Abstract
Several imaging modalities have been introduced over recent years to better screen for and stage breast cancer. Positron emission mammography (PEM) has been approved by the US Food and Drug Administration and introduced into clinical use as a diagnostic adjunct to mammography and breast ultrasonography. PEM has higher resolution and a more localized field of view than positron emission tomography–computed tomography and can be performed on patients to stage a newly diagnosed malignancy. Review of mammograms together with magnetic resonance or PEM images improves detection of disease.
Breast cancer remains the most prevalent cancer in women of developed countries with great social and economic impact. The scientific community is therefore focused on improving imaging methods for screening and staging of breast cancer. Conventional screening has traditionally included a combination of breast self-examination, clinical breast examination, and screening mammography (1).
Among the imaging modalities introduced over recent years is breast magnetic resonance imaging (MRI), a promising technique for the detection, diagnosis, and staging of breast carcinoma. Generally accepted indications for MRI include evaluating response in patients treated with chemotherapy, screening high-risk patients, aiding the detection of primary tumors in patients with nodal metastases of unknown character, and staging known breast carcinoma (2). There are, however, contraindications to MRI, including pacemakers, a metallic foreign body in the orbit, some aneurysm clips, claustrophobia, renal disease, and an allergy to gadolinium. Other factors, such as orthopedic spinal hardware, can degrade the image quality and interpretation.
Another imaging modality that has been utilized in breast cancer patients is positron emission tomography–computed tomography (PET-CT), which has allowed the merging of morphological and functional images, thereby providing information about the metabolic activity of neoplastic tissue and tumor biology. This information can be used to differentiate between benign and malignant lesions, identify metastasis for disease staging, evaluate treatment response, and assess tumor aggressiveness. However, due to the limited resolution of PET equipment and the space limitations of the current protocols for CT acquisition, small breast tumors are not visible with this technique (3).
Positron emission mammography (PEM) is a new imaging modality that has higher resolution than PET-CT and can be performed on patients unable to have an MRI scan. PEM uses a pair of dedicated gamma radiation detectors placed above and below the breast and mild breast compression to detect coincident gamma rays after administration of fluorine-18 fluorodeoxyglucose (18F-FDG), the positron-emitting radionuclide used in whole-body PET studies for the detection of metastatic disease (4). There is currently only one commercially available PEM scanner, the PEM Flex Solo II (5, 6) (Figure 1). This article provides an overview of the technology of PEM and describes its advantages and potential disadvantages for future applications in the clinical setting.
Figure 1.
Positron emission mammography scanner. Photo courtesy of Naviscan, Inc.
POSITRON EMISSION MAMMOGRAPHY TECHNOLOGY
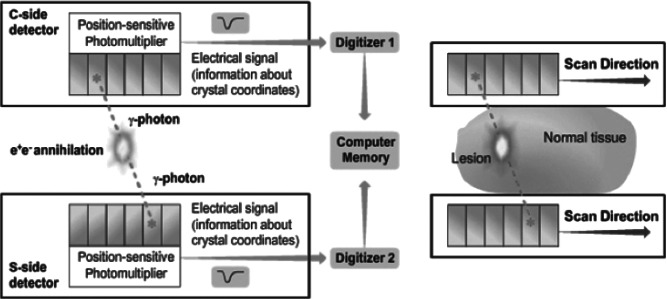
PEM is a high-resolution tomographic method for molecular imaging of positron-emitting isotopes. The principle behind this technology is that cancer cells demonstrate increased utilization of glucose. Through use of isotope fluorine-18 attached to the delivery compound deoxyglucose to produce the radiopharmaceutical 18F-FDG, this utilization of glucose can be visualized. 18F-FDG is taken up into the cancer cell via glucose transporter 1. Once inside the cell, the radiopharmaceutical becomes phosphorylated and cannot be transported back out of the cell, leading to its accumulation. The fluorine-18 nucleus is unstable, and as it decays a positron is emitted. The collision of the positron with an electron results in the production of two 511 keV gamma rays, which are emitted 180 degrees from each other. In PEM, these gamma rays are detected by striking a pair of dedicated gamma radiation detectors placed above and below the breast. Once the gamma rays are detected, they are amplified by photon-sensitive photomultipliers and translated into an electrical signal that becomes digitized and is stored as computer memory (7) (Figure 2).
Figure 2.
The signal received by the detectors is stored as computer memory and utilized to determine spatial information about the lesion. Photo courtesy of Naviscan, Inc.
The technology of PEM and PET are similar in that they both provide functional imaging employing 18F-FDG. However, PEM is optimized for small body parts and utilizes gentle immobilization of the breast (Figure 3) to attain higher spatial resolution (1–2 mm for PEM vs 4–6 mm for PET), as well as minimize the radiation dose by reducing breast thickness (8). The crystal detectors in PEM are constructed to provide this improved spatial resolution (1.5 mm in-plane, 5 mm between planes) and count rate efficiency (1 M reconstructed counts in 10 minutes).
Figure 3.
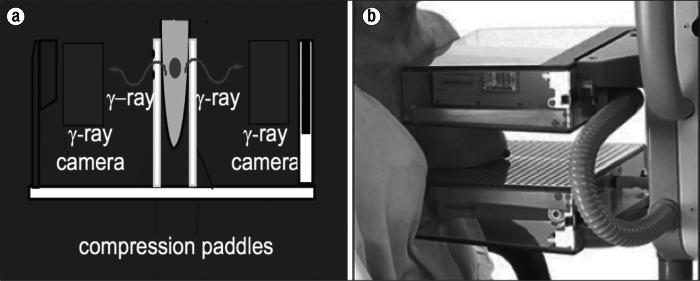
PEM utilizes compression paddles placed on both sides of the breast with positioning similar to mammography, as can be seen (a) schematically and (b) in a patient. Photo courtesy of Naviscan, Inc.
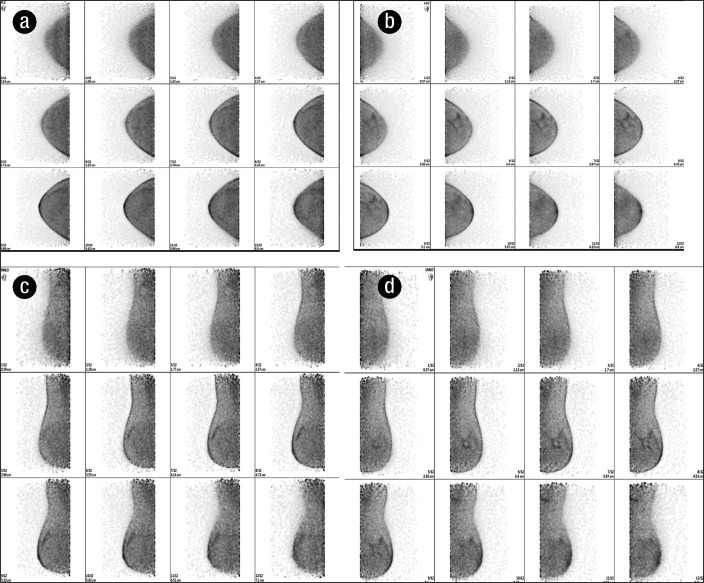
The result of PEM imaging is a set of 12 slices each in the craniocaudal and mediolateral oblique positions, analogous to mammography (Figure 4). The three-dimensional tomographic image set provides detailed location of normal and abnormal FDG uptake as well as features or architectural patterns of any abnormal uptake (9). The smaller overall detector size facilitates the use of high-resolution components and allows closer proximity to the source, enhancing annihilation photon sensitivity (10).
Figure 4.
PEM produces a set of 12 slices in the (a) right craniocaudal, (b) left craniocaudal, (c) right mediolateral oblique, and (d) left mediolateral oblique positions.
ADVANTAGES AND DISADVANTAGES OF POSITRON EMISSION MAMMOGRAPHY
Relative to whole-body PET, PEM's advantage lies in its ability to detect small hypermetabolic lesions. PEM can detect lesions measuring <2 cm as a result of its higher spatial resolution of up to 2.4 mm (Figure 5). Even in very small tumors measuring <1 cm, the imaging sensitivity of PEM has been reported to be 60% to 70% (2). When PEM has been directly compared with PET and MRI, the reported sensitivity of PEM was 93% for known index lesions and 85% for unsuspected additional lesions. The sensitivity was comparable to that of MRI and significantly higher than that of PET, particularly in small tumors (11). In another study, PEM and MRI were both shown to have index lesion sensitivity of 92.8%. Whole-body PET demonstrated a sensitivity of only 67.9% (12).
Figure 5.
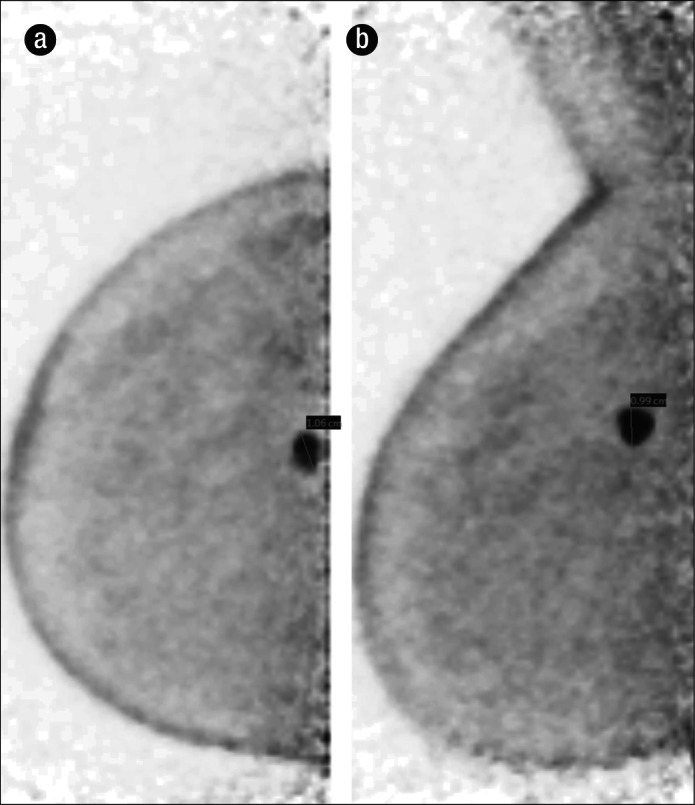
Infiltrating ductal carcinoma, grade 3, in a 69-year-old woman with a strong family history of breast cancer. (a) Right craniocaudal projection PEM shows an intense focus of FDG uptake in the right breast at the 12:00 position, measuring 1.06 cm. (b) Right mediolateral oblique projection PEM shows the same intense focus, measuring <1 cm on this view. This case highlights the ability of PEM to detect very small lesions.
As both MRI and PEM have similar sensitivities, PEM's role in clinical practice mirrors that of MRI. Detection and characterization of primary breast lesions in preoperative surgical planning or prechemotherapy evaluation remain primary indications for the exam. The utility of PEM has been demonstrated in staging both the ipsilateral and contralateral breasts in newly diagnosed patients as an alternative to MRI (Figures 6–7). MRI has been shown to be more sensitive than PEM in the detection of malignancy, although particularly for ipsilateral lesions, PEM is more specific. It can therefore be concluded that in patients in whom MRI may be contraindicated, PEM can still play a valuable role in the detection of additional foci of malignancy (13, 14).
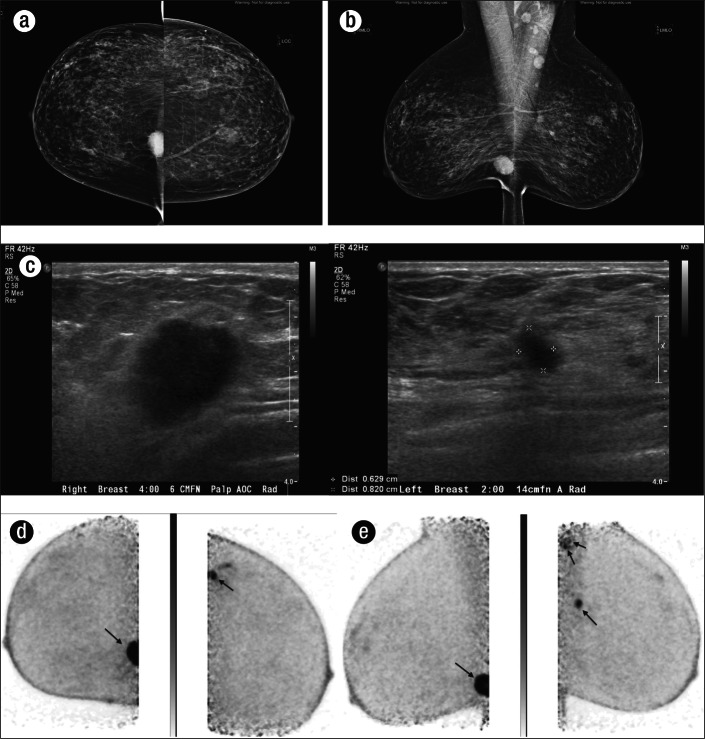
Figure 6.
Bilateral infiltrating grade 1 carcinoma with mixed lobular and ductal features in a 56-year-old woman who was not a candidate for breast MRI secondary to aneurysm clip placement. The cancer was first detected on a bilateral screening mammogram, with both (a) craniocaudal and (b) mediolateral oblique projections, which demonstrated an irregular hyperdense mass with spiculated margins in the lower inner quadrant of the right breast, as well as a smaller irregular spiculated mass within the upper outer quadrant of the left breast, seen only on the mediolateral oblique projection. Prominent lymph nodes were also seen in the left axilla. (c) Further evaluation of these lesions was done with ultrasound, which demonstrated an irregular hypoechoic mass in the right breast at the 4:00 position 6 cm from the nipple and an irregular spiculated mass in the left breast at the 2:00 position 14 cm from the nipple. Ultrasound-guided biopsy was recommended and demonstrated bilateral grade 1 carcinoma with mixed lobular and ductal features. PEM was then performed for disease staging in the (d) craniocaudal and (e) mediolateral oblique projections and showed the two foci of disease as well as increased uptake within the left axilla (e, double arrows), which proved to be malignant.
Figure 7.
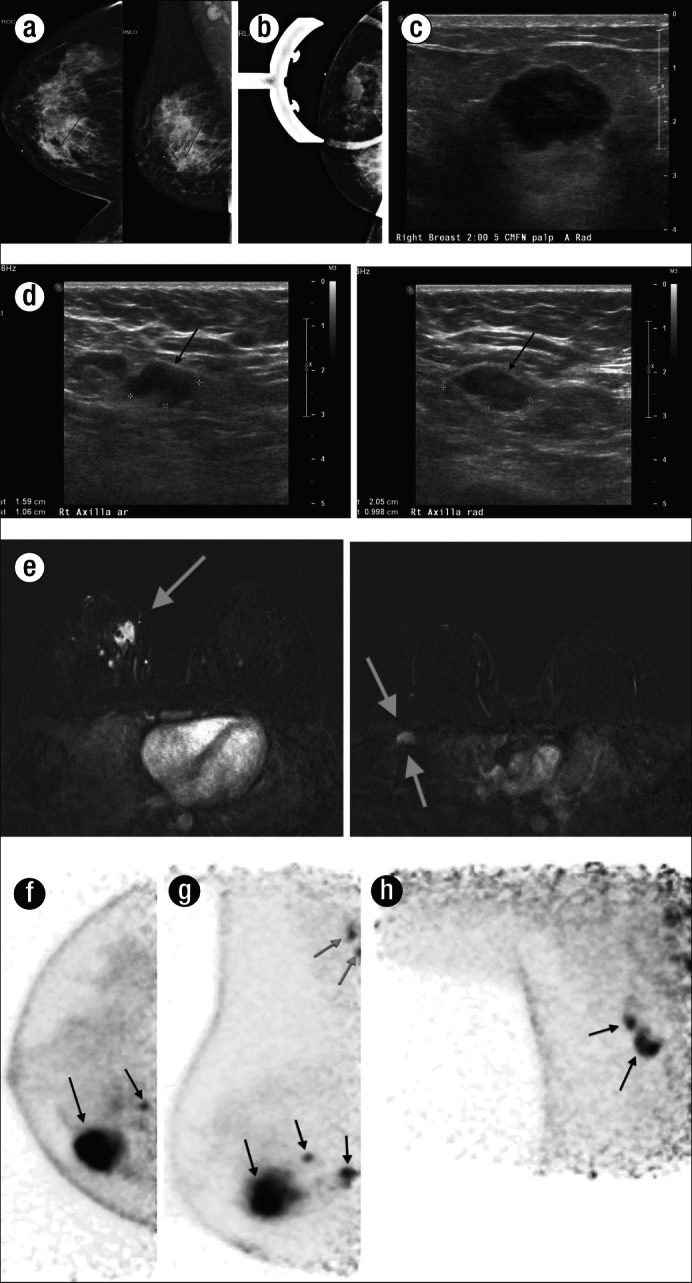
High-grade invasive ductal carcinoma in a 39-year-old woman who presented with a palpable mass in the right breast. The patient had (a) a diagnostic mammogram that demonstrated a 2-cm mass in the right breast that corresponded to the palpable area of concern, seen on both the craniocaudal and mediolateral oblique views, and persisted on (b) a magnification view. Prominent lymph nodes were also noted in the right axilla on the mediolateral oblique view (a). (c) The lesion was then further evaluated with right breast sonogram, which showed a highly suspicious hypoechoic solid mass in the region of palpable concern. (d) A sonogram of the axilla demonstrated two prominent lymph nodes, which were concerning for metastatic involvement. A sonographic-guided biopsy of the dominant mass was performed and demonstrated high-grade invasive ductal carcinoma. (e) An MRI was then performed to evaluate the local extent of disease, which showed a dominant irregular enhancing mass within the right breast as well as multiple additional satellite nodules (single arrow) within the upper inner quadrant of the right breast and right axillary lymphadenopathy (double arrows). PEM was then performed to aid in further staging the patient's disease. The (f) craniocaudal and (g) mediolateral oblique views demonstrated a hypermetabolic round mass within the right breast with two adjacent subcentimeter satellite lesions, as well as increased uptake within two axillary lymph nodes (gray arrows). The axillary lymph nodes were better seen on (h) an axillary view and were felt most compatible with metastatic disease given their intense uptake on PEM. These findings were confirmed during surgery.
Despite the high sensitivities described for both exam types, PEM suffers from the same specificity issues as those seen in breast MRI. The specificity for detecting carcinoma ranges from 85% to 92% for MRI and from 92% to 97% for PEM (14). A number of nonmalignant lesions can accumulate radionuclide, such as fibroadenoma, fibrocystic change, and fat necrosis (Figure 8). To address this issue, commercially available biopsy systems can be used, which allow vacuum-assisted biopsy of PEM-detected lesions prior to altering surgical management. Positive predictive values of these biopsies have been similar to those seen for MRI-guided biopsy and higher that that seen for mammography.
Figure 8.
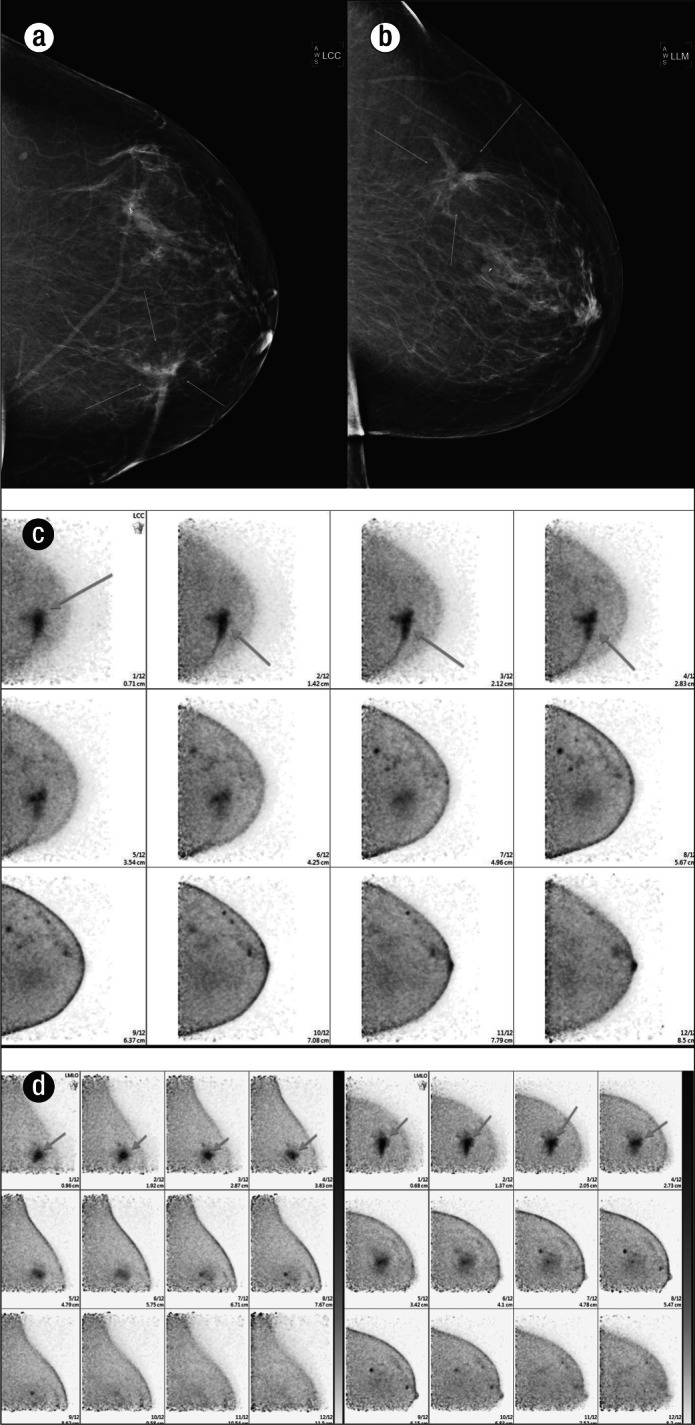
A 51-year-old woman who had a previous left lumpectomy for ductal carcinoma in situ was noted to have a changing appearance of lumpectomy bed on mammogram seen on both (a) craniocaudal and (b) lateral views of the left breast. She subsequently had a PEM scan that showed a region of increased uptake of radiotracer within the lumpectomy bed on both (c) craniocaudal and (d) mediolateral oblique views. Although this was felt to most likely reflect postsurgical change, the presence of residual malignancy could not be excluded. The patient therefore underwent ultrasound-guided biopsy, which demonstrated fat necrosis, fibrosis, and granulation tissue, without evidence of residual neoplasm. This case highlights the inability of PEM to differentiate between metabolically active lesions of varying etiology.
Additionally, a disadvantage of PEM is the radiation exposure. In terms of relative risk to a 40-year-old woman, a single PEM study involving the use of a label-recommended radionuclide dose is associated with a 15-fold higher risk of cancer induction than a single screen film or digital mammogram. Overall, there is also a 25-fold higher risk of cancer-related mortality. In mammography, fibroglandular tissue is the only tissue exposed to a substantial level of ionizing radiation. In PEM, however, all body organs are irradiated with radionuclides. Therefore, the risk from mammography is essentially only that of induced breast cancer, while PEM can lead to cancer induction in any number of radiosensitive organs. This is caused primarily by a reliance on both radioactive decay (fluorine-18 positron emissions having a 110-minute half-life) and biologic clearance for the excretion of radionuclide. The highest radiation dose and cancer risk with PEM is to the bladder (4).
FUTURE OF POSITRON EMISSION MAMMOGRAPHY
PEM was approved by the US Food and Drug Administration and has been introduced into clinical use as a diagnostic adjunct to mammography and breast ultrasonography. PEM is currently under clinical investigation in hopes of improving the sensitivity of breast cancer screening (1). Indications for PEM include initial staging evaluation of patients with newly diagnosed cancer (i.e., determining extent of disease, but not including axillary node staging), distinguishing recurrent carcinoma from scar, and monitoring response to neoadjuvant chemotherapy (15). Currently, PEM is used specifically in patients diagnosed with breast cancer who are considering breast conservation surgery to evaluate for multifocal or multicentric disease (9).
Whereas PEM has high imaging sensitivity for breast lesions, its clinical utility requires further investigation. PEM cannot provide the anatomical detail that is provided by MRI. It can, however, be an alternative form of staging for patients who cannot tolerate MRI, have scheduling constraints due to their hormonal status/menstrual cycle, or have no access to breast MRI, as well as for communities struggling to implement a successful breast MRI program (12). Due to the radiation dose received by a patient during a PEM scan, it is likely that PEM will be employed as an alternative for women who are not candidates for MRI. PEM should be considered a strong adjunct to conventional imaging in those patients unable to undergo MRI who qualify for staging of newly diagnosed malignancy.
References
- 1.Feldman ED, Oppong BA, Willey SC. Breast cancer screening: clinical, radiologic, and biochemical. Clin Obstet Gynecol. 2012;55(3):662–670. doi: 10.1097/GRF.0b013e31825ca884. [DOI] [PubMed] [Google Scholar]
- 2.Tejerina Bernal A, Tejerina Bernal A, Rabadán Doreste F, De Lara González A, Roselló Llerena JA, Tejerina Gómez A. Breast imaging: how we manage diagnostic technology at a multidisciplinary breast center. J Oncol. 2012;2012:213–421. doi: 10.1155/2012/213421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, Weber W, Ziegler S, Graeff H, Schwaiger M. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18(20):3495–3502. doi: 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- 4.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257(1):246–253. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]
- 5.Springer A, Mawlawi OR. Evaluation of the quantitative accuracy of a commercially available positron emission mammography scanner. Med Phys. 2011;38(4):2132–2139. doi: 10.1118/1.3560881. [DOI] [PubMed] [Google Scholar]
- 6.Shkumat NA, Springer A, Walker CM, Rohren EM, Yang WT, Adrada BE, Arribas E, Carkaci S, Chuang HH, Santiago L, Mawlawi OR. Investigating the limit of detectability of a positron emission mammography device: a phantom study. Med Phys. 2011;38(9):5176–5185. doi: 10.1118/1.3627149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinyak JE. Positron emission mammography: a new tool in the fight against breast cancer. Naviscan, Inc.; [Google Scholar]
- 8.Niklason LT, Kopans DB, Hamberg LM. Digital breast imaging: tomosynthesis and digital subtraction mammography. Breast Dis. 1998;10(3–4):151–164. doi: 10.3233/bd-1998-103-415. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan D, Madsen KS, Kalinyak JE, Berg WA. Interpretation of positron emission mammography: feature analysis and rates of malignancy. AJR Am J Roentgenol. 2011;196(4):956–970. doi: 10.2214/AJR.10.4748. [DOI] [PubMed] [Google Scholar]
- 10.Wang CL, MacDonald LR, Rogers JV, Aravkin A, Haseley DR, Beatty JD. Positron emission mammography: correlation of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status and 18F-FDG. AJR Am J Roentgenol. 2011;197(2):W247–W255. doi: 10.2214/AJR.11.6478. [DOI] [PubMed] [Google Scholar]
- 11.Eo JS, Chun IK, Paeng JC, Kang KW, Lee SM, Han W, Noh DY, Chung JK, Lee DS. Imaging sensitivity of dedicated positron emission mammography in relation to tumor size. Breast. 2012;21(1):66–71. doi: 10.1016/j.breast.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Schilling K, Narayanan D, Kalinyak JE, The J, Velasquez MV, Kahn S, Saady M, Mahal R, Chrystal L. Positron emission mammography in breast cancer presurgical planning: comparisons with magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2011;38(1):23–36. doi: 10.1007/s00259-010-1588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, Narayanan D, Ozonoff A, Miller JP, Kalinyak JE. Breast cancer: comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology. 2011;258(1):59–72. doi: 10.1148/radiol.10100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, Narayanan D, Kalinyak JE. Comparative effectiveness of positron emission mammography and MRI in the contralateral breast of women with newly diagnosed breast cancer. AJR Am J Roentgenol. 2012;198(1):219–232. doi: 10.2214/AJR.10.6342. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan D, Madsen KS, Kalinyak JE, Berg WA. Interpretation of positron emission mammography and MRI by experienced breast imaging radiologists: performance and observer reproducibility. AJR Am J Roentgenol. 2011;196(4):971–981. doi: 10.2214/AJR.10.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]