tegument proteins are attached to the capsid and may have cis-acting functions, such as stabilizing capsid and mediating intra-cellular capsid transport. Identification of these inner tegument proteins represents an essential step towards understanding HCMV capsid/tegument assembly, cellular capsid transport, and delivery of the viral genome to the nucleus of the newly infected cell.
Of the about 20 tegument proteins identified in HCMV, only 6 are present in sufficiently large quantities and can be considered as candidates for the ordered inner tegument density: pp150, UM, LM, pp28, HMWP and its binding protein HMWBP (Figs. 2c and 3). Of these proteins, pp150 and HMWP are tightly bound to the capsid and were essentially unaffected by the chemical treatments (Table 2). In contrast, UM and pp28 were completely removed by the chemical treatment (Table 2). The residual amount of HMWPB in the tegumented capsid is insufficient to account for even the minimal copy number (320 copies/particle) of icosahedrally-ordered tegument densities. Although 10% of LM remains after treatment, LM is unlikely to be part of the capsid-interacting tegument densities for the following reasons. Over 90% protein mass of HCMV dense body is due to LM, yet dense body does not contain a capsid. LM plays key roles in immune evasion either by blocking the major histocompatibility complex class I presentation of viral immediately-early protein and the recognition of infected cells by cytotoxic T lymphocytes within the cells (Gilbert et al. 1996) or by inhabiting the natural killer cell cytotoxicity by binding to the activating receptor NKp30 (Arnon et al. 2005). Those functions would require LM to be a free or loosely bound protein (i.e., a “cargo” protein as discussed above), not immobilized as those in the capsid-bound inner tegument protein. Taken together, we suggest that the candidates for forming the ordered tegument densities observed in our reconstructions of intact HCMV particles should be pp150 and/or HMWP.
It has been shown that only the N-terminal one-third of pp150 is essential for binding to capsid (Baxter and Gibson 2001) and the C-terminal portion of pp150 is modified by glycosylation through O-GlcNAc (Greis et al. 1994), which could mediate binding of pp150 to HMWP. The copy number of HMWP in each HCMV as estimated from Fig. 3 is less than that of pp150. It is conceivable that the low occupancy and possible flexibility of the C-terminal region of pp150 have made HMWP invisible in our single particle cryoEM map. Indeed, extended tether-like projections can be seen in the cryoEM images of the tegumented capsids (arrows in Fig. 2a). Our tomograms of intact HCMV particles and tegumented capsids also show that the inner tegument proteins have pleomorphic projections extending outwards from the capsid shell, which could be HMWP (Fig.1b-e and Fig. 6b). The contributions of pp150 and HMWP to the formation of the inner tegument layer and their exact stoichiometry in the virion await further biochemical and structural analyses.
Among all herpesviruses, HCMV has the largest DNA genome. The volume available inside the capsids of HCMV and other herpesviruses are similar, thus the DNA genome inside HCMV is more tightly packed (Bhella et al. 2000), leading to stronger static electronic repulsion inside the HCMV capsid. It is conceivable that the betaherpesvirus-specific pp150 might play the role of strengthening the capsid by directly binding to its outer surface to form a reinforcing net structure, thus stabilizing the genome-containing capsid during virion assembly. Indeed, inhibition of the pp150 expression prevented virion formation; but did not affect capsid assembly, viral DNA packaging and formations of non-infectious enveloped particles (Meyer et al. 1997).
A hallmark of herpesvirus structure is the large tegument compartment. One of the central issues in the study of herpesviruses has been the structural organization of the tegument proteins. Our results provide the first direct evidence suggesting that pp150 and HMWP are tightly associated with the HCMV capsid. In addition to the role of stabilizing genome-containing capsid, these inner tegument proteins could also serve to direct incorporation of essential tegument proteins into the infectious particle through protein:protein interactions. Further structural studies, especially higher resolution cryo-electron tomography structures of particles containing different kind of tegument proteins, are needed to further define the role of these proteins in the replication of HCMV and provide additional insight into the assembly of HCMV infectious particles.
Materials and Methods
Virus purification
Intact HCMV particles were purified directly from human foreskin fibroblasts infected with HCMV strain AD169 (ATCC, Rockville, MD) using a protocol modified from published procedures (Baldick and Shenk 1996; Bhella et al. 2000). Briefly, human foreskin fibroblasts were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum and propagated in roller bottles. The cells were initially infected with HCMV at an MOI of 0.1–0.5 and incubated for 7–10 d until the cells reached 100% cytopathic effect. The media containing the infected cells from a total of 50 two-liter roller bottles were collected and centrifuged at 2000×g for 1 h at 4°C to remove cells and large cellular debris. The supernatant was then spun at 60,000×g for 1 h at 4°C to pellet HCMV virus particles. The pellet was resuspended in 20 mM phosphate-buffered saline (PBS) at pH 7.4 and then subjected to centrifugation through a 15–50% (w/w) sucrose density gradient at 60,000×g for 1 h at 4°C. The fraction containing intact HCMV particles (judged by negative-stain EM to have intact viral envelopes) was collected, pelleted, and further purified through a glycerol-tartrate gradient (15–35% potassium tartrate and 30–0% glycerol, w/v) at 170,000×g for 20 min at 4°C. After the centrifugation, the fraction containing intact virus particles was collected, pelleted, and resuspended in PBS. The nuclear capsids were isolated from the nuclei of infected cells as previously described (Butcher et al. 1998).
Chemical treatments, SDS-polyacrylamide gel electrophoresis and quantification
Purified HCMV particles were incubated in a solution with different concentrations of NP-40 or Triton X-100, dithiothreitol (DTT), and SDS at 4°C for different incubation times. The effects of each treatment, including the integrity of the HCMV particles and the number of resulting tegumented capsids, were first assessed by negative stain EM and, when necessary, subsequently by cryoEM (see below). The incubation with 0.02% SDS, 1% Triton X-100, and 10 mM DTT in PBS at 4°C for 8 h resulted in the most tegumented capsids, which were analyzed by SDS-PAGE. After the incubation, the sample was centrifuged over a 45% and 65% (w/v) double sucrose cushion, containing the same concentration of detergents and other reagents, at 90,000×g for 90 min. The tegumented capsids were collected from the interface between the 45% and 65% sucrose, immediately diluted with PBS, and centrifuged at 50,000×g for 60 min. Some of the pellet was suspended in PBS for cryoEM analyses, while some was dissolved in an equivalent volume of SDS sample buffer [100 mM Tris-HCl (pH 6.8), 200 mM DTT, 4% SDS, 0.2% bromophenol blue and 20% glycerol], boiled at 100°C for 5 min, run on 10–20% pre-cast PAGE, and stained using a silver staining kit (Bio-Rad, Hercules, CA).
For densitometry analysis, the silver-stained SDS-PAGE gels were digitized on a Zeiss SCAI high accuracy microdensitometer (Z/I Imaging, Huntsville, AL). The densities across individual bands were averaged horizontally to create a stain density profile for each lane. SigmaPlot2000 (SPSS Inc.) was used to estimate the baseline, by selecting the set of data points defining the bases of the profile and fitting them into a smooth curve. Due to local variations in the backgrounds of the gels, the baselines of the two profiles were slightly different (notably in the high molecular weight regions of the gels; see Fig. 2c) and were thus subtracted from the original profiles to create normalized density profiles. Because mCP is a median-sized capsid protein and its shape and mass density remained the same in the 3D reconstructions obtained from intact particles and tegumented capsids (compare triplexes in Fig. 4a, b), we used mCP as a reference to scale the density profiles so that the mCP bands in the tegumented capsids and intact HCMV particles lanes had the same relative heights.
Cryo-electron tomography
Cryo-electron tomography of intact HCMV particles and tegumented capsids labeled with anti-pp150 antibodies were performed in a 300 kV transmission cryo-electron microscope from FEI (Hillsboro, Oregon), equipped with a 4k × 4k pixel TVIPS CCD camera. A 34μl aliquot of each of the HCMV preparations was applied to a 3/1μm Quantifoil holey carbon grid, quickly blotted with filter paper, and plunged into liquid nitrogen-cooled ethane so that particles were embedded in a thin layer of vitreous ice across the holes of the carbon film. 200 mesh grids were used so that the edge of the holes would not block the beam when the stage is tilted in the range of −70 to 70°. Computer-controlled tilting, sample tracking, and image recording were performed using the EMMENU3.0 image acquisition software package (TVIPS, Germany) using a 2° incremental interval and a dosage of about 1 electron/Å2. The accumulative dosage for each tilt series is about 100 electrons/Å2. The magnification used is 38,180x, corresponding to a pixel size of 3.93 Å/pixel at the specimen level.
For antibody labelling of tegumented capsids, intact particles were first subjected to the above chemical treatment to generate tegumented capsids. Then the tegumented capsids were incubated overnight with anti-pp150 antibody before grid preparation by flash freezing. The rabbit anti-pp150 antibody used here was described in a previous study of the intracellular localization of pp150 during HCMV assembly (Sanchez et al. 1998).
Data processing for tomographic reconstruction was carried out using the Protomo program package (Winkler and Taylor 1996; Taylor et al. 1997; Taylor et al. 1999). Briefly, a preliminary tomogram was obtained by merging the aligned images in the series after low-pass filtering to the first zero of the contrast transfer function, using the goniometer readings as the tilt angles. Translational and rotational (tilt angle) alignment and tilt axis refinement were subsequently performed using the preliminary tomogram. Several rounds of refinement of the alignment and area matching were carried out until no improvement in the tomogram was detectable. Tomograms were segmented using Amira (Mercury Computer Systems, Inc., Chelmsford, MA) and segmented structures were visualized using UCSF Chimera (Pettersen et al. 2004).
Electron microscopy and 3D reconstruction by single particle analysis
For negative stain EM, a 2.5-μl aliquot of the sample was applied to a carbon-coated copper EM grid and stained by washing twice with an excess of 2% uranyl acetate solution. The effects of each of these treatments on the HCMV envelope, tegument layer, and capsid integrity were assessed by examining the negatively stained samples in a Philips CM12 electron microscope operated at 100 kV.
CryoEM imaging was performed as described previously (Wu et al. 2000). Briefly, a 3-μl aliquot of each of the HCMV preparations was applied to a 400 mesh copper grid coated with holey carbon film, quickly blotted with filter paper, and plunged into liquid nitrogen-cooled ethane. CryoEM images were recorded at a magnification of 30,000× using an electron dose of ~10 electrons/Å2/micrograph as previously described (Chen et al. 1999). Focal pairs of cryoEM micrographs were recorded, with the first, close-to-focus micrograph aimed at ~1.5 μm underfocus and the second, far-from-focus micrograph at ~3 μm underfocus.
For 3D reconstruction, selected micrographs were digitized on a Zeiss SCAI microdensitometer using a step size equivalent to 4.67 Å/pixel on the specimen. Data processing and visualization were carried the IMIRS package (Liang et al. 2002), which were based on Fourier common-lines (Crowther 1971; Fuller 1987; Fuller et al. 1996). The particles from far-from-focus micrographs were used to determine the initial orientation parameters of the corresponding particles in the close-to-focus micrographs, which were subsequently refined. The final 3D reconstructions were generated by merging only the close-to-focus particles (with defocus values ranging from 1.0–3.3 μm) using the Fourier-Bessel synthesis (Crowther 1971) with correction of the contrast transfer function (Zhou et al. 1999). The final 3D reconstruction of the chemically treated particles was computed to an effective resolution of 25 Å by combining 535 close-to-focus particle images. The 3D structure of the intact particles was determined to 15 Å resolution from 2106 particles and was subsequently filtered to 25 Å for direct comparison with treated particles. The nuclear capsid was reconstructed to similar resolution. The structures were visualized with UCSF Chimera (Pettersen et al. 2004).
ACKNOWLEDGMENTS
This research is supported in part by grants from the National Institutes of Health (AI069015 and GM071940 to ZHZ and AI035602 to WJB). We thank Yong-Hwan Kim for assistance with HCMV sample preparation, Ivo Atanasov for assistance with electron imaging and Jun Liu for advices in cryo-electron tomography.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, Gazit R, Gonen-Gross T, Hanna J, Nahari E, Porgador A, Honigman A, Plachter B, Mevorach D, Wolf DG, Mandelboim O. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- Baker TS, Newcomb WW, Booy FP, Brown JC, Steven AC. Three-dimensional structures of maturable and abortive capsids of equine herpesvirus 1 from cryoelectron microscopy. J Virol. 1990;64:563–573. doi: 10.1128/jvi.64.2.563-573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick CJ, Jr., Shenk T. Proteins associated with purified human cytomegalovirus particles. J. Virol. 1996;70:6097–6105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MK, Gibson W. Cytomegalovirus basic phosphoprotein (pUL32) binds to capsids in vitro through its amino one-third. J Virol. 2001;75:6865–6873. doi: 10.1128/JVI.75.15.6865-6873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhella D, Rixon FJ, Dargan DJ. Cryomicroscopy of human cytomegalovirus virions reveals more densely packed genomic DNA than in herpes simplex virus type 1. J. Mol. Biol. 2000;295:155–161. doi: 10.1006/jmbi.1999.3344. [DOI] [PubMed] [Google Scholar]
- Booy FP, Newcomb WW, Trus BL, Brown JC, Baker TS, Steven AC. Liquid-crystalline, phage-like packing of encapsidated DNA in herpes simplex virus. Cell. 1991;64:1007–1015. doi: 10.1016/0092-8674(91)90324-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst EM, Mathys S, Wagner M, Muranyi W, Messerle M. Genetic evidence of an essential role for cytomegalovirus small capsid protein in viral growth. J Virol. 2001;75:1450–1458. doi: 10.1128/JVI.75.3.1450-1458.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt WJ, Alford CA. Cytomegalovirus. In: Fields BN, Knipe DM, Howley PM, et al., editors. Fields Virology. Vol. 2. Lippincott-Raven Publishers; Philadephia: 1996. pp. 2493–2523. [Google Scholar]
- Britt WJ, Boppana S. Human cytomegalovirus virion proteins. Hum Immunol. 2004;65:395–402. doi: 10.1016/j.humimm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Butcher SJ, Aitken J, Mitchell J, Gowen B, Dargan DJ. Structure of the human cytomegalovirus B capsid by electron cryomicroscopy and image reconstruction. J Struct Biol. 1998;124:70–76. doi: 10.1006/jsbi.1998.4055. [DOI] [PubMed] [Google Scholar]
- Chee M, Rudolph SA, Plachter B, Barrel B, Jahn G. Identification of the major capsid protein gene of human cytomegalovirus. Journal of Virology. 1989;63:1345–1353. doi: 10.1128/jvi.63.3.1345-1353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison C. A. r., Kouzarides T, Martignetti JA. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. e. al. [DOI] [PubMed] [Google Scholar]
- Chen DH, Jakana J, McNab D, Mitchell J, Zhou ZH, Dougherty M, Chiu W, Rixon FJ. The pattern of tegument-capsid interaction in the herpes simplex virus type 1 virion is not influenced by the small hexon-associated protein VP26. J Virol. 2001;75:11863–11867. doi: 10.1128/JVI.75.23.11863-11867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DH, Jiang H, Lee M, Liu F, Zhou ZH. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology. 1999;260:10–16. doi: 10.1006/viro.1999.9791. [DOI] [PubMed] [Google Scholar]
- Crowther RA. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971;261:221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Gatherer D, Hilfrich B, Baluchova K, Dargan DJ, Thomson M, Griffiths PD, Wilkinson GW, Schulz TF, Davison AJ. Sequences of complete human cytomegalovirus genomes from infected cell cultures and clinical specimens. J Gen Virol. 2010;91:605–615. doi: 10.1099/vir.0.015891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A, Cunningham C, Hector RD, Hassan-Walker AF, Lee L, Addison C, Dargan DJ, McGeoch DJ, Gatherer D, Emery VC, Griffiths PD, Sinzger C, McSharry BP, Wilkinson GW, Davison AJ. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100:14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller SD. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987;48:923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Fuller SD, Butcher SJ, Cheng RH, Baker TS. Three-dimensional reconstruction of icosahedral particles--the uncommon line. J Struct Biol. 1996;116:48–55. doi: 10.1006/jsbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- Gibson W, Baxter MK, Clopper KS. Cytomegalovirus “missing” capsid protein identified as heat-aggregable product of human cytomegalovirus UL46. J Virol. 1996a;70:7454–7461. doi: 10.1128/jvi.70.11.7454-7461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Clopper KS, Britt WJ, Baxter MK. Human cytomegalovirus (HCMV) smallest capsid protein identified as product of short open reading frame located between HCMV UL48 and UL49. Journal of Virology. 1996b;70:5680–5683. doi: 10.1128/jvi.70.8.5680-5683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MJ, Riddell SR, Plachter B, Greenberg PD. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- Greis KD, Gibson W, Hart GW. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J Virol. 1994;68:8339–8349. doi: 10.1128/jvi.68.12.8339-8349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ke EY, Zhou ZH. IMIRS: a high-resolution 3D reconstruction package integrated with a relational image database. J Struct Biol. 2002;137:292–304. doi: 10.1016/s1047-8477(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Lo P, Yu X, Atanasov I, Chandran B, Zhou ZH. Three-Dimensional Localization of pORF65 in Kaposi’s Sarcoma-Associated Herpesvirus Capsid. J Virol. 2003;77:4291–4297. doi: 10.1128/JVI.77.7.4291-4297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HH, Ripalti A, Landini MP, Radsak K, Kern HF, Hensel GM. Human cytomegalovirus late-phase maturation is blocked by stably expressed UL32 antisense mRNA in astrocytoma cells. J Gen Virol. 1997;78(Pt 10):2621–2631. doi: 10.1099/0022-1317-78-10-2621. [DOI] [PubMed] [Google Scholar]
- Mocarski ES, Shenk T, Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields Virology. Lippincott-William & Wilkins; Philadelphia, Pa.: 2007. pp. 2701–2772. [Google Scholar]
- Murphy E, Yu D, Grimwood J, Schmutz J, Dickson M, Jarvis MA, Hahn G, Nelson JA, Myers RM, Shenk TE. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon K, Newcomb WW, Pray TR, Craik CS, Brown JC, Kedes DH. Lytic replication of Kaposi’s sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J Virol. 2001;75:2866–2878. doi: 10.1128/JVI.75.6.2866-2878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Juhas RM, Thomsen DR, Homa FL, Burch AD, Weller SK, Brown JC. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J Virol. 2001;75:10923–10932. doi: 10.1128/JVI.75.22.10923-10932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Angeletti PC, Engler JA, Britt WJ. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J Virol. 1998;72:3321–3329. doi: 10.1128/jvi.72.4.3321-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag J, Prasad B, Rixon F, Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989;56:561. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Steven AC, Spear PG. Herpesvirus capsid assembly and envelopment. In: Chiu W, Burnett RM, Garcea R, editors. Structural Biology of Viruses. Oxford University Press; New York: 1997. pp. 312–351. [Google Scholar]
- Taylor KA, Schmitz H, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, Tregear RT, Poole K, Lucaveche C, Edwards RJ, Chen LF, Winkler H, Reedy MK. Tomographic 3D reconstruction of quick-frozen, Ca2+−activated contracting insect flight muscle. Cell. 1999;99:421–431. doi: 10.1016/s0092-8674(00)81528-7. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Tang J, Cheng Y, Winkler H. The use of electron tomography for structural analysis of disordered protein arrays. J Struct Biol. 1997;120:372–386. doi: 10.1006/jsbi.1997.3932. [DOI] [PubMed] [Google Scholar]
- Trus BL, Gibson W, Cheng N, Steven AC. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J Virol. 1999;73:2181–2192. doi: 10.1128/jvi.73.3.2181-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Heymann JB, Nealon K, Cheng N, Newcomb WW, Brown JC, Kedes DH, Steven AC. Capsid structure of Kaposi’s sarcoma-associated herpesvirus, a gammaherpesvirus, compared to those of an alphaherpesvirus, herpes simplex virus type 1, and a betaherpesvirus, cytomegalovirus. J Virol. 2001;75:2879–2890. doi: 10.1128/JVI.75.6.2879-2890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Newcomb WW, Booy FP, Brown JC, Steven AC. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simples virus capsid. Proc. Natl. Acad. Sci. USA. 1992;89:11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Newcomb WW, Cheng N, Cardone G, Marekov L, Homa FL, Brown JC, Steven AC. Allosteric signaling and a nuclear exit strategy: binding of UL25/UL17 heterodimers to DNA-Filled HSV-1 capsids. Mol Cell. 2007;26:479–489. doi: 10.1016/j.molcel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, 2nd, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J Virol. 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H, Taylor KA. Three-dimensional distortion correction applied to tomographic reconstructions of sectioned crystals. Ultramicroscopy. 1996;63:125–132. doi: 10.1016/0304-3991(96)00024-1. [DOI] [PubMed] [Google Scholar]
- Wu L, Lo P, Yu X, Stoops JK, Forghani B, Zhou ZH. Three-dimensional structure of the human herpesvirus 8 capsid. J Virol. 2000;74:9646–9654. doi: 10.1128/jvi.74.20.9646-9654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Shah S, Atanasov I, Lo P, Liu F, Britt WJ, Zhou ZH. Three-dimensional localization of the smallest capsid protein in the human cytomegalovirus capsid. J Virol. 2005;79:1327–1332. doi: 10.1128/JVI.79.2.1327-1332.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Chen DH, Jakana J, Rixon FJ, Chiu W. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J Virol. 1999;73:3210–3218. doi: 10.1128/jvi.73.4.3210-3218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Dougherty M, Jakana J, He J, Rixon FJ, Chiu W. Seeing the herpesvirus capsid at 8.5 Å. Science. 2000;288:877–880. doi: 10.1126/science.288.5467.877. [DOI] [PubMed] [Google Scholar]
- Zhou ZH, Prasad BV, Jakana J, Rixon FJ, Chiu W. Protein subunit structures in the herpes simplex virus A-capsid determined from 400 kV spot-scan electron cryomicroscopy. J Mol Biol. 1994;242:456–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]
- Zhu H, Cong J-P, Shenk T. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]