Abstract
Rats suppress intake of a normally preferred 0.15% saccharin conditioned stimulus (CS) when it is paired with an aversive agent like lithium chloride (LiCl) or a preferred substances such as sucrose or a drug of abuse. The reward comparison hypothesis suggests that rats avoid intake of a saccharin cue following pairings with a drug of abuse because the rats are anticipating the availability of the rewarding properties of the drug. The present study used bilateral ibotenic acid lesions to examine the role of the gustatory cortex in the suppression of CS intake induced by cocaine, morphine, and LiCl. The results show that bilateral lesions of the insular gustatory cortex (1) fully prevent the suppressive effects of both a 15- and a 30-mg/kg dose of morphine, (2) attenuate the suppressive effect of a 10 mg/kg dose of cocaine, but (3) are overridden by a 20 mg/kg dose of the drug. Finally, these same cortical lesions had no impact on LiCl-induced conditioned taste aversion. The current data show that the insular taste cortex plays an integral role in drug-induced avoidance of a gustatory CS.
Keywords: reward comparison, drugs of abuse, anticipatory contrast, avoidance, CTA
Introduction
Rats avoid intake of a saccharin cue when paired with an aversive, illness-inducing agent, such as lithium chloride (LiCl) or x-radiation (Barker & Smith, 1974; Nachman & Ashe, 1973). They also avoid intake of the same saccharin conditioned stimulus (CS) when paired with a drug of abuse (Goudie, Dickins, & Thornton, 1978; Kulkosky, Sickel, & Riley, 1980; Kumar, Pratt, & Stolerman, 1983; LeBlanc & Cappell, 1975; Wise, Yokel, & DeWit, 1976). With further testing, however, the parallels between LiCl and drugs of abuse break down. Although LiCl and drugs of abuse both suppress CS intake, LiCl promotes conditioned place aversion and suppression of operant responding for the emetic agent (Mucha, van der Kooy, O’Shaughnessy, & Bucenieks, 1982; Paredes-Olay & Lopez, 2002; Schalomon, Robertson, & Laferriere, 1994; Turenne, Miles, Parker, & Siegel, 1996). Drugs of abuse, on the other hand, produce conditioned place preferences and increased operant responding for the abused substance (D’Mello, Goldberg, Goldberg, & Stolerman, 1981; Gomez, 2001; LeBlanc & Cappell, 1975; Mackey, Keller, & van der Kooy, 1986; Schenk & Partridge, 1999; Wakonigg, Sturm, Saria, & Zernig, 2003; Wise et al., 1976; Zito, Bechara, Greenwood, & van der Kooy, 1988).
In fact, the simultaneous display of a “positive” instrumental response (i.e., increased running speed, place preference, and drug-seeking) and a “negative” consummatory response (i.e., avoidance of the associated gustatory CS) was thought to be provoked by simultaneous opposing positive and negative properties of abused drugs, thereby creating a theoretical paradox (Corrigall, Linseman, D’Onofrio, & Lei, 1986; Hunt & Amit, 1987; Parker, 1995; White, Sklar, & Amit, 1977). The reward comparison hypothesis attempts to resolve this paradox by postulating that the same rewarding properties that drive increased operant responding for drugs of abuse also drive the decrease in intake of an associated gustatory cue (Grigson, 1997). This hypothesis is based on a phenomenon, referred to as anticipatory contrast, in which rats reduce intake of a normally preferred saccharin CS when it comes to predict access to more preferred 32% sucrose solution (Flaherty & Checke, 1982). In support, the suppressive effect of a rewarding sucrose unconditioned stimulus (US) and a drug of abuse, but not LiCl, are similarly affected by the deprivation state of the rat, the strain of the rat, and by a history of chronic exposure to morphine (Glowa, Shaw, & Riley, 1994; Grigson & Freet, 2000; Grigson, Lyuboslavsky, Tanase, & Wheeler, 1999; Grigson, Twining, & Carelli, 2000; Grigson, Wheeler, Wheeler, & Ballard, 2001).
Data from lesion experiments also support the reward comparison hypothesis by demonstrating that different central gustatory pathways mediate reward contrast effects and learned aversions. For example, bilateral lesions of the gustatory thalamus (THLX) have no impact on the development of a LiCl-induced CTA, but fully prevent morphine- and sucrose-induced suppression of CS intake (Flynn, Grill, Schulkin, & Norgren, 1991; Grigson, Lyuboslavsky, & Tanase, 2000; Mungarndee, Lundy, & Norgren, 2006; Reilly & Pritchard, 1996b, 1997; Scalera, Grigson, & Norgren, 1997; Schroy et al., 2005). Given the strong projections from taste thalamus to the insular cortex, however, it is not clear whether an intact gustatory thalamus is critical for the phenomenon, or whether the taste thalamus is involved simply because it serves as a conduit to the taste cortex (Benjamin & Pfaffmann, 1955; Norgren & Leonard, 1971; Norgren & Wolf, 1975; Wolf, 1968). Evidence in support of the latter conclusion is provided by reports showing that bilateral lesions of the taste cortex, like lesions of the taste thalamus, selectively disrupt sucrose- and morphine-, but not LiCl-induced suppression of CS intake (Kiefer & Orr, 1992; Mackey et al., 1986; Zito et al., 1988).
Despite these data, a firm conclusion regarding the function of the gustatory cortex in these phenomena is hampered by methodological constraints, a paucity of data, and mixed results. In fact, other data suggest that the insular gustatory cortex is required for normal acquisition and retention of a CTA (Dunn & Everitt, 1988; Escobar, Fernandez, Guevara-Aguilar, & Bermudez-Rattoni, 1989; Schafe & Bernstein, 1996; Yamamoto, Yuyama, Kato, & Kawamura, 1984). Regarding the apparent disruptive effect of lesions of the taste cortex on drug-induced suppression of CS intake, only morphine has been investigated and, only at a single dose of the drug (Mackey et al., 1986; Zito et al., 1988). In the single known study that specifically examined the role of the taste cortex in sucrose-induced suppression of CS intake (i.e., anticipatory contrast), Kesner and Gilbert (2007) failed to include an “unshifted” control group, thus complicating the interpretation of the data. Finally, the disruptive effect of lesions of the gustatory insular cortex on CTA learning depends on the training procedure (i.e., intra-oral infusion versus bottle licking) and the type of CS (e.g., saccharin or sucrose) employed (Fresquet, Angst, & Sandner, 2004; Koh & Bernstein, 2005; Wilkins & Bernstein, 2006; Takashi Yamamoto, Sako, Sakai, & Iwafune, 1997).
The current experiments sought to resolve these conflicting data. Thus, Experiment 1 tested whether lesions of the taste cortex would disrupt the suppressive effect of cocaine, another drug of abuse. Experiment 2 tested whether the same cortex lesions would disrupt drug-induced suppression of CS intake when a high dose of morphine, served as the US. Finally, Experiment 3 tested whether these lesions would disrupt CTA learning when using a relatively low or the standard dose of LiCl.
Experiment 1
The objective of Experiment 1 was to test whether the gustatory cortex, the main projection target of the gustatory thalamus (Krettek & Price, 1977; Norgren & Wolf, 1975), is essential for the conditioned suppression of CS intake that follows taste-drug pairings. As mentioned, both bilateral gustatory thalamic (electrophysiologically-guided) and gustatory insular cortical (stereotaxically-placed) excitotoxic lesions fully prevent morphine- but not LiCl-induced suppression of CS intake (Grigson, Lyuboslavsky et al., 2000; Mackey et al., 1986; Zito et al., 1988). Furthermore, drug-induced suppression of CS intake, but not LiCl-induced CTA, was blocked by (1) pretreatment with centrally-acting cholinergic antagonist atropine or (2) by injecting a dopamine antagonist directly in the insular cortex (Hunt, Segal, & Amit, 1987; Hunt, Switzman, & Amit, 1985). Unpublished data from our laboratory suggest that lesions of the taste thalamus also disrupt cocaine-induced suppression of intake of a taste cue. The disruptive effect of the lesion, however, was over-ridden when using a higher 20 mg/kg dose of cocaine (Baldwin, Palomo, Han, Horvath, & Grigson, 2002). The present study, then, was designed to test whether bilateral ibotenic acid lesions of the gustatory cortex also will disrupt the suppressive effect of a standard dose of cocaine (i.e., 10 mg/kg), but not that induced by a higher dose of the drug (i.e., 20 mg/kg).
Methods
Subjects and apparatus
The subjects were 70 naïve male Sprague-Dawley rats (Charles River) weighing 250–350 g at the beginning of the experiment. Experiment 1 was run in two complete replications consisting of 33 and 37 rats, respectively. The rats were individually housed in standard suspended stainless steel wire-mesh cages in a temperature and humidity controlled colony room maintained on a 12/12 hour light/dark cycle. Food (Teklad #6068) and distilled water (dH2O) were available ad libitum, unless otherwise noted. During all experiments, acquisition trials were conducted in the home cages using inverted Nalgene graduated cylinders with silicone stoppers and stainless steel spouts attached to the front of the cage with springs. Intake was measured to the nearest 0.5 ml.
Drugs and solutions
The CS was a 0.03 M Polycose solution (Sigma Chemical Company, St. Louis, MO) prepared 24-h in advance and presented at room temperature. This concentration of Polycose is highly preferred by rats and readily supports CTA learning (Sako et al., 1994; Smith, Norgren, & Grigson, 2004). The US, cocaine hydrochloride was generously provided by the National Institute on Drug Abuse (NIDA) and mixed in 0.9% saline every morning, approximately 1 h prior to the CS-US pairings. The cocaine was prepared as a stock solution (1.5 mg/ml) in saline and the dose (injected subcutaneously (s.c.)) was adjusted for bodyweight. This regimen diluted the solution to avoid necrosis at the injection site (Durazzo, Gauvin, Goulden, Briscoe, & Holloway, 1994; Mayer & Parker, 1993).
Surgery
Thirty-six rats received bilateral injections of 0.2 microliters (μl) of ibotenic acid over 10-min in the gustatory cortical region of each cortex (group GCTX), 16 more received similar infusions of sodium phosphate buffer (PBS), and 18 served as non-surgical controls (NSC). The coordinates for the stereotaxically placed lesions are modified from those of Mackey and colleagues (Mackey et al., 1986). Prior to surgery, each rat was injected with atropine sulfate (0.25 mg/ i.p.) and Gentamicin (6 mg/kg, i.p.). Twenty minutes later each rat was injected with pentobarbital sodium (50 mg/kg, i.p.), which was supplemented throughout surgery as necessary. Body temperature was maintained at 37±1 °C. After the rat reached a surgical level of anesthesia, the head was shaved, secured in a stereotaxic instrument, and the skull leveled between lamda and bregma and then the nose bar was set at −3.3 mm. The skin was cleaned with Betadine and a midline incision was made. Then a hole, centered at 0.5 mm anterior to bregma, was drilled in the skull on either side of the midline, using a ball tip burr. The dura mater was exposed and kept moist with physiological saline as needed. Lesions were placed at the following coordinates: AP +0.5 mm from bregma, ML +/− 4.8 mm, and DV −5.5 mm from the dura mater using a 1μl Hamilton microsyringe, with a glass pipette (tip: 5–10 μm) glued to its tip. The pipette microsyringe assembly was attached to a carrier and lowered into the target area. Immediately thereafter, 0.2 μl (20 μg/ml) of ibotenic acid was infused over a 10-min interval. After the infusion, the pipette remained in place for an additional 10-min. This procedure was then repeated in the contralateral hemisphere. Surgical controls were treated identically to the GCTX rats except, instead of ibotenic acid, rats were bilaterally infused with 0.2 μl of PBS (pH = 7.4). After surgery, the holes in the skull were filled with Gelfoam and the incision closed with wound clips.
Procedure
Following at least 5 d recovery, the rats were placed on a restricted water regimen (5 min a.m. and 1 h p.m.). Daily distilled water (dH2O) intake stabilized within a week. Morning 5 min water intake was used to match and assign rats, in a counterbalanced manner, to one of two US conditions: saline or 10 mg/kg cocaine (s.c.). Thus, 16 SHAM rats (8 PBS and 8 NSC) and 18 GCTX rats received saline injections, while the remaining 18 SHAM rats (8 PBS and 10 NSC) and 18 GCTX rats received cocaine as the US. During conditioning, all rats were given 5 min access to 0.03 M Polycose and, after a 5 min interval, were injected (s.c.) with either saline or cocaine. There was a total of 9 CS-US pairings, with one dH2O day elapsing between each, at which time rats received 5 min access to water in the morning and 1-h each afternoon. The concentration of cocaine was increased from 10 mg/kg to 20 mg/kg on trials 8–9. All rats were given 1 h access to water each afternoon to maintain hydration.
Histology
After completion of all behavioral experiments, the rats were deeply anesthetized with pentobarbital sodium (100 mg/kg (i.p.) and perfused transcardially for 5–10 min with physiological saline, followed by cold 4% paraformaldehyde in 0.1 M phosphate buffer for 15–20 min. The brains were stored overnight in 20% sucrose in phosphate buffer (20 g/100 ml) at 4 °C. The next day, the brains were cut into 50-micron thick coronal sections using a freezing microtome. This tissue was immunostained for neuron-specific nuclear protein (NeuN) (Jongen-Relo & Feldon, 2002). This staining procedure permits assessment of possible retrograde damage in the gustatory thalamus due to the cortical lesion. The sections were collected in phosphate buffer (PB) overnight. Sections were transferred to a PBS solution, incubated for 30 min in 0.5% H2O2, 1 h in blocking solution, and then 24-h in primary antibody. The secondary antibody incubation, avidin-biotin complex (ABC Elite kit) step, and DAB incubation were each preceded and followed by 3 rinses in PBS for 5 min. Finally, the sections were mounted on gelatin-coated slides, allowed to dry overnight, and then cover-slipped using Permount. When processing could not occur immediately, the sections were collected in 30% ethylene glycol glycerol in 50 mM phosphate buffer and stored at −20 °C for approximately 1-week before being thawed and placed in PBS overnight.
Results and Discussion
Histology
Histological analysis of the 36 rat brains from the GCTX group revealed 28 well-placed bilateral lesions of the gustatory cortex. The remaining eight rats were eliminated from the experiment, five due to misplaced lesions and three because extensive cortical lesions led to marked retrograde degeneration of the corresponding thalamic taste relay. Thus, for Experiment 1, the behavioral data from 34 SHAM rats and 28 GCTX rats contributed to the current statistical analyses. In Figure 1, panels A-C depict photomicrographs of NeuN stained coronal sections (2x) of an intact rat brain at 3 of the 5 levels analyzed during cortical lesion assessment. Levels 1–5 roughly correspond to Plates 26, 28, 30, 32 and 34 (Paxinos & Watson, 2005b). In the labeled photomicrograph shown in panel B of Figure 1, the region identified as AI/DI represents a section of the insular cortex where gustatory neurons were previously found to have maximal responsiveness to taste stimuli applied to the anterior tongue in rats (Benjamin & Pfaffmann, 1955; Kosar, Grill, & Norgren, 1986a; Yamamoto et al., 1984; Yamamoto, Yuyama, & Kawamura, 1981). On the surface of the brain, this region lies just dorsal to where the middle cerebral artery traverses the rhinal sulcus. In Figure 1, Levels 1 (panel A) and 5 (panel C) are approximately 1 millimeter anterior and posterior to Level 3 (panel B), respectively.
Figure 1.
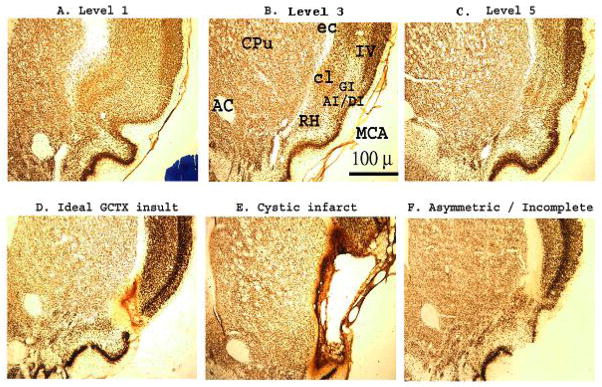
Photomicrographs of NeuN-stained coronal brain sections (2x) through the insular gustatory cortex in Sprague-Dawley rats. As a histological control, the cytoarchitectonic organization of the gustatory and surrounding nuclei was assessed at 5 levels throughout the insular cortex. Sections in A–C, represents the rostral (Level 1), middle (Level 3) and caudal (Level 5) limits of the gustatory cortical zone in an intact SHAM-operated Sprague-Dawley rat, respectively (Levels 2 & 4 not shown). Bilateral ibotenic acid lesions of the gustatory cortex (GCTX) were systematically rated from anterior to posterior in 5 brain sections corresponding to the Levels 1 through 5 (data shown from Levels 1, 3, and 5). D–F. While most ibotenic acid lesions were (D) ideal (limited to the gustatory cortical nuclei), some were (E) overwhelmingly large resulting in cystic infarction, and others (F) were misplaced or asymmetrical (unilateral), thus sparing some gustatory cortical neurons and rated as incomplete. Of the 19 GCTX rats only the behavioral data for 11 GCTX rats, with complete gustatory lesions, were retained for statistical analysis. In total, the data for 8 GCTX rats were discarded due to incomplete lesions (n=3), extensive retrograde damage (see Figure 2 panel D) to the taste thalamus (n=3), or both (n=2). AC: anterior commissure, AI/DI/GI: agranular/ dysgranular/ granular insular cortex, cl: claustrum, Cpu: caudate putamen, ec: external capsule, MCA: (anterior branch of) middle cerebral artery, RH: rhinal horn, IV: cortical layer 4.
Panel D of Figure 1 depicts a representative section of an ideal gustatory cortex lesion with damage limited primarily to the gustatory cortex and little to no retrograde degeneration of the corresponding gustatory thalamus (thalamus not shown). As mentioned above, data from three GCTX rats were discarded due to large cortical cysts (Figure 1, panel E) accompanied by extensive cell loss in the thalamic nuclei (i.e, VPMpc, VPM/VPL, CM, and Po nuclei of the thalamus, see Figure 2, panel D). In three rats the cortical lesions were too small or asymmetric (sparing cortical taste nuclei in either hemisphere) and, therefore, incomplete (Figure 1, panel F). The data from two GCTX rats also were omitted when the histology revealed a complete lack of cortical injury. In an intact brain is shown in Fig 2A and B under low and higher magnification, respectively), NeuN stained cell bodies can readily be seen throughout the gustatory thalamus (VPMpc) and other structures of the thalamus (i.e., VPM and Po). Figure 2, panel C is a representative section from a GCTX rat that incurred minimal retrograde neuronal cell loss in the taste thalamus. Similar to the SHAM operated rat brain, the thalamic taste nucleus of these GCTX rats were evenly stained with the NeuN marker indicating that the vast majority of neurons survived. Finally, Figure 2 panel D depicts a representative brain section of extensive retrograde gustatory thalamic neuronal degeneration. The data from these rats were, of course, eliminated from the analysis.
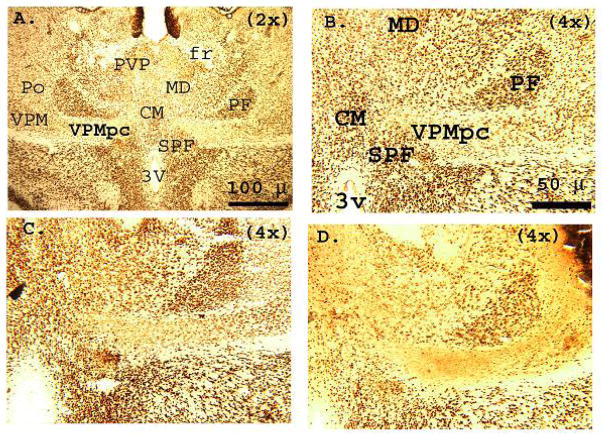
Figure 2.
Photomicrographs of NeuN-stained coronal brain sections at the level of the ventral posteriomedial (gustatory) thalamus (VPMpc) in Sprague-Dawley rats. Histological analysis of the gustatory thalamus was conducted at 4 levels, roughly corresponding to Figures 31–34 in the standard laboratory rat stereotaxic atlas (Paxinos & Watson, 2005a). A. SHAM-operated rat brain (2x). Note the cytoarchitectonic organization of the gustatory thalamus and surrounding nuclei, the bilateral symmetry of the region (between hemispheres), and the distinct distribution of NeuN stained cell bodies B. Higher (4x) magnification of the gustatory thalamus in the right hemisphere shown in panel A. Despite the dense appearance of other thalamic nuclei (i.e., CM, MD, PF, SPF) within the gustatory thalamic nuclei, NeuN staining was relatively abundant, organized and uniformed. C. Similar NeuN staining was observed in the thalamus of GCTX rats having ideal lesions of the gustatory cortex (4x). These GCTX rat brains appeared anatomically identical to SHAM-operated rat brains at the level of the gustatory thalamus. In some cases, upon closer inspection, minor cell loss was observed in the gustatory thalamus of the GCTX rat brains, which were otherwise indistinguishable from SHAM rat brains and thus were retained for analysis. In contrast, D. depicts a representative section of GCTX rat brains with extensive retrograde degeneration (4x). The total absence of cell bodies indicates extensive neuronal degradation throughout the gustatory thalamus. This damage extends laterally into the VPM/ VPL thalamic nucleus. Note the loss of cytoarchitectal organization overall. As previously mentioned the behavioral data for these GCTX rats were omitted from statistical analysis. CM: centromedial thal., fr: fronix, MD: mediodorsal thal., PF: parafasciulus thal., Po: posterior thal., PVP: paraventricular posterior thal, SPF: subparafasciulus thal., VPM/VPL: ventro-posteriomedial/ventro-posteriolateral thal., VPMpc: ventro-posteriomedial (gustatory) thal., 3v:3rd ventricle.
CS intake
Intake of the gustatory cue serves as the dependent measure. The intake data from PBS and NSC rats did not significantly differ, F < 1. Consequently, the data from these two groups were collapsed and will hereafter be referred to as group SHAM. The results of a 2 × 2 × 2 × 10 mixed factorial ANOVA varying replication (1 or 2), lesion (GCTX or SHAM), US (saline vs. cocaine), and trials (1 – 10) also failed to demonstrate a significant effect of replication, F<1. Thus, the behavioral data from the two replications were combined and analyzed using a 2 × 2 × 10 mixed factorial ANOVA. The results revealed a significant Lesion x Drug x Trials interaction, F (9,522) = 2.77, p<.05, (Figure 3).
Figure 3.
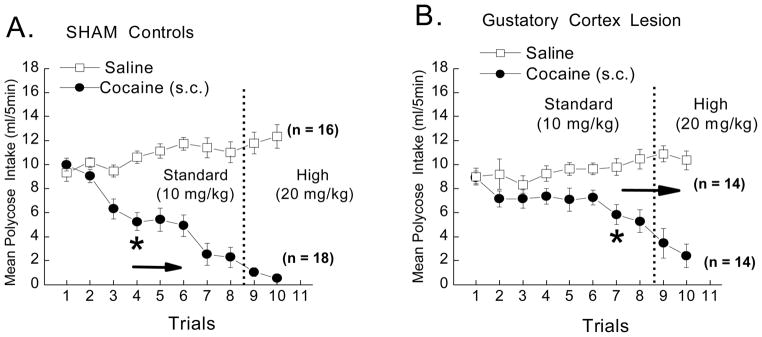
Mean CS intake (ml/5 min) of the 0.03M Polycose CS in SHAM and gustatory cortex lesioned (GCTX) rats following pairings with saline (open squares) or 10 and then 20 mg/kg cocaine (closed circles). SHAM rats significantly reduced intake of the CS cue following pairings first the 10 and then the 20 mg/kg dose of cocaine (see panel A). Lesions of the cortex reduced the magnitude of suppression induced by the 10 mg/kg dose of cocaine (see panel B). The effect of the lesion, however, was overridden by the use of a 20 mg/kg dose of the drug. Post hoc analyses of a significant 3-way ANOVA, F(9,522) 2.76, p<.05, revealed that SHAM rats suppressed CS cue intake by the 4th trial, while GCTX rats took almost twice as long (7 trials), ps < .05 to reduce CS intake relative to their saline controls. n = 14–18 per group. * indicate significant difference between US (saline vs. cocaine) groups.
Following the significant 3-way ANOVA, post-hoc comparisons revealed that, while SHAM rats in the Polycose-cocaine condition suppressed intake of the taste cue by the 4th trial, p<.05 (see Figure 3, left panel), the GCTX rats did not show a significant reduction in CS intake, relative to their saline controls, until the seventh taste-drug pairing (see Figure 3, right panel). This finding suggests that bilateral lesions of the gustatory cortex disrupt the suppressive effects of a 10, but not a 20, mg/kg dose of cocaine. Post-hoc analysis also revealed that gustatory cue intake did not significantly differ between the SHAM and GCTX saline-treated groups across trials 1 – 6, p>.05. As such, the cortical lesion-induced deficit in cocaine-induced suppression of CS intake could not simply be attributed to the reduction in overall fluid intake by the saline treated rats. Of course, it is possible that the 10 mg/kg dose would have exerted a greater reduction in CS intake in the GCTX rats if training had continued with the lower dose of the drug. While it may be important to address this possibility in a future study, here we opted to raise the dose on the 8th pairing because we thought it most critical to fully challenge the effectiveness of these gustatory cortical lesions. Regardless (i.e., whether mediated by more trials or new trials with the higher dose), the insular cortex lesions delay, but do not eliminate the suppressive effects of cocaine on intake of the drug-associated taste cue. Therefore, it appears that, as with bilateral lesions of the gustatory thalamus (Baldwin et al., 2002; Liu, Showalter, & Grigson, In Prep), lesions of the gustatory cortex also reduce the suppressive effects of a lower, but not a higher dose of cocaine.
Experiment 2
Although the effects of the cortex lesion closely paralleled those associated with damage to the gustatory thalamus (Geddes, Han, Baldwin, & Grigson, 2004; Geddes, Han, & Grigson, 2003; Mackey et al., 1986; Zito et al., 1988), the gustatory cortex lesion appears less disruptive than bilateral lesions of the gustatory thalamus (Baldwin et al., 2002; Grigson, Lyuboslavsky et al., 2000; Reilly & Trifunovic, 1999). This suggests that the suppressive effects of cocaine may differ from those of morphine. Alternatively, the same data could reflect insufficient lesions of the gustatory cortex, leaving cocaine-induced suppression of CS intake partially intact. Mackey, Keller and Van der Kooy (1986) demonstrated that, in adult male Wistar rats, bilateral ibotenic acid lesions of the gustatory cortex fully disrupted the suppressive effect of a 15 mg/kg dose of morphine on intake of a saccharin CS, up to 5 CS-US pairings. Unlike morphine, when paired with an injection of a 15 or 75 mg/kg dose of LiCl (i.p.), these same rats acquired an aversion to a similar saccharin cue (Mackey et al., 1986). In our Experiment 2, the rats from Experiment 1 were used to replicate the finding of Mackey and colleagues on the highly disruptive effect of bilateral cortical lesions on morphine-induced suppression of CS intake. Moreover, since the high dose of cocaine in Experiment 1 appeared to override the disruptive effect of the cortical lesion, the dose of morphine was increased after 6 trials from 15 to 30 mg/kg. The results of this study speak to the adequacy of the lesion and also test whether the suppressive effects of cocaine and morphine might be mediated, at least in part, by different neural substrates.
Methods
Subjects, surgery, and apparatus
Thirty-seven rats (18 SHAM and 19 GCTX) from the second replication of Experiment 1 were counterbalanced as a function of drug-history(see Diagram 1) and used to examine Morphine-induced suppression of CS intake in Experiment 2. The rats were housed and maintained as described for Experiment 1.
Drugs and Solutions
The CS was sodium saccharin purchased from Sigma Chemical Co., St. Louis, MO, mixed in dH2O and presented at room temperature. A 0.15% saccharin solution is highly preferred and readily supports drug-induced suppression of CS intake, anticipatory contrast, and CTA learning (Bardo & Valone, 1994; Flaherty & Grigson, 1988; Flaherty & Mitchell, 1999; Grigson, 2000; Grigson & Hajnal, 2007; Nachman & Ashe, 1973; Reilly & Pritchard, 1997; Schroy et al., 2005; Yamamoto & Fujimoto, 1991). The US morphine sulfate was generously provided by NIDA and mixed in 0.9% saline every morning, approximately 1 h prior to the CS-US pairings (LeBlanc & Cappell, 1975; Wise et al., 1976).
Procedure
Approximately 2 weeks following the end of Experiment 1, the rats were returned to the water deprivation regimen (5 min a.m., 1 h p.m.). Based on their drug experience in Experiment 1, a mixed cross over design was used to match rats into 2 US conditions: saline or 15 mg/kg morphine, see Diagram 1. Treatment groups were balanced by placing half the SHAM and GCTX rats with previous cocaine history in the saline group and the other half in the morphine group for the present experiment. Thus, of the eighteen SHAM rats, eight received saline and 10 now received morphine as the US. Out of the 19 GCTX rats, 9 GCTX rats served in the saline group and 10 GCTX rats were placed in the morphine group. During testing, all rats were allowed 5 min access to 0.15% saccharin, and after a 5 min interval, were injected i.p. with the appropriate US. There were a total of 8 CS-US pairings with one dH2O day (5 min a.m., 1 h p.m.) elapsing between each. The concentration of morphine was increased from 15 mg/kg to 30 mg/kg on trials 7–8, followed by one CS only test day. All of the rats were given 1 h access to water each afternoon to maintain hydration.
Results and Discussion
Histology
As previously mentioned, the data for 8 of the 19 GCTX rats were eliminated from the statistical analysis. Thus only 11 GCTX rats (5 saline, 6 drug) contributed data to Experiment 2. See the discussion of the Histological results above.
CS intake
Mean intake of the 0.15% saccharin solution (ml/5 min) served as the dependent measure. Intake by the PBS and NSC rats did not significantly differ (p>.05. Consequently, these groups were collapsed. The resultant data were reanalyzed using a 2 × 2 × 9 repeated measures ANOVA varying lesion (GCTX or SHAM), US (saline or morphine), and trials (1–9). Post hoc analyses were conducted on significant ANOVAs using the Newman-Keuls test. Figure 4 shows the mean +/− SEM intake of the saccharin CS for all groups across the 9 trials. The SHAM but not the GCTX rats suppressed intake of the saccharin cue following saccharin-morphine pairings.
Figure 4.
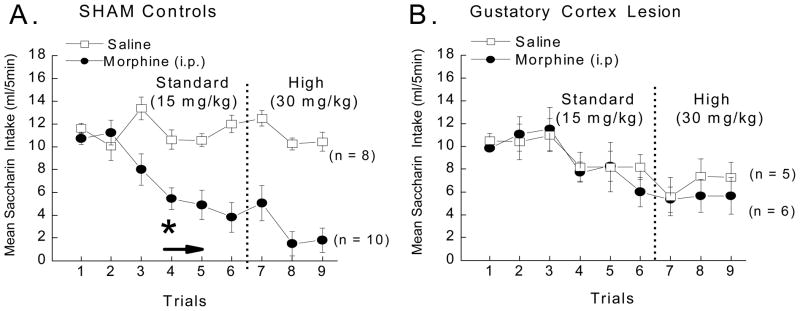
Mean CS intake (ml/5 min) of the 0.15% saccharin CS in SHAM and gustatory cortex lesioned (GCTX) rats following pairings with saline (open squares) or 15 and then 30 mg/kg morphine (closed circles) across trials. A 3-way ANOVA varying Drug (saline or morphine) x Lesion (SHAM or GCTX) x Trials (1–9) revealed that bilateral lesions of the gustatory cortex disrupted morphine-induced contrast such that SHAM (A), but not GCTX rats (B), suppressed intake of the 0.15% saccharin cue when paired with the passive administration of the low or high dose of morphine, F (8,200) = 2.37, p < .05. The SHAM rats suppressed intake of the 0.15% saccharin CS cue by the 4th CS-morphine pairing, p < .05 (indicated by an *), while the GCTX rats never significantly reduced intake of the saccharin CS relative to the saline treated GCTX controls, ps > .05 (n = 5–10 per group).
This observation was confirmed by a significant Lesion x US x Trials interaction, F (8,200) = 2.37, p<.05. Post hoc analysis confirmed that SHAM rats, but not GCTX rats, suppressed intake of the CS cue beginning with the 4th saccharin-morphine pairing, ps <.05. The GCTX rats treated with morphine, on the other hand, never significantly reduced intake relative to their saline treated controls. It should also be noted that post-hoc analysis comparing the CS intake of vehicle treated SHAM versus vehicle treated GCTX rats revealed no significant differences on trials 1 – 6, p>.05. These data confirm that the lesion-induced deficit in morphine-induced suppression of CS intake can not be simply attributed to the slightly lower intake by vehicle treated GCTX. Moreover, the GCTX rats also tended to drink less water on the days between conditioning trials (data not shown), but this effect also was not statistically significant, and essentially identical for both the morphine and saline treated subgroups. A similar decrement in intake has been seen in rats with bilateral thalamic lesions (Grigson, Lyuboslavsky et al., 2000). Therefore, it appears that fluid consumption by fluid deprived GCTX rats (in both the drug and the saline groups) is generally lower than that of SHAM rats. In sum, despite minor differences in general fluid intake, the results show that bilateral lesions of the insular cortex fully abolish morphine-induced suppression of a saccharin cue.
The outcome of the current experiment is critical on a number of levels. First, in Sprague-Dawley rats, we replicated the finding that bilateral lesions of the taste cortex disrupt morphine-induced contrast with a standard dose of the drug (Mackey et al., 1986). Second, we demonstrated that the lesion also fully disrupted the suppressive effects of even a relatively high 30 mg/kg dose of morphine. This finding differs from the dose dependent effect obtained with cocaine in Experiment 1. Thus, the neural mechanisms mediating the suppressive effects of morphine and high doses of cocaine may differ. Potential experimental approaches to address this possibility are discussed below (see General Discussion). An alternative consideration, however, is that prior experience with either Polycose-saline or Polycose-cocaine in Experiment 1 may have retarded the development of a subsequent saccharin-morphine association in Experiment 2 and that the magnitude of this effect may have been augmented in rats with lesions of the gustatory cortex. Bilateral lesions of the ventral tegmental area (VTA), that do not affect drug-induced suppression of CS intake in naive rats, nevertheless retard the development of this effect in rats with a history of either saccharin-morphine or saccharin-saline pairings (Twining, Hajnal, Bruno, Hess, & Grigson, 2005). Bilateral lesions of the gustatory cortex, then, also may facilitate this latent inhibition-like effect (Lubow & De la Casa, 2005; Lubow, Markman, & Allen, 1968). If so, these taste-drug experienced rats with cortical lesions also should demonstrate deficits in CTA learning when a novel cue is paired with the illness-inducing agent, LiCl. This hypothesis was addressed in Experiment 3.
Experiment 3
Bilateral lesions of the parabrachial nucleus (PBN) have been shown to fully prevent CTA learning (Grigson, Reilly, Shimura, & Norgren, 1998; Grigson, Shimura, & Norgren, 1997; Spector, Norgren, & Grill, 1992; Spector, Scalera, Grill, & Norgren, 1995). Gustatory information from the medial PBN, is transmitted to cells in the taste thalamus, which in turn send projections to the gustatory insular cortex (Kosar, Grill, & Norgren, 1986b; Norgren & Leonard, 1971; Norgren & Pfaffmann, 1975; Shi & Cassell, 1998). Given the gustatory PBN connection to the gustatory thalamocortical pathway, the possibility remains, that PBN lesions blocks CTA learning by preventing pertinent information about the CS cue from reaching the insular gustatory cortex (Allen, Saper, Hurley, & Cechetto, 1991). Thus, Experiment 3 tested whether these same lesions of the gustatory cortex also disrupt acquisition of a LiCl-induced conditioned taste aversion. As described, there are data in support of this possibility (Dunn & Everitt, 1988; Koh & Bernstein, 2005; Naor & Dudai, 1996; Ramirez-Amaya et al., 1996), and data that, either directly or indirectly argue against it (Flynn et al., 1991; Geddes et al., 2004; Geddes et al., 2003; Grigson, Lyuboslavsky et al., 2000; Lasiter & Glanzman, 1982; Mackey et al., 1986; Reilly & Pritchard, 1996a; Scalera et al., 1997; Zito et al., 1988). In the present study we used low (0.009 M) and standard (0.15 M) dose of LiCl because, when passively administered to intact rats, these doses suppress CS intake in a manner similar to the standard and higher doses of cocaine and morphine (Grigson, 1997).
Methods
Subjects, surgery and apparatus
All thirty-seven rats from Experiment 2 served as subjects (n=18 SHAM and n=19 GCTX).
Drugs and Solutions
The CS, 0.1 M sodium chloride (NaCl) (Sigma Chemical Co., St. Louis, MO), was mixed in water and presented at room temperature. As a gustatory cue, NaCl has been shown to be fairly preferred in rats and to readily support CTA learning (Baird, St John, & Nguyen, 2005; Grill & Norgren, 1978; Spector, Grill, & Norgren, 1993; Yamamoto, 1984; Yamamoto et al., 1984). The US, lithium chloride (LiCl) was purchased from Sigma Chemical Company, St. Louis, MO and, prepared in a stock solution and maintained at 4°C between trials. Over the course of the experiment 0.009 M or 0.15 M doses were injected ip.
Procedure
Approximately 2 weeks following the end of Experiment 2, the rats were returned to the water deprivation regimen (5 min a.m., 1 h p.m.). Based on their drug experience from Experiments 1 and 2, we used a mixed cross over design to match rats into 2 US conditions: saline or LiCl. Treatment groups were balanced by placing half the SHAM and GCTX rats with previous cocaine and morphine history in the saline group and the other half in the LiCl group for the present experiment, see Diagram 1 above. Thus, of the 18 SHAM rats, 9 received saline and 9 now received LiCl as the US. Out of the 19 GCTX rats, 9 GCTX rats served in the saline group and 10 GCTX rats were placed in the LiCl group. As previously mentioned, the data for 8 of the 19 GCTX rats were discarded based on histological analysis of lesion placement and retrograde thalamic degeneration (see the Results section in Experiment 1 above). Thus, 6 GCTX rats served in the saline condition and the remaining 5 GCTX rats received LiCl as the US. During testing, all rats were allowed 5 min access to 0.1 M NaCl and, after a 5 min interval, were injected i.p. with the appropriate US. There was a total of 8 CS-US pairings with one dH2O day between each pairing. The concentration of LiCl was increased from 0.009 M to 0.15 M on trials 7–8, followed by one CS only test. All of the rats were given 1 h access to water each afternoon to maintain hydration.
Results and discussion
CS intake
Intake of the 0.1 M NaCl CS (ml/5 min) served as the dependent measure. Intake by the PBS and NSC groups did not significantly differ, p>.05, so the data were collapsed and reanalyzed using a 2 × 2 × 9 mixed factorial ANOVA varying lesion (GCTX or SHAM), US (saline or LiCl), and trials (1–9). Post hoc analyses were conducted on significant interactions using the Newman-Keuls test. The results of the CTA test with SHAM and GCTX rats are shown in Figure 5, panels A and B, respectively.
Figure 5.
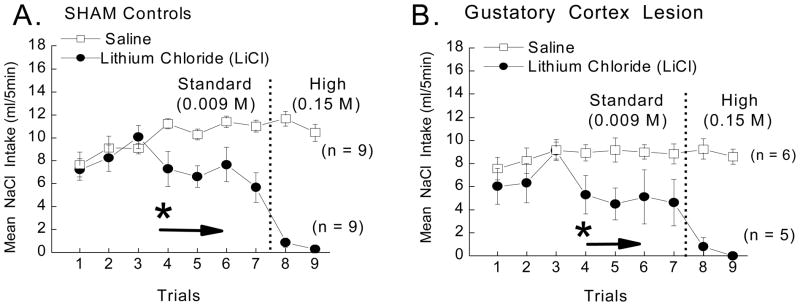
Mean CS intake (ml/5 min) of the 0.1 M NaCl CS in SHAM and gustatory cortex lesioned (GCTX) rats following pairings with saline (open squares) or 0.009 M or 0.15 M LiCl (closed circles). A 3-way ANOVA varying US (saline or LiCl) x Lesion (SHAM or GCTX) x Trials (1–9) revealed that GCTX failed to disrupt LiCl-induced CTA such that both the SHAM (panel A) and GCTX rats (panel B) learned to suppress intake of the CS when repeatedly paired with either dose of LiCl, F (8,200) = .669, p > .05. Post hoc tests on a significant US x Trials interaction, F (8,200) = 18.75, p<.05, showed that intake of the CS cue was suppressed by the fourth trial in both SHAM and GCTX rats alike, ps < .05 (n = 5–10 per group). * indicate significant difference between US (saline vs. LiCl) groups.
The 3 way ANOVA varying Lesion x Drug x Trials was not significant, F<1, nor were any analyses involving lesion as a factor, ps >.05. The US x Trials interaction, however, did attain statistical significance, F (8,200) = 18.75, p<.05, and post hoc tests revealed that all rats (SHAM and GCTX combined) suppressed intake of the NaCl taste cue following four taste-drug pairings, ps <.05. These findings confirm that rats with bilateral ibotenic acid lesions of the gustatory cortex retain the ability to demonstrate a LiCl-induced CTA. Suppression of CS intake in the CTA paradigm by GCTX rats is apparent over several acquisition trials and is marked, as in the SHAM rats, on the final two trials after having been switched to the higher 0.15 M dose of the drug. Since the rate of acquisition between the two lesion groups is the same, this demonstrates that lesions of the gustatory cortex not only have no effect on the LiCl-induced CTA, but also that they have no effect on the potential disruptive effects of prior experience with other CSs and USs (e.g., on latent inhibition-like effect) (De la Casa & Lubow, 1995).
General Discussion
The results confirm that Sprague-Dawley rats with bilateral ibotenic acid lesions of the gustatory cortex can learn a LiCl-induced CTA, even at very low US doses. The same lesions, however, attenuated the development of cocaine-induced suppression of CS intake and prevented the suppressive effects of the standard 15 mg/kg and higher 30 mg/kg dose of morphine. These findings suggest that the thalamocortical gustatory system is not necessary for rats to experience the LiCl US exposure as an aversive event. Nor is the pathway necessary to learn an association between the taste cue and the unconditioned stimulus in a CTA paradigm. As summarized in Table 1, this pattern of data mirrors that obtained from rats with bilateral lesions of the gustatory thalamus.
Table 1.
Comparison of bilateral gustatory thalamus and gustatory cortex lesion-induced deficits
Comparison of the effect of bilateral lesions of the gustatory thalamus (THLX) and the gustatory cortex (GCTX). Table depicts the disruptive effect of a bilateral thalamic and cortical lesions on LiCl-induced conditioned taste aversion (CTA), the suppressive effects of various drugs of abuse (i.e., morphine or cocaine), and sucrose-induced anticipatory contrast effects (ACE). Neither type of bilateral thalamic (THLX) or cortical (GCTX) lesion was sufficient to block CTA learning produced by an aversive US. Both lesion types, however, did disrupt the suppressive effects of a rewarding US (e.g., the standard doses of morphine and cocaine, the higher dose of morphine (only tested in GCTX rats), and a highly reinforcing sucrose solution, ACE). Despite the ineffectiveness of the either THLX or GCTX lesions at the 20 mg/kg dose of cocaine, taken together these findings serve to further dissociate these phenomena, while strongly implicating a specific role for the dorsal taste pathway in suppression of intake of a taste CS by a rewarding US.
| + = can learn to suppress in take of CS cue − = fail to suppress taste cue |
Thalamic Lesions (THLX) | Gustatory Cortex (GCTX) |
|---|---|---|
| Low dose LiCl-induced CTA | + | + |
| High dose LiCl-induced CTA | + | + |
| Standard Morphine (15 mg/kg) | − | − |
| Standard Cocaine (10 mg /kg) | − | − |
| High Morphine (30 mg/kg) | untested | − |
| High Cocaine (20 mg/kg) | +(in pre preparation) | + |
| (sucrose-induced) ACE | − | −(in preparation) |
The plus sign (+) indicates behaviors not affected by the lesion, thus rats with the particular lesion in question learn to suppress CS intake in the given situation. In contrast, the minus sign (−) indicates behaviors that are disrupted by lesions of the dorsal taste pathway (i.e., the rats do not show evidence of having learned to association).
Specifically, we have shown that THLX rats suppress intake of a gustatory CS when paired with a low or standard dose of LiCl but, fail to suppress intake of the same gustatory cue when paired with the standard doses of morphine or cocaine. In THLX rats, the higher dose of morphine remains untested, while unpublished data suggest that the higher dose cocaine, overrides the disruptive effective of these lesions. Similarly, in the present experiment, the higher dose of cocaine also was sufficient to override the disruptive effect of bilateral lesions of the taste cortex, but morphine was not. Finally, as discussed above, rats with lesions of the taste thalamus also fail to suppress intake of the 0.15% saccharin solution when paired with a normally preferred sucrose solution (Reilly & Pritchard, 1997; Schroy et al., 2005). Other researcher have reported that in Long-Evans rats, sucrose anticipatory contrast is also disrupted by bilateral lesions of the gustatory insular cortex (Kesner & Gilbert, 2007; Ragozzino & Kesner, 1999). Similar to Kesner & Gilbert (2007), but with the appropriate unshifted control group, we also have found that bilateral lesions of the gustatory insular cortex block the suppressive effects of a high concentration of sucrose on intake of a lesser saccharin or sucrose cue (Geddes, Han, & Grigson, 2005). Thus, taken together, the data show that both the taste thalamus and the taste cortex need to be intact for drug- and sucrose-induced suppression of CS intake, but not for LiCl-induced ‘CTA’.
What is the role of the gustatory cortex in the comparison of a natural reward cue with a drug of abuse? Evidence suggests that the gustatory cortex plays a role in responding to both the absolute and the relative properties of reward. Regarding absolute properties, similar insular cortex lesions disrupt short-term working memory for the magnitude of a food reward, but not its spatial location (DeCoteau, Kesner, & Williams, 1997; Ragozzino & Kesner, 1999). Utilizing neuroimaging techniques, human data suggest that the temporal-insular cortex is involved in the processing of naturally (appetitive) rewarding taste stimuli (Kobayakawa et al., 1996; O’Doherty, Dayan, Friston, Critchley, & Dolan, 2003; Small, 2006). Human studies also have shown that neuronal activity in this region is preferentially affected by changes in reward value (O’Doherty, Rolls, Francis, Bowtell, & McGlone, 2001; Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001; Yamamoto et al., 2006). If the reward comparison hypothesis is correct and avoidance of the taste cue is due to the relatively rewarding properties of the drug US, then an intact gustatory cortex also must be essential for the comparison of the relative value of the taste cue with not only sweets, but also with the drug US. Specifically, the rat must (1) appropriately detect the taste cue and experience the drug consequence, (2) associate the taste cue with the effects of the drug, (3) remember the value of the drug experience upon presentation of the taste cue, (4) compare the value of the available gustatory CS with the memory of the value of the subsequent drug US, and finally (5) reduce intake of the lesser valued CS. Conditioned taste aversion follows a similar paradigm, but CTA learning does not involve comparing the relative reward values of the CS cue and US experience, nor does it require the thalamocortical gustatory pathway. As such, the taste cortex appears critical for making the explicit comparison between a less rewarding taste cue and a more rewarding drug of abuse that is anticipated in the near future and expressing the consequence of these comparisons in ingestive behavior.
While an intact taste cortex was essential for avoidance of the taste cue when paired with a low and a high dose of morphine and the low dose of cocaine, the disruptive effect of the lesion was overridden by the use of a relatively higher 20 and 40 mg/kg dose of cocaine. Baldwin et al. (in preparation) have obtained a similar pattern in rats with bilateral lesions of the taste thalamus (see Table 1: column 2 - row 5). The suppressive effect of the higher dose of cocaine may represent a quantitative (i.e., moderate to highly rewarding) and/or a qualitative (i.e., reward vs. aversion) change in the suppression of CS intake. For instance, the reinforcing properties of 20 mg/kg cocaine, but not 10 mg/kg, were sufficiently rewarding for rats with THLX or GCTX lesions to suppress intake of the CS cue. Such an increase in the magnitude of the US reward value may increase the number of neuronal systems recruited during the US exposure, thus rendering the gustatory thalamus and cortex less critical.
As for changes is the quality of the drug US, it is possible that at the higher dose of the cocaine, the suppression of CS intake may reflect an aversion, similar to a LiCl-CTA. This could occur if this dose of drug is toxic, thus creating an aversive US consequence. The 20 mg/kg (i.p.) dose of cocaine, however, has been shown to be sufficient to decrease the time taken to traverse a runway and to support the development of a conditioned place preference (Ettenberg & Geist, 1993; Hansen-Trench, Segar, & Barron, 1996; Knackstedt & Ettenberg, 2005; Lett, 1989; Mucha et al., 1982; Wakonigg et al., 2003; Zernig et al., 2002). Furthermore, 0.33 mg/infusion (the interventricular (i.v.) equivalent of the 20 mg/kg (i.p.) dose of cocaine), not only caused suppression of the 0.3M Polycose cue in both groups, but this dose was also readily self-administered by SHAM and THLX rats, alike (Baldwin et al., 2002). More recently, we have attempted to address this question in rats with asymmetric lesions of the gustatory thalamus and gustatory cortex using cocaine self-administration in operant chambers. We have found that in adult male Sprague-Dawley rats, disconnecting the taste thalamus from the gustatory insular cortex specifically blocks avoidance of the taste cue, but not the ability of the gustatory cues to induced drug seeking or instrumental responding for 0.33 mg i.v. infusions of cocaine (Geddes, Han, & Grigson, 2007). These data suggest that cocaine retains its positive attributes in rats with lesions of the thalamocortical gustatory system. A final possibility is that, while the drug is not aversive, per se, the state elicited by the drug-associated cue is. In this case, the drug-associated taste cue may elicit cue-induced withdrawal, for example, which is an aversive state known to support taste aversion (Frumkin, 1976; McDonald & Hong, 2004; McDonald, Parker, & Siegel, 1997; Siegel, 1975, 1999; Weise-Kelly & Siegel, 2001; Wheeler & Miller, 2007; Wheeler et al., 2008). Taken together, the development of an aversive state (perhaps, the most likely interpretation) also would be expected to recruit other neuronal circuits. Future studies will test the merits of these hypotheses.
In sum, the gustatory cortex appears to play a major role in comparing the relative value of an available taste reward with the memory of the alternative reward that is anticipated in the near future. Bilateral lesions of the gustatory cortex parallel those of the gustatory thalamus, see Table 1. In addition to the thalamic afferent axons, insular gustatory cortical cells have been shown to project back to the gustatory thalamus, forming a gustatory thalamocortical loop (Allen et al., 1991; Kosar et al., 1986a, 1986b; Norgren & Wolf, 1975; Shi & Cassell, 1998; Wolf, 1968). These observations support our recent hypothesis that drug-induced suppression of CS intake depends upon communication between the two nuclei in this thalamocortical loop. Current experiments are testing this hypothesis (Geddes, Han, Baldwin, & Grigson, 2006; Geddes, Han, & Grigson, 2005). Reciprocal connections between the cerebral cortex and the thalamus have been studied, in other modalities including vision, odor, touch and pain (de Carvalho, 1994; Ghazanfar & Nicolelis, 1997; Kuroda, Murakami, Oda, Shinkai, & Kishi, 1993). Understanding the relationship between the thalamus and cortex during higher-order behaviors, such as attention and sleep, may give some insight to the role and manner by which the gustatory thalamocortical loop contributes to relative reward learning (Mayer, Schuster, & Claussen, 2006; Smythies, 1997).
Figure 6.
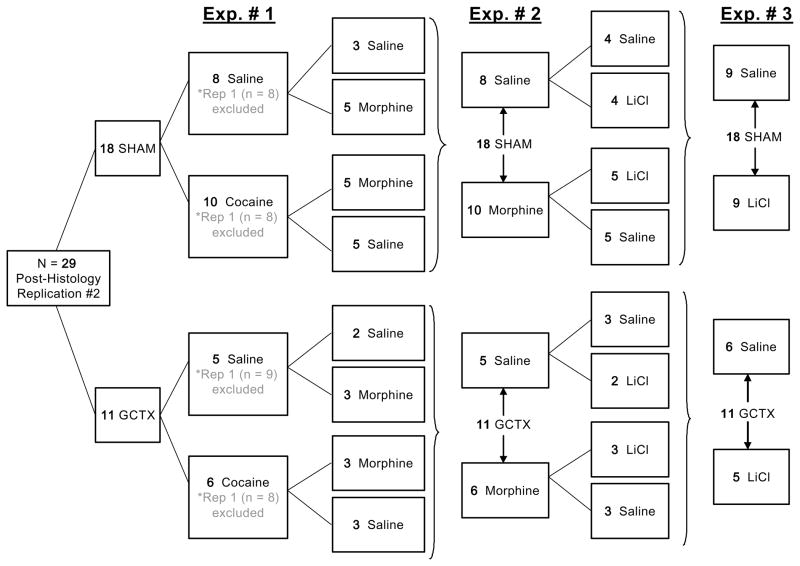
Diagram 1. Unconditioned Stimulus History: Cross-over methods by treatment group. This diagram shows the sample size, lesion type, and unconditioned stimulus treatment groups for the rats who only served in the 1st cocaine study (Replication #1). This information (SHAM-saline (n = 8), SHAM-cocaine (n = 8), GCTX-saline (n = 9) and GCTX-cocaine (n = 8) is shaded gray and marked with an asterisk (*), under Exp. 1. Second, this diagram further illustrates the manner in which SHAM and GCTX rats (n = 29, post-histology) from the 2nd cocaine study, referred to as replication #2, were crossed-over based on their prior cocaine experience in order to subsequently test the effects of bilateral gustatory cortical (GCTX) lesions on Morphine contrast (Exp. 2) and LiCl-induced CTA (Exp. 3) learning in the same set of animals. Post-histological data from Rep 1 (n = 33) and Rep 2 (n = 29) were combined for analysis and presented as such in the present manuscript. Together (by adding the black and gray #’s, under Exp.1) the total sample size for the present cocaine study were as follows: SHAM-saline (n = 16), SHAM-cocaine (n = 18), GCTX-saline (n = 14), and GCTX-cocaine (n = 14).
Acknowledgments
This research was supported by U.S. Public Health Services Grants DA09815, DA12473, DA017146, and DC00240. Special thanks to Kathy Matayas and Nellie Horvath for their careful assistance with processing bran sections used for histological analysis. Thanks to Drs. Robert Lundy and Sam Mungarndee for their assistance with lesion coordinate verification and photomicrograph imagery, respectively. Thanks to Dr. Robert C. Twining and Chris Freet for their comments on one or several drafts of the manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
References
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311(1):1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Baird JP, St John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 2005;119(4):983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Palomo A, Han L, Horvath N, Grigson PS. Program No. 501.4. 2002 Abstract Viewer/Itinerary Planner. Vol. 2002. Washington, DC: Society for Neuroscience; 2002. GUSTATORY THALAMUS LESIONS BLOCK THE SUPPRESSIVE EFFECTS OF LOW, BUT NOT HIGH, DOSES OF COCAINE FOLLOWING PASSIVE OR SELF-ADMINISTRATION. Online. [Google Scholar]
- Bardo MT, Valone JM. Morphine-conditioned analgesia using a taste cue: dissociation of taste aversion and analgesia. Psychopharmacology (Berl) 1994;114(2):269–274. doi: 10.1007/BF02244848. [DOI] [PubMed] [Google Scholar]
- Barker LM, Smith JC. A comparison of taste aversions induced by radiation and lithium chloride in CS-US and US-CS paradigms. J Comp Physiol Psychol. 1974;87(4):644–654. doi: 10.1037/h0036962. [DOI] [PubMed] [Google Scholar]
- Benjamin RM, Pfaffmann C. Cortical localization of taste in albino rat. J Neurophysiol. 1955;18(1):56–64. doi: 10.1152/jn.1955.18.1.56. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Linseman MA, D’Onofrio RM, Lei H. An analysis of the paradoxical effect of morphine on runway speed and food consumption. Psychopharmacology (Berl) 1986;89(3):327–333. doi: 10.1007/BF00174369. [DOI] [PubMed] [Google Scholar]
- de Carvalho LA. Modeling the thalamocortical loop. Int J Biomed Comput. 1994;35(4):267–296. [PubMed] [Google Scholar]
- De la Casa G, Lubow RE. Latent inhibition in conditioned taste aversion: the roles of stimulus frequency and duration and the amount of fluid ingested during preexposure. Neurobiol Learn Mem. 1995;64(2):125–132. doi: 10.1006/nlme.1995.1051. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP, Williams JM. Short-term memory for food reward magnitude: the role of the prefrontal cortex. Behav Brain Res. 1997;88(2):239–249. doi: 10.1016/s0166-4328(97)00044-2. [DOI] [PubMed] [Google Scholar]
- D’Mello GD, Goldberg DM, Goldberg SR, Stolerman IP. Conditioned taste aversion and operant behavior in rats: effects of cocaine, apomorphine and some long-acting derivatives. J Pharmacol Exp Ther. 1981;219(1):60–68. [PubMed] [Google Scholar]
- Dunn LT, Everitt BJ. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav Neurosci. 1988;102(1):3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gauvin DV, Goulden KL, Briscoe RJ, Holloway FA. Cocaine-induced conditioned place approach in rats: the role of dose and route of administration. Pharmacol Biochem Behav. 1994;49(4):1001–1005. doi: 10.1016/0091-3057(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Escobar M, Fernandez J, Guevara-Aguilar R, Bermudez-Rattoni F. Fetal brain grafts induce recovery of learning deficits and connectivity in rats with gustatory neocortex lesion. Brain Res. 1989;478(2):368–374. doi: 10.1016/0006-8993(89)91519-9. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44(1):191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Checke S. anticipation of incentive gain. Animal learning and Behavior. 1982;(10):171–182. [Google Scholar]
- Flaherty CF, Grigson PS. From contrast to reinforcement: role of response contingency in anticipatory contrast. J Exp Psychol Anim Behav Process. 1988;14(2):165–176. [PubMed] [Google Scholar]
- Flaherty CF, Mitchell C. Absolute and relative rewarding properties of fructose, glucose, and saccharin mixtures as reflected in anticipatory contrast. Physiol Behav. 1999;66(5):841–853. doi: 10.1016/s0031-9384(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105(6):944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Fresquet N, Angst MJ, Sandner G. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose delivery. Behavioural Brain Research. 2004;153(2):357–365. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Frumkin K. Differential potency of taste and audiovisual stimuli in the conditioning of morphine withdrawal in rats. Psychopharmacologia. 1976;46(3):245–248. doi: 10.1007/BF00421109. [DOI] [PubMed] [Google Scholar]
- Geddes RI, Han L, Baldwin A, Grigson PS. Asymmetric lesions of the gustatory thalamocortical loop selectively disrupt morphine-induced contrast, while sparing LiCl-induced conditioned taste aversion (CTA) learning. Appetite. 2006 In Press, Corrected Proof. [Google Scholar]
- Geddes RI, Han L, Grigson PS. Program No. 801.15. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. MORPHINE-INDUCED SUPPRESSION OF INTAKE OF A TASTE CUE DEPENDS UPON AN INTACT THALAMOCORTICAL LOOP IN THE DORSAL TASTE PATHWAY. Online. [Google Scholar]
- Geddes RI, Han L, Grigson PS. Lesions of the gustatory thalamocortical loop block drug-induced devaluation of a natural saccharin reward cue, while leaving instrumental responding for the drug intact. Appetite. 2007;49(1):292–311. [Google Scholar]
- Geddes RI, Han LB, Baldwin AE, Grigson PS. Program No. 119.20. 2004 Abstract Viewer/Itinerary Planner. Vol. 2004 Washington, DC: Society for Neuroscience; 2004. THE ROLE OF THE GUSTATORY CORTEX IN DRUG-INDUCED SUPPRESSION OF CONDITIONED STIMULUS INTAKE IN SPRAGUE-DAWLEY RATS. [Google Scholar]
- Geddes RI, Han LB, Grigson PS. Vol. Program No. 420.8. 2003 Abstract Viewer/Itinerary Planner. Vol. 2003. Washington, DC: Society for Neuroscience; 2003. IBOTENIC ACID LESIONS OF THE GUSTATORY CORTEX DISRUPT COCAINE-INDUCED SUPPRESSION OF SACCHARIN INTAKE IN RATS. Online. [Google Scholar]
- Ghazanfar AA, Nicolelis MA. Nonlinear processing of tactile information in the thalamocortical loop. J Neurophysiol. 1997;78(1):506–510. doi: 10.1152/jn.1997.78.1.506. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology (Berl) 1994;114(2):229–232. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Gomez F. Induction of conditioned taste aversion with a self-administered substance in rats. Brain Res Brain Res Protoc. 2001;8(2):137–142. doi: 10.1016/s1385-299x(01)00097-6. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Dickins DW, Thornton EW. Cocaine-induced conditioned taste aversions in rats. Pharmacol Biochem Behav. 1978;8(6):757–761. doi: 10.1016/0091-3057(78)90279-4. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci. 1997;111(1):129–136. [PubMed] [Google Scholar]
- Grigson PS. Drugs of abuse and reward comparison: a brief review. Appetite. 2000;35(1):89–91. doi: 10.1006/appe.2000.0334. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci. 2000;114(2):353–363. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once Is Too Much: Conditioned Changes in Accumbens Dopamine Following a Single Saccharin-Morphine Pairing. Behavioral Neuroscience. 2007;121(6):1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine- but not LiCl-induced intake suppression in rats: evidence against the conditioned taste aversion hypothesis. Brain Res. 2000;858(2):327–337. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky PN, Tanase D, Wheeler RA. Water-deprivation prevents morphine-, but not LiCl-induced, suppression of sucrose intake. Physiol Behav. 1999;67(2):277–286. doi: 10.1016/s0031-9384(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav Neurosci. 1998;112(1):160–171. [PubMed] [Google Scholar]
- Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: III. The role of the nucleus of the solitary tract and the parabrachial nucleus in retention of a conditioned taste aversion in rats. Behav Neurosci. 1997;111(1):180–187. [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Carelli RM. Heroin-induced suppression of saccharin intake in water-deprived and water-replete rats. Pharmacol Biochem Behav. 2000;66(3):603–608. doi: 10.1016/s0091-3057(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Wheeler RA, Wheeler DS, Ballard SM. Chronic morphine treatment exaggerates the suppressive effects of sucrose and cocaine, but not lithium chloride, on saccharin intake in Sprague-Dawley rats. Behav Neurosci. 2001;115(2):403–416. doi: 10.1037/0735-7044.115.2.403. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Hansen-Trench LS, Segar TM, Barron S. Neonatal cocaine and/or ethanol exposure: effects on a runway task with suckling reward. Neurotoxicol Teratol. 1996;18(6):651–657. doi: 10.1016/s0892-0362(96)00130-4. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev. 1987;11(1):107–130. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Hunt T, Segal R, Amit Z. Differential involvement of central cholinergic mechanisms in the aversive stimulus properties of morphine and amphetamine. Pharmacol Biochem Behav. 1987;28(3):335–339. doi: 10.1016/0091-3057(87)90449-7. [DOI] [PubMed] [Google Scholar]
- Hunt T, Switzman L, Amit Z. Involvement of dopamine in the aversive stimulus properties of cocaine in rats. Pharmacology Biochemistry and Behavior. 1985;22(6):945–948. doi: 10.1016/0091-3057(85)90300-4. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Feldon J. Specific neuronal protein: a new tool for histological evaluation of excitotoxic lesions. Physiol Behav. 2002;76(4–5):449–456. doi: 10.1016/s0031-9384(02)00732-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88(1):82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106(1):140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Ettenberg A. Ethanol consumption reduces the adverse consequences of self-administered intravenous cocaine in rats. Psychopharmacology (Berl) 2005;178(2–3):143–150. doi: 10.1007/s00213-004-1996-2. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, et al. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett. 1996;212(3):155–158. doi: 10.1016/0304-3940(96)12798-1. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav Neurosci. 2005;119(2):388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986a;379(2):329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986b;379(2):342–352. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171(2):157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Sickel JL, Riley AL. Total avoidance of saccharin consumption by rats after repeatedly paired injections of ethanol or LiCl. Pharmacology Biochemistry and Behavior. 1980;13(1):77–80. doi: 10.1016/0091-3057(80)90123-9. [DOI] [PubMed] [Google Scholar]
- Kumar R, Pratt JA, Stolerman IP. Characteristics of conditioned taste aversion produced by nicotine in rats. Br J Pharmacol. 1983;79(1):245–253. doi: 10.1111/j.1476-5381.1983.tb10518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Murakami K, Oda S, Shinkai M, Kishi K. Direct synaptic connections between thalamocortical axon terminals from the mediodorsal thalamic nucleus (MD) and corticothalamic neurons to MD in the prefrontal cortex. Brain Res. 1993;612(1–2):339–344. doi: 10.1016/0006-8993(93)91683-j. [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Glanzman DL. Cortical substrates of taste aversion learning: dorsal prepiriform (insular) lesions disrupt taste aversion learning. J Comp Physiol Psychol. 1982;96(3):376–392. doi: 10.1037/h0077894. [DOI] [PubMed] [Google Scholar]
- LeBlanc AE, Cappell H. Antagonism of morphine-induced aversive conditioning by naloxone. Pharmacol Biochem Behav. 1975;3(2):185–188. doi: 10.1016/0091-3057(75)90146-x. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98(3):357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Liu C, Showalter J, Grigson PS. Ethanol-Induced Conditioned Taste Aversion: Reward or Aversion? Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2008.00865.x. (In Prep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, De la Casa LG. There is a time and a place for everything: bidirectional modulations of latent inhibition by time-induced context differentiation. Psychon Bull Rev. 2005;12(5):806–821. doi: 10.3758/bf03196774. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Markman RE, Allen J. Latent inhibition and classical conditioning of the rabbit pinna response. J Comp Physiol Psychol. 1968;66(3):688–694. doi: 10.1037/h0026547. [DOI] [PubMed] [Google Scholar]
- Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24(1):71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Mayer J, Schuster HG, Claussen JC. Role of inhibitory feedback for information processing in thalamocortical circuits. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;73(3 Pt 1):031908. doi: 10.1103/PhysRevE.73.031908. [DOI] [PubMed] [Google Scholar]
- Mayer LA, Parker LA. Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology (Berl) 1993;112(2–3):189–194. doi: 10.1007/BF02244909. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS. A dissociation of dorso-lateral striatum and amygdala function on the same stimulus-response habit task. Neuroscience. 2004;124(3):507–513. doi: 10.1016/j.neuroscience.2003.11.041. [DOI] [PubMed] [Google Scholar]
- McDonald RV, Parker LA, Siegel S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol Biochem Behav. 1997;58(4):1003–1008. doi: 10.1016/s0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243(1):91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Mungarndee SS, Lundy RF, Jr, Norgren R. Central gustatory lesions and learned taste aversions: Unconditioned stimuli. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10(1):73–78. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- Naor C, Dudai Y. Transient impairment of cholinergic function in the rat insular cortex disrupts the encoding of taste in conditioned taste aversion. Behav Brain Res. 1996;79(1–2):61–67. doi: 10.1016/0166-4328(95)00262-6. [DOI] [PubMed] [Google Scholar]
- Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173(2):1136–1139. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91(1):99–117. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92(1):123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Paredes-Olay C, Lopez M. Lithium-induced outcome devaluation in instrumental conditioning: dose-effect analysis. Physiol Behav. 2002;75(5):603–609. doi: 10.1016/s0031-9384(02)00663-7. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19(1):143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR. Rat brain in stereotaxic coordinates (CD-ROM) J Neurosci Methods. 2005a;3(2):129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR. The Rat brain in stereotaxic coordinates (CD-ROM) 2. 2005b. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Kesner RP. The role of the agranular insular cortex in working memory for food reward value and allocentric space in rats. Behav Brain Res. 1999;98(1):103–112. doi: 10.1016/s0166-4328(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Alvarez-Borda B, Ormsby CE, Martinez RD, Perez-Montfort R, Bermudez-Rattoni F. Insular cortex lesions impair the acquisition of conditioned immunosuppression. Brain Behav Immun. 1996;10(2):103–114. doi: 10.1006/brbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: I. Innate taste preferences and aversions. Behav Neurosci. 1996a;110(4):737–745. doi: 10.1037//0735-7044.110.4.737. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behav Neurosci. 1996b;110(4):746–759. [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: III. Simultaneous contrast and autoshaping. Physiol Behav. 1997;62(6):1355–1363. doi: 10.1016/s0031-9384(97)00352-1. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Progressive ratio performance in rats with gustatory thalamus lesions. Behav Neurosci. 1999;113(5):1008–1019. doi: 10.1037//0735-7044.113.5.1008. [DOI] [PubMed] [Google Scholar]
- Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav. 1994;56(4):741–745. doi: 10.1016/0031-9384(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav Neurosci. 1997;111(3):633–645. [PubMed] [Google Scholar]
- Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain Res. 1996;741(1–2):109–116. doi: 10.1016/s0006-8993(96)00906-7. [DOI] [PubMed] [Google Scholar]
- Schalomon PM, Robertson AM, Laferriere A. Prefrontal cortex and the relative associability of taste and place cues in rats. Behav Brain Res. 1994;65(1):57–65. doi: 10.1016/0166-4328(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147(3):285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Role of gustatory thalamus in anticipation and comparison of rewards over time in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R966–980. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399(4):440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89(5):498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Siegel S. Drug anticipation and drug addiction. The 1998 H. David Archibald Lecture. Addiction. 1999;94(8):1113–1124. doi: 10.1046/j.1360-0443.1999.94811132.x. [DOI] [PubMed] [Google Scholar]
- Small DM. Central gustatory processing in humans. Adv Otorhinolaryngol. 2006;63:191–220. doi: 10.1159/000093761. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith ME, Norgren R, Grigson PS. A mixed design reveals that glucose moieties facilitate extinction of a conditioned taste aversion in rats. Learn Behav. 2004;32(4):454–462. doi: 10.3758/bf03196041. [DOI] [PubMed] [Google Scholar]
- Smythies J. The functional neuroanatomy of awareness: with a focus on the role of various anatomical systems in the control of intermodal attention. Conscious Cogn. 1997;6(4):455–481. doi: 10.1006/ccog.1997.0315. [DOI] [PubMed] [Google Scholar]
- Spector AC, Grill HJ, Norgren R. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol Behav. 1993;53(2):277–283. doi: 10.1016/0031-9384(93)90205-t. [DOI] [PubMed] [Google Scholar]
- Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci. 1992;106(1):147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci. 1995;109(5):939–954. [PubMed] [Google Scholar]
- Turenne SD, Miles C, Parker LA, Siegel S. Individual differences in reactivity to the rewarding/aversive properties of drugs: assessment by taste and place conditioning. Pharmacol Biochem Behav. 1996;53(3):511–516. doi: 10.1016/0091-3057(95)02042-x. [DOI] [PubMed] [Google Scholar]
- Twining RC, Hajnal A, Bruno K, Hess EJ, Grigson PS. Lesions of the Ventral Tegmental Area disrupt drug-induced appetite stimulating effects but spare reward comparison. International journal of Comparative Psychology. 2005;18:372–396. [Google Scholar]
- Wakonigg G, Sturm K, Saria A, Zernig G. Opioids, cocaine, and food change runtime distribution in a rat runway procedure. Psychopharmacology (Berl) 2003;169(1):52–59. doi: 10.1007/s00213-003-1488-9. [DOI] [PubMed] [Google Scholar]
- Weise-Kelly L, Siegel S. Self-administration cues as signals: drug self-administration and tolerance. J Exp Psychol Anim Behav Process. 2001;27(2):125–136. [PubMed] [Google Scholar]
- Wheeler DS, Miller RR. Interactions between retroactive-interference and context-mediated treatments that impair Pavlovian conditioned responding. Learn Behav. 2007;35(1):27–35. doi: 10.3758/bf03196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- White N, Sklar L, Amit Z. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology (Berl) 1977;52(1):63–66. doi: 10.1007/BF00426601. [DOI] [PubMed] [Google Scholar]
- Wilkins EE, Bernstein IL. Conditioning method determines patterns of c-fos expression following novel taste-illness pairing. Behav Brain Res. 2006;169(1):93–97. doi: 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191(4233):1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- Wolf G. Projections of thalamic and cortical gustatory areas in the rat. J Comp Neurol. 1968;132(4):519–530. doi: 10.1002/cne.901320403. [DOI] [PubMed] [Google Scholar]
- Yamamoto C, Nagai H, Takahashi K, Nakagawa S, Yamaguchi M, Tonoike M, et al. Cortical representation of taste-modifying action of miracle fruit in humans. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Taste responses of cortical neurons. Prog Neurobiol. 1984;23(4):273–315. doi: 10.1016/0301-0082(84)90007-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y. Brain mechanisms of taste aversion learning in the rat. Brain Res Bull. 1991;27(3–4):403–406. doi: 10.1016/0361-9230(91)90133-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neuroscience Letters. 1997;226(2):127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol. 1984;51(4):616–635. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kawamura Y. Cortical neurons responding to tactile, thermal and taste stimulations of the rat’s tongue. Brain Res. 1981;221(1):202–206. doi: 10.1016/0006-8993(81)91075-1. [DOI] [PubMed] [Google Scholar]
- Zernig G, Harbig P, Weiskirchner I, Auffinger M, Wakonigg G, Saria A. Reinforcing effect of subcutaneous morphine in a modified Ettenberg runway. J Mol Neurosci. 2002;18(1–2):135–142. doi: 10.1385/JMN:18:1-2:135. [DOI] [PubMed] [Google Scholar]
- Zito KA, Bechara A, Greenwood C, van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacol Biochem Behav. 1988;30(3):693–699. doi: 10.1016/0091-3057(88)90086-x. [DOI] [PubMed] [Google Scholar]