Abstract
Background
Owing to the low therapeutic index of barbiturates, benzodiazepines (BZDs) became popular in this country and worldwide many decades ago for a wide range of conditions. Because of an increased understanding of pharmacology and physiology, the mechanisms of action of many BZDs are now largely understood, and BZDs of varying potency and duration of action have been developed and marketed. Although BZDs have many therapeutic roles and BZD-mediated effects are typically well tolerated in the general population, side effects and toxicity can result in morbidity and mortality for some patients. The elderly; certain subpopulations of patients with lung, liver, or kidney dysfunction; and patients on other classes of medication are especially prone to toxicity.
Methods
This review details the present knowledge about BZD mechanisms of action, drug profiles, clinical actions, and potential side effects. In addition, this review describes numerous types of BZD-mediated central nervous system effects.
Conclusion
For any patient taking a BZD, the prescribing physician must carefully evaluate the risks and benefits, and higher-risk patients require careful considerations. Clinically appropriate use of BZDs requires prudence and the understanding of pharmacology.
Keywords: Adverse effects, benzodiazepines, central nervous system
INTRODUCTION
Benzodiazepines (BZDs) are one of the most widely prescribed pharmacologic agents in the United States (more than 112 million prescriptions in 2007).1 BZDs are used for numerous indications, including anxiety, insomnia, muscle relaxation, relief from spasticity caused by central nervous system pathology, and epilepsy. BZDs are also used intraoperatively because of their amnesic and anxiolytic properties. However, these properties become undesired side effects in nearly all other clinical instances.
The severity of BZD-induced adverse effects forces physicians to exercise caution and pay attention to side effects when prescribing this class of agents. Tolerance, dependence, age-related physiological changes, and drug-drug interactions are all important considerations. This review explains the mechanisms of action of BZDs, compares and contrasts popular BZDs on the market today, and describes specific BZD-mediated effects and side effects.2
BENZODIAZEPINE PHARMACOLOGY
General/Pharmacodynamics
BZDs act as positive allosteric modulators on the gamma amino butyric acid (GABA)-A receptor. The GABA-A receptor is a ligand-gated chloride-selective ion channel.
GABA is the most common neurotransmitter in the central nervous system, found in high concentrations in the cortex and limbic system. GABA is inhibitory in nature and thus reduces the excitability of neurons. GABA produces a calming effect on the brain.2 The 3 GABA receptors are designated A, B, and C. This article focuses primarily on the GABA-A receptor, with which BZDs interact.
The GABA-A receptor complex is composed of 5 glycoprotein subunits, each with multiple isoforms (Figure 1). GABA-A receptors contain 2 α subunits, 2 β subunits, and 1 γ subunit. Each receptor complex has 2 GABA-binding sites but only 1 BZD-binding site. The benzodiazepine binding site is in a specific pocket at the pairing (intersection) of the α and γ subunits. Within the α subunit of isoforms 1, 2, 3, and 5 resides a histidine residue (H101, H101, H126, and H105, respectively) that possesses a high affinity for BZDs.3 Isoforms 4 and 6 of the α subunit contain an arginine residue and do not have an affinity for BZDs.3 BZDs bind to the pocket created by the α and γ subunits and induce a conformational change in the GABA-A receptor, allowing GABA to bind. BZDs bind to the pocket created by α and γ subunits and induce a conformational change in the GABA-A receptor. This alteration, in turn, induces a conformational change in the GABA-A receptor's chloride channel that hyperpolarizes the cell and accounts for GABA's inhibitory effect throughout the central nervous system.3
Figure 1.

Gamma amino butyric acid receptor with target sites. Adapted from http://ccforum.com/content/12/S3/S4/figure/F1.
Specific Benzodiazepine Receptors
The BZD receptor has been classified into several types, based on α subunit isoforms and clinical effects related to each type. The BZ1 receptor contains the α1 isoform. The BZ1 receptor is highly concentrated in the cortex, thalamus, and cerebellum;4,5 it is responsible for the BZDs' sedative effects6 and anterograde amnesia and for some of the anticonvulsive effects of diazepam.7 Sixty percent of GABA-A receptors contain the α1 subunit. Therefore, amnesia is a common side effect of BZD use because the majority of GABA-A receptors contain the BZ1 receptor responsible for amnesia.8 A major factor in predicting amnesia risk is lipid solubility; the greater the lipid solubility, the greater the risk of amnesia. BZDs with high lipid solubility have higher absorption rates and faster onset of clinical effects than BZDs with low lipid solubility.2
BZ2 receptors contain the α2 isoform4 and mediate the anxiolytic and, to a large extent, the myorelaxant effects of BZDs.6 BZ2 receptors are highly concentrated in areas such as the limbic system, motor neurons, and the dorsal horn of the spinal cord.7 The anxiolytic effects of BZDs are believed to be mediated through BZ2 receptors located in the limbic system, and myorelaxant properties are mediated via α2-containing receptors in the spinal cord and motor neurons.7 Not all BZDs interact with the same type of BZ receptor or with equal affinity to a specific receptor. These differences in α subunit isoforms, BZ receptor type affinity, and location within the central nervous system account for the different effects of the various BZDs.7
Benzodiazepine Pharmacokinetics
The pharmacokinetic properties of a drug determine its onset of action and the duration of its effect. Specifically, pharmacokinetics describes the absorption, distribution, metabolism, and excretion of a drug (ie, what the body does to the drug). Pharmacodynamics describes the responsiveness of receptors to a drug and the mechanism by which these effects occur (ie, what the drug does to the body). Individuals respond differently to the same drug, and often these different responses reflect the pharmacokinetics and/or pharmacodynamics among different patients.
Pharmacokinetics (determination of the onset of action and the duration of drug effect) is affected by route of administration, absorption, and volume of distribution. BZDs can be administered via intramuscular, intravenous, oral, sublingual, intranasal, or rectal gel forms. Characteristics of the drug—including lipid solubility, binding to plasma proteins, and molecular size—influence the volume of distribution. Pharmacodynamics and pharmacologic drug effects are described in terms of dose-response curves that depict the relationship between the dose and the resulting pharmacologic effect. Dose-response curves predict the effect of the drug on the patient as doses increase. Titration of a drug should proceed based on the expected pharmacodynamics. Key considerations during titration of medications include making the appropriate choice for the patient's condition (eg, renal failure, liver failure, previous drug exposure), appropriate choice of incremental dosing (ie, time and quantity), and periodic monitoring.9
Preexisting disease processes and age-related changes affect elimination half-life, an especially important consideration when administering BZDs. Elimination half-life is the time necessary for plasma concentration of a drug to decrease to 50% during the elimination phase. Because elimination half-life is directly proportional to the volume of distribution and inversely proportional to its clearance, renal and hepatic disease (altered volume of distribution and/or clearance) affect elimination half-life.
Elimination half-life does not reflect time to recovery from drug effects. Elimination half-life is an estimate of the time needed to reduce the drug concentration in the plasma by half. After about 5 elimination half-lives, a drug is nearly totally eliminated from the body. Therefore, drug accumulation is likely if dosing intervals are less than this period of time.
From a pharmacological perspective, BZDs are usually well absorbed by the gastrointestinal tract after oral administration. After intravenous administration, BZDs quickly distribute to the brain and central nervous system. BZD activity is terminated by redistribution similar to that of the lipid-soluble barbiturates. Following intramuscular injection, absorption of diazepam or chlordiazepoxide is slow and erratic, whereas absorption of intramuscular administration of lorazepam or midazolam appears to be rapid and complete. Lorazepam is well absorbed after sublingual administration, reaching peak levels in 60 minutes.2
BZDs and their metabolites are highly protein bound. They are widely distributed in the body and preferentially accumulate in lipid-rich areas such as the central nervous system and adipose tissue. As previously mentioned, the more lipophilic agents generally have the highest rates of absorption and fastest onset of clinical effects. Most BZDs are oxidatively metabolized by the cytochrome P450 enzymes (phase I), conjugated with glucuronide (phase II), and excreted almost entirely in the urine.
Some BZDs exert additional action via production of active metabolites, an important consideration when prescribing these agents. Midazolam, one of the short-acting BZDs, produces no active metabolites. However, diazepam, a long-acting BZD, produces the active metabolites oxazepam, desmethyldiazepam, and temazepam; these metabolites further increase the duration of drug action and should be a serious consideration in some patient groups, especially the elderly and those with extensive hepatic disease.2
BENZODIAZEPINES IN CLINICAL PRACTICE
General
BZDs are classified in terms of their elimination half-life. Short-acting BZDs have a median elimination half-life of 1-12 hours, intermediate-acting BZDs have an average elimination half-life of 12-40 hours, and long-acting BZDs have an average elimination half-life of 40-250 hours.2 As noted earlier, 5 half-lives are generally necessary for an agent to be eliminated from the body, making the number of hours that a drug is in the body considerably longer. The table lists various BZDs and their characteristics.2
Table.
Benzodiazepines Commonly Prescribed in Clinical Practice

Another way to characterize BZDs is by relative potency. The first BZDs were low to medium potency. These include long-acting chlordiazepoxide, the first BZD discovered, as well as oxazepam and temazepam. Because of their effectiveness and relatively low toxicity, they became first-line agents for conditions such as insomnia and anxiety. Later, high-potency BZDs (alprazolam, lorazepam, and clonazepam) were discovered. These new drugs led to new indications for usage: as a treatment for panic disorders,10 as adjuncts to selective serotonin reuptake inhibitors for treatment of obsessive-compulsive disorder, and as adjuncts to antipsychotics for treatment of acute mania or agitation.11 The newer high-potency BZDs showed improved therapeutic effects as well as faster onset of action, making them the preferred BZDs for most applications. However, with increased potency comes an increase in the risk of undesired effects. Therefore, when prescribing drugs in this BZD group, clinicians must consider individual properties such as absorption, distribution, elimination half-life, and lipid solubility.
Alprazolam
Alprazolam (Figure 2) is a short-acting high-potency BZD with an elimination half-life of 6-27 hours (Table). Alprazolam was first studied for use in panic disorders and was proven to be well tolerated and effective.12 Alprazolam is commonly prescribed for panic disorders and anxiety. The recommended dose for anxiety starts with 0.25-0.5 mg tablets, administered by mouth 3 times per day. The maximum recommended daily dose of alprazolam for anxiolysis should not exceed 4 mg. For panic disorders, the same tablet form and route of administration are recommended at a maximum recommended dose of 6-10 mg/d. A common issue with alprazolam is rebound anxiety that occurs with abrupt discontinuation because of the drug's short elimination half-life.
Figure 2.
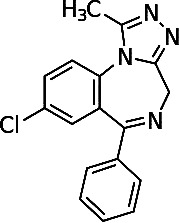
Chemical structure of alprazolam.
Clonazepam
Clonazepam (Figure 3) was the second high-potency BZD discovered. Clonazepam behaves both as a GABA-A receptor agonist in a highly-potent, long-acting manner and also as a serotonin agonist.11 Clonazepam has anticonvulsant and anxiolytic effects. One study proved clonazepam to be at least as effective as lithium for treating acute mania.11 In association with serotonin reuptake blockers, clonazepam appears to accelerate treatment response to panic disorder.12 In another study, clonazepam proved as effective for treating panic disorders as alprazolam, and termination did not cause rebound anxiety symptoms13 because of clonazepam's long elimination half-life. Because clonazepam displays low lipid solubility, it is less likely to cause anterograde amnesia compared to the other high-potency BZDs. For example, clonazepam is half as lipid soluble as alprazolam, so patients' amnesic side effects are reduced. Clonazepam also has a relatively weaker binding affinity for GABA-A receptors than the other high-potency BZDs.11 Clonazepam, when used to treat panic disorders, should be initiated at a dose of 0.25 mg tablets, taken orally twice a day for 3 days, after which the dose should be increased to 0.5 mg tablets twice daily. The maximum daily dose should not exceed 1-4 mg. For treating seizure disorders, adults should start with 0.5 mg tablets taken orally 3 times per day. For this indication, the maximum daily dose should not exceed 20 mg. In the pediatric population, beginning with a dose of 0.01-0.03 mg/kg orally divided into 2 or 3 doses is recommended. The maximum dose in this population should not exceed 0.1-0.2 mg/kg in 3 doses.
Figure 3.

Chemical structure of clonazepam.
Lorazepam
Lorazepam (Figure 4) is another high-potency BZD that displays short-acting characteristics. It is slightly less lipid soluble compared with alprazolam, suggesting a lower risk of amnesic side effects compared to alprazolam. Lorazepam binds GABA-A with less affinity than alprazolam but with greater affinity than clonazepam. Lorazepam has proven effective as an anticonvulsant and also works well as an adjunct to antipsychotics in the treatment of acute agitation and mania.14,15 Following intramuscular injection, lorazepam is absorbed rapidly and completely. After sublingual administration, lorazepam reaches peak plasma levels in roughly 60 minutes.2 Lorazepam is unique in that it undergoes direct glucuronidation without prior cytochrome p450 metabolism. Because of this characteristic, lorazepam can be used in patients with hepatic or renal dysfunction with only minor effects on the drug's pharmacokinetics.16
Figure 4.

Chemical structure of lorazepam.
Lorazepam dosing largely depends on the indication. For alcohol withdrawal, clinicians prescribe 2 mg tablets orally every 6 hours for a total of 4 doses, followed by 1 mg every 6 hours for a total of 8 doses. For anxiolysis, dosing begins with 2-3 mg/d orally, divided into 3 doses per day. Maximum daily doses should not exceed 10 mg. The safety and effectiveness of oral forms have not been established in children under the age of 12. However, the same dosing recommendations for adults apply to children over the age of 12. For sedation, such as in the intensive care unit (ICU), 0.01-0.1 mg/kg/h intravenously is recommended.
Midazolam
Midazolam (Figure 5), a short-acting BZD, is roughly 1.5-2 times as potent as diazepam17 and has a greater hypnotic effect than diazepam because it interferes with GABA reuptake. Midazolam is primarily used preoperatively as an anxiolytic and sedative hypnotic agent (not as a long-term drug in a clinic setting mainly because of its short duration of action). It is available in intravenous, intramuscular, oral, sublingual, rectal, and intranasal preparations. The parenteral preparation used in clinical practice has an acidic pH of 3.5, making it water soluble.18,19 The water solubility of the drug makes the addition of propylene glycol unnecessary, decreasing the pain of injections compared with diazepam. This characteristic explains why midazolam causes less venous irritation and less thrombophlebitis than diazepam.20 At physiologic pH, however, midazolam is one of the most lipophilic BZDs. This lipophilia accounts for midazolam's rapid absorption and crossing of the blood-brain barrier and, hence, the rapid onset of clinical effects. Midazolam is rapidly redistributed, leading to a short duration of action and a short elimination half-life. This short half-life makes midazolam suitable for continuous infusion. Midazolam, like other BZDs, causes peripheral vasodilation and a subsequent decrease in arterial blood pressure. This effect is more pronounced than that seen with diazepam. Midazolam is primarily metabolized by cytochrome p450 into the inactive metabolite 1-hydroxymidazolam. For this drug, anterograde amnesia is usually a desired effect, especially in an operative setting. The amnesic effects of midazolam are more intense than diazepam's but shorter than lorazepam's.21
Figure 5.

Chemical structure of midazolam.
Recommended dosing of midazolam in the preoperative setting for sedation/anxiolysis is usually 1-5 mg intravenously up to 1 hour before surgery in otherwise healthy patients. In higher-risk patients, such as those older than 60 years or those with chronic obstructive pulmonary disease, no more than 3 mg intravenously up to 1 hour before surgery is recommended. In pediatric patients, the safety and efficacy of oral midazolam syrup has not been established for those under 6 months. Therefore, the oral syrup form should only be given to children older than 6 months, and up to 1 mg/kg (maximum dose 20 mg) administered orally is recommended for patients between 6 months and 6 years. Another option for pediatric preoperative sedation is intramuscular or intranasal delivery in a dose of 0.1-0.5 mg/kg (maximum dose 10 mg). Midazolam can also be given intravenously to pediatric patients at a maximum recommended total dose of 10 mg. Most children require substantially lower dosages to achieve desired effects.
Diazepam
Diazepam (Figure 6) is a long-acting, medium-potency BZD that is used as an anticonvulsant and for anxiolysis, sedation, and myorelaxation. Diazepam, one of the most common BZDs used for anxiety,2 is available in intramuscular, intravenous, oral, and rectal gel forms. Diazepam interacts with equal affinity on all BZD-sensitive receptors in the central nervous system.7 Anxiolytic effects are seen at low doses because of diazepam's interaction with α2-containing receptors in the limbic system. At higher doses, diazepam may provide myorelaxation in addition to anxiolysis; the myorelaxant effect is primarily mediated through α2-containing receptors in the spinal cord and motor neurons and to a lesser extent through interaction with α3-containing receptors.7 Of course, at higher doses, sedation and anterograde amnesia are also noted, but these effects are α1-mediated.22 Diazepam is unique in that its metabolism in the liver produces the active metabolites oxazepam, temazepam, and desmethyldiazepam, each of which exerts its own action. These metabolites and their actions account for diazepam's long elimination half-life, which increases approximately 1 hour for each year of age over 40 (eg, the diazepam elimination half-life in a 75-year-old would be approximately 75 hours). Thus, when prescribing this drug, clinicians must consider potential side effects related to active metabolite buildup, such as oversedation and anterograde amnesia. These side effects can be serious and long-lasting, especially in the elderly and in those with hepatic or renal dysfunction. For intravenous administration, diazepam must be prepared in solution with propylene glycol to be water soluble; this solution can cause pain on injection and, in some cases, thrombophlebitis.
Figure 6.

Chemical structure of diazepam.
Diazepam, when used for anxiety, can be given as 2-10 mg orally, 2-4 times per day depending on symptom severity and the patient's age. Both intramuscular and intravenous forms are also available for anxiolysis and should be given in doses of 2-10 mg every 3-4 hours, depending on symptom severity and age considerations. As an adjunct to antiseizure therapy or for muscle relaxation, 2-10 mg orally up to 4 times per day is recommended. For status epilepticus, physicians initially administer 5-10 mg intravenously every 15 minutes up to a maximum dose of 30 mg. If needed, this dose may be repeated in 2-4 hours.
Side Effects
Common side effects among all BZDs include drowsiness, lethargy, and fatigue. At higher dosages, impaired motor coordination, dizziness, vertigo, slurred speech, blurry vision, mood swings, and euphoria can occur, as well as hostile or erratic behavior in some instances. BZDs are eliminated slowly from the body, so repeated doses over a prolonged period can result in significant accumulation in fatty tissues. Thus, some symptoms of overmedication (impaired thinking, disorientation, confusion, slurred speech) can appear over time. Tolerance, dependence, and withdrawal are adverse effects associated with long-term use.2
Drug interaction is another issue with BZDs. They are metabolized in the liver via the cytochrome p450 system and subsequently glucuronidated and renally excreted. Drugs that either attenuate (oral contraceptive pills, antifungals, and some antibiotics) or potentiate (carbamazepine, phenytoin, rifampin, St. John's wort) cytochrome p450 enzymes will either increase or decrease the elimination half-life of BZDs, respectively.2
Severe adverse effects may occur when BZDs are administered with other drugs such as opioids. In combination with opioids, cardiovascular and hemodynamic perturbations become more significant. Respiratory depressant effects on spontaneous ventilation are enhanced dramatically when opioids are used in combination with BZDs, and these effects are dose dependent. Respiratory depressant effects are also exaggerated in patients with chronic obstructive pulmonary disease.
Venoirritation may occur with diazepam and lorazepam; both agents are commonly administered in a hospital or palliative care setting in intravenous formulations.2
AGE-RELATED PATHOPHYSIOLOGIC CHANGES
As people age, they experience a steady decline in the function of homeostatic mechanisms in the body, notably the central nervous system, liver, and kidneys. Research has demonstrated that as a function of aging, numerous central nervous system changes occur, including the death of neurons and their replacement with proliferating glial cells, decreases in intracellular enzymes, and reductions in dendritic synapses.23,24 The physiological changes of aging in the liver result in prolonged clearance of drugs. Decline in renal function begins after the age of 40 at a rate of approximately 1% per year, or a 1 mL/min/y decline in creatinine clearance.25 In the aggregate, these aging-related physiological changes are particularly important in terms of BZD accumulation. In general, there is increased sensitivity—including greater confusion and disorientation—to the effects of BZDs in the elderly compared with the young. This increased sensitivity is directly related to the accumulation of BZDs and related active metabolites. The elderly have subsequent increased intensity of BZD-mediated responses and the duration of BZD-mediated effects.
BENZODIAZEPINE-INDUCED CENTRAL NERVOUS SYSTEM TOXICITY AND ALTERED STATES
General
Cognitive impairment is a broad term that encompasses several symptoms of BZD-induced central nervous system toxicity, such as anterograde amnesia, sedation, drowsiness, motor impairment, inattentiveness, and ataxia. These symptoms are usually more prominent in elderly populations because of the metabolic changes associated with normal aging. In 1991, Beers compiled the famous “Beers List” of pharmacological agents that can be hazardous to the elderly. Numerous BZDs were placed on this list because of their cognition-impairing qualities observed when drug levels accumulate.26 The effects of cognitive impairment can result in serious consequences, including an increased risk of falls and higher rates of fractures, as well as a higher incidence of motor vehicle accidents. Injury is one of the leading causes of death in the elderly, and most fatal injuries are a result of falls.27
In addition to cognitive impairment, BZDs have the risk of dependence. Withdrawal symptoms are commonly observed following abrupt cessation, especially at higher doses. The following sections further expand on the numerous effects and the consequences of high doses of BZDs or poorly tolerated BZDs.25
Anterograde Amnesia
The 3 broad memory classes are sensory, short term, and long term. Sensory and short-term memory seem to be unaffected by BZD usage.27 Long-term memory, on the other hand, is affected by BZDs. The subcategories of long-term memory are explicit (intentional, conscious memories) and implicit (unconscious, unintentional memories).27
Within explicit memory is a subcategory called episodic memory; it is the memory of personally experienced events, involving the recall and recognition of information such as words, stories, pictures, etc. BZDs impair episodic memory.27 The other type of explicit memory is semantic memory; it involves the stored knowledge of information such as language and rules that does not need to be remembered in any specific context. Semantic memory is not impaired by BZDs.27
Implicit memory is also impaired by BZDs, but not in the same manner as explicit memory. Numerous studies to assess BZD-induced memory impairment have all demonstrated a “differential time course” of BZD-induced impairments in implicit and explicit memory,27 meaning that impairments in implicit memory tend to coincide with peak plasma levels of BZDs and do not last as long as the impairments in explicit memory. Impairments in explicit memory occur earlier (in reference to drug administration) and last longer than implicit memory impairments. Two hypotheses have aimed to explain this observation: (1) impairments in implicit memory require relatively higher drug levels in serum than explicit memory impairments, and (2) a specific type of BZD receptor is activated only at higher drug levels, making this receptor at least partly responsible for implicit memory impairments.27 Thus, BZDs impair long-term memory, more specifically, anterograde memory (amnesia for events occurring after the inciting event [drug absorption]). Currently no literature supports any significant evidence of BZDs causing retrograde amnesia (amnesia for events occurring before the inciting event).
In the perioperative setting, BZDs are used specifically for their amnesic properties, but in nearly all other instances, amnesia is an undesired side effect. Although amnesia can occur in any patient, it is especially worrisome among the aging population, because age-related organ decline reduces the ability to metabolize and eliminate drugs, including BZDs. This problem can lead to toxic accumulation of BZDs and their breakdown products, the result of which may manifest in morbidity and even mortality. In many instances, a patient prescribed a BZD for an approved indication (such as anxiety, muscle spasms, or sleep disorders) in a dose that appears to be safe experiences severe memory loss or confusion after several doses. This effect occurs because many BZDs are relatively slowly eliminated from the body because of their lipophilic properties, and they accumulate in fatty tissues. As a result, a patient taking a typical standard dose may suffer significant memory loss. The patient may be unable to recognize loved ones and/or friends and may have difficulty remembering significant portions of his or her life, sometimes as much as several years. Cognitive impairment may also limit the patient's ability to work effectively as well.
Another unfortunate potential consequence that can occur as a result of the amnesic effects of BZD is sexual abuse. Sexual abuse, in general, occurs at an estimated rate of 64/100,000 women each year.28 Many of these cases occur via the administration of a pharmacological agent (eg, a date rape drug), some of which are BZDs.28 This scenario most commonly occurs in conjunction with alcohol consumption either when the victim knowingly ingested a BZD or when an assailant surreptitiously placed the drug into the victim's drink. The latter is commonly referred to as drug-facilitated sexual assault (DFSA). In 1 case, a woman ingested an alcoholic beverage that, unknown to her, contained 1 mg of flunitrazepam. She was subsequently sexually assaulted and, because of flunitrazepam's anterograde amnesic effects, had no recollection of the event. Police later found the major metabolite of flunitrazepam, 7-amino flunitrazepam, in a urine sample collected from the patient for toxicological examination.29 An important clinical observation is that BZDs are a class of drugs used in DFSAs and their amnesic effects are potentially strong enough to facilitate such crimes.29
Disinhibition
Another concerning effect of toxic accumulation of BZDs and their metabolic byproducts is loss of inhibition that can lead one to behave out of character, placing the patient in dangerous situations because of an impaired perception of inherent risk. Common scenarios involve high-risk sexual behavior and reckless driving. One study suggested that BZD usage approximately doubles the risk of motor vehicle accidents.30
A PubMed literature search using the keywords driving and anxiety and the results' cross-references identified 14 placebo-controlled, double-blind studies that examined the effects of anxiolytic drugs on driving ability by conducting on-the-road driving tests during normal traffic. One review concluded that after single-dose administration of BZDs and related compounds, driving performance was significantly impaired.31 Furthermore, driving studies showed that the impairing effects of BZDs and related compounds may still be present after 1 week of daily treatment (demonstrated for diazepam, lorazepam, alpidem, and suriclone), although tolerance may develop. Not surprisingly, the review recommended that patients using BZDs exercise caution when operating a motor vehicle.31
Delirium
Another adverse effect of BZDs, commonly seen in the intensive care setting, is delirium, an acute condition characterized by impaired attention and cognition. BZDs increase the risk of delirium, especially in elderly patients in the intensive care unit.32 Studies have demonstrated an astonishing 78%-87% incidence rate of delirium in elderly patients in the ICU.33-37 Delirium is a serious problem and can lead to increased morbidity, increased mortality, and longer hospital stays.33 Morbidity and mortality increase because the risk of nosocomial infections increases the longer the patient stays in the hospital. Another study has demonstrated that BZDs given prior to intensive care admission were associated with delirium within the first 48 hours of admission.38 Clinicians must be mindful of the risks of delirium when administering BZDs to hospitalized patients.32
CONCLUSION
BZDs are commonly prescribed for a wide range of conditions, including use as sleep aides, muscle relaxants, and anxiolytics. However, dose-related side effects can be seen, including amnesia and central respiratory depression. Other drugs—including opioids, alcohol, and over-the-counter sleep aids—can have additive or synergistic effects on the central nervous system and respiratory function. Certain subpopulations of patients can have significant and severe BZD-mediated effects. The prudent clinician should weigh the risks and benefits of these agents before prescribing.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Cascade E, Kalali AH. Use of benzodiazepines in the treatment of anxiety. Psychiatry (Edgmont) 2008 Sep;5(9):21–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Fox C, Liu H, Kaye AD. Manchikanti L, Trescot AM, Christo PJ, et al, eds. Clinical Aspects of Pain Medicine and Interventional Pain Management: A Comprehensive Review. Paducah, KY: ASIP Publishing;; 2011. Antianxiety agents; pp. 543–552. In. [Google Scholar]
- 3.Kelly MD, Smith A, Banks G, et al. Role of the histidine residue at position 105 in the human alpha 5 containing GABA(A) receptor on the affinity and efficacy of benzodiazepine site ligands. Br J Pharmacol. 2002 Jan;135(1):248–256. doi: 10.1038/sj.bjp.0704459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sieghart W. Pharmacology of benzodiazepine receptors: an update. J Psychiatry Neurosci. 1994 Jan;19(1):24–29. [PMC free article] [PubMed] [Google Scholar]
- 5.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999 Oct 21;401(6755):796–800. doi: 10.1038/44579. Erratum in: Nature. 2000 Apr 6;404(6778):629. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann WA, Humpel C, Alheid GF, Marksteiner J. Compartmentation of alpha 1 and alpha 2 GABA(A) receptor subunits within rat extended amygdala: implications for benzodiazepine action. Brain Res. 2003 Feb 21;964(1):91–99. doi: 10.1016/s0006-8993(02)04082-9. [DOI] [PubMed] [Google Scholar]
- 7.Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001 Mar;59(3):442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 8.Mattila-Evenden M, Bergman U, Franck J. A study of benzodiazepine users claiming drug-induced psychiatric morbidity. Nord J Psychiatry. 2001;55(4):271–278. doi: 10.1080/080394801681019138. [DOI] [PubMed] [Google Scholar]
- 9.Kaye AD, Gayle K, Kaye AM. Pharmacological agents in moderate and deep sedation. In: Urman RD, Kaye AD, editors. Moderate and Deep Sedation. New York, NY: Cambridge University Press;; 2012. pp. 8–32. In. eds. [Google Scholar]
- 10.Chouinard G, Annable L, Fontaine R, Solyom L. Alprazolam in the treatment of generalized anxiety and panic disorders: a double-blind placebo-controlled study. Psychopharmacology (Berl) 1982;77(3):229–233. doi: 10.1007/BF00464571. [DOI] [PubMed] [Google Scholar]
- 11.Chouinard G, Young SN, Annable L. Antimanic effect of clonazepam. Biol Psychiatry. 1983 Apr;18(4):451–466. [PubMed] [Google Scholar]
- 12.Nardi AE, Perna G. Clonazepam in the treatment of psychiatric disorders: an update. Int Clin Psychopharmacol. 2006 May;21(3):131–142. doi: 10.1097/01.yic.0000194379.65460.a6. [DOI] [PubMed] [Google Scholar]
- 13.Chouinard G, Labonte A, Fontaine R, Annable L. New concepts in benzodiazepine therapy: rebound anxiety and new indications for the more potent benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7((4-6)):669–673. doi: 10.1016/0278-5846(83)90043-x. [DOI] [PubMed] [Google Scholar]
- 14.Lenox RH, Modell JG, Weiner S. Acute treatment of manic agitation with lorazepam. Psychosomatics. 1986 Jan;27((1 Suppl)):28–32. doi: 10.1016/s0033-3182(86)72736-9. [DOI] [PubMed] [Google Scholar]
- 15.Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985 Apr;5(2):109–113. doi: 10.1097/00004714-198504000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;(182):335–360. doi: 10.1007/978-3-540-74806-9_16. [DOI] [PubMed] [Google Scholar]
- 17.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985 Mar;62(3):310–324. [PubMed] [Google Scholar]
- 18.Pieri L. Preclinical pharmacology of midazolam. Br J Clin Pharmacol. 1983;16((Suppl 1)):17S–27S. doi: 10.1111/j.1365-2125.1983.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerecke M. Chemical structure and properties of midazolam compared with other benzodiazepines. Br J Clin Pharmacol. 1983;16((Suppl 1)):11S–16S. doi: 10.1111/j.1365-2125.1983.tb02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Midtling JI. Midazolam: a new drug for intravenous sedation. Anesth Prog. 1987 May-Jun;34(3):87–89. [PMC free article] [PubMed] [Google Scholar]
- 21.Kothary SP, Brown AC, Pandit UA, Samra SK, Pandit SK. Time course of antirecall effect of diazepam and lorazepam following oral administration. Anesthesiology. 1981 Dec;55(6):641–644. doi: 10.1097/00000542-198155060-00007. [DOI] [PubMed] [Google Scholar]
- 22.van Rijnsoever C, Täuber M, Choulli MK, et al. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004 Jul 28;24(30):6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long DM. Aging in the nervous system. Neurosurgery. 1985 Aug;17(2):348–354. doi: 10.1227/00006123-198508000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987 Nov-Dec;8(6):521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 25.Stiff JL. Evaluations of the geriatric patient. In: MC Rogers, Covino BG, Tinker JH., editors. Principles and Practice of Anesthesiology. St. Louis, MO: Mosby Year Book;; 1993. pp. 480–492. In. eds. [Google Scholar]
- 26.Stuart B, Kamal-Bahl S, Briesacher B, et al. Trends in the prescription of inappropriate drugs for the elderly between 1995 and 1999. Am J Geriatr Pharmacother. 2003 Dec;1(2):61–74. doi: 10.1016/s1543-5946(03)90002-x. [DOI] [PubMed] [Google Scholar]
- 27.Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45–58. doi: 10.2174/1381612023396654. [DOI] [PubMed] [Google Scholar]
- 28.Negrusz A, Juhascik M, Gaensslen RE. Estimate of the incidence of drug-facilitated sexual assault in the U.S. Final Report. 2005 Washington, DC. https://www.ncjrs.gov/pdffiles1/nij/grants/212000.pdf. Accessed March 5, 2013. [Google Scholar]
- 29.Ohshima T. A case of drug-facilitated sexual assault by the use of flunitrazepam. J Clin Forensic Med. 2006 Jan;13(1):44–45. doi: 10.1016/j.jcfm.2005.05.006. Epub 2005 Aug 8. [DOI] [PubMed] [Google Scholar]
- 30.Thomas RE. Benzodiazepine use and motor vehicle accidents. Systematic review of reported association. Can Fam Physician. 1998 Apr;44:799–808. [PMC free article] [PubMed] [Google Scholar]
- 31.Verster JC, Veldhuijzen DS, Volkerts ER. Is it safe to drive a car when treated with anxiolytics? Evidence from on-the-road driving studies during normal traffic. Curr Psychiatry Rev. 2005 Jun;1(2):215–225. [Google Scholar]
- 32.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009 Jan;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001 Dec 5;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 34.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003 May;51(5):591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001 May;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 36.Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121. doi: 10.1186/cc5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balas MC, Deutschman CS, Sullivan-Marx EM, Strumpf NE, Alston RP, Richmond TS. Delirium in older patients in surgical intensive care units. J Nurs Scholarsh. 2007;39(2):147–154. doi: 10.1111/j.1547-5069.2007.00160.x. [DOI] [PubMed] [Google Scholar]
- 38.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007 Aug 13-27;167(15):1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]


