Abstract
Background
ML 3000 ([2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid) is an inhibitor of both cyclooxygenase and 5-lipoxygenase in vitro, and shows promise as a novel non-steroidal anti-inflammatory drug (NSAID). Unlike conventional NSAIDs which are associated with gastric ulcerogenic effects, ML 3000 causes little or no damage to the gastric mucosa, even though it significantly depresses gastric prostaglandin synthesis.
Methods
As part of an effort to clarify mechanisms underlying the gastric sparing properties of ML 3000, we studied the effects of ML 3000 on H,K-ATPase activity in vitro, on acid accumulation in isolated gastric parietal cells, and on IL-8 secretion by gastric epithelial cells in culture.
Results
SCH28080-sensitive H,K-ATPase activity in highly-purified pig gastric microsomes was dose-dependently inhibited by ML 3000 (IC50 = 16.4 μM). Inhibition was reversible, and insensitive to ML 3000 acidification in the pH range 2.0–8.0. In rabbit gastric parietal cells,
ML 3000 dose-dependently inhibited histamine-stimulated acid accumulation (IC50 = 40 μM) and forskolin-stimulated acid accumulation (IC50 = 45 μM). Lastly, in human gastric adenocarcinoma (AGS) cells, ML 3000 dose-dependently inhibited both baseline and IL-1β-stimulated (20 ng/ml) IL-8 secretion with IC50s of 0.46 μM and 1.1 μM respectively.
Conclusion
The data indicate that ML 3000 affects acid-secretory mechanisms downstream of cAMP mobilization induced by histamine H2 receptor activation, that it directly inhibits H,K-ATPase specific activity, and that baseline gastric epithelial cell IL-8 secretory inhibition may be mediated by ML 3000 inhibition of 5-lipoxygenase activity. We conclude that these gastric function inhibitory data may underlie the gastric sparing properties of ML 3000.
Background
The pharmacological properties of nonsteroidal anti-inflammatory drugs (NSAIDs) arise from their suppression of prostaglandin synthesis from arachidonic acid [1]. Inhibition of gastric prostaglandin syntheses is accompanied however by decreased gastric mucosal blood flow, with concomitant mucosal sensitivity to topical injury by a variety of irritants [2]. A promising pharmacological strategy to avoid gastric complications of NSAID administration focuses on concurrent cyclooxygenase and 5-lipoxygenase inhibition, with the objective of suppressing leukotriene synthesis. 5-lipoxygenase converts arachidonic acid to leukotrienes, among which leukotriene B4 is chemotactic for leukocytes and thereby contributes to inflammatory mechanisms leading to gastrointestinal ulcers [3]. The pyrrolizine derivative ML 3000 ([2,2-dimethyl-6-(4-chlorophen-yl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid) is an inhibitor of both cyclo-oxygenase and 5-lipoxygenase in vitro [4,5], and shows promise as a novel NSAID. ML 3000 reduces gastric prostaglandin E2 synthesis in rat stomach and blood, although significant inhibition of gastric or blood leukotriene B4 synthesis was not detected [6]. Most importantly, ML 3000 has no effect on leukocyte adherence in mesenteric venules, and causes no gastric mucosal injury [6].
In animal models, ML 3000 was found to be pharmacologically benign. Thus, the compound did not affect rat locomotor activity or hexobarbital-induced sleep, had no cardiovascular or respiratory effects in rat and dogs, was without affect on neuromuscular function in cats, and caused neither gastric damage nor peristaltic disturbance [7]. ML 3000 evoked a weak spasmogenic response in guinea-pig ileum, and caused a small transient reduction in urine output in rats [7]. Genotoxicity studies indicate that ML 3000 has no effects on gene mutation frequencies in bacteria or mammalian cells, on unscheduled DNA synthesis, or on frequency of chromosomal aberrations in rat bone marrow cells [8]. 14C-labeled ML 3000 distribution and excretory studies in rats indicate enterohepatic circulation and glucuronide conjugation of ML 3000 [9].
The pharmacological profile of ML 3000 as studied in animal models shows anti-inflammatory, analgesic, antipyretic, antiasthmatic, and antiaggregative activity at a dosage that causes no gastric damage [4,10]. The acute and chronic anti-inflammatory properties of ML 3000 studied in the adjuvant-induced rat paw edema model [11] show that although indomethacin is somewhat more potent as an anti-inflammatory agent, no gastric damage was noted with ML 3000 up to 100 mg/kg/d p.o., whereas the ulcerogenic dose (UD50) for indomethacin was 7 mg/kg/d p.o.
Gastric ulceration induced by NSAIDs significantly limits the utility of these drugs. Clearly, the combination of anti-inflammatory and gastric sparing properties exhibited by ML 3000 makes this compound an attractive candidate for further study. Two gastric mucosal functions which play important roles in gastric ulceration are acid secretion and interleukin-8 secretion. Acid secretory inhibitors are among the most highly-prescribed medications, reflecting the physiological adage "No acid, no ulcer". Pharmacological or pathophysiological breaching of the gastric mucosal barriers to back diffusion of HCl leads to rapid erosion of the gastric epithelial monolayer and consequent ulceration of the mucosa. Inhibition of acid secretion by antagonism at the parietal cell histamine H2 receptor (cimetidine), or by direct covalent derivatization and inactivation of the gastric proton pump (omeprazole, lansoprazole, rabeprazole, esomeprazole, etc) is routine for amelioration and promotion of healing of gastric ulcers. Thus, the gastric sparing effects of ML 3000 may be related to acid secretory inhibition by the compound.
A complementary gastric sparing effect of ML 3000 may involve modulation of gastric secretion of the pro-inflammatory cytokine IL-8. Aspirin-induced gastric injury has been reported to be associated with increased antral production of IL-8 [12]. The ulcerogenic bacterium Helicobacter pylori has been shown to increase the rate and amplitude of IL-8 secretion both in vitro and in vivo [13-15], and to up-regulate IL-8 gene expression [16]. Similar IL-8 prosecretory effects are seen as a result of IL-1β stimulation of gastric epithelial cells. Comparison of the effects of ML 3000 and of other NSAIDs on these gastric secretory activities may elucidate the mechanism by which ML 3000 prevents the formation of gastric mucosal lesions.
In order to clarify mechanisms underlying the gastric sparing properties of ML 3000, we investigated the effect of ML 3000 on two gastric mucosal secretory functions with roles in ulcerogenesis: secretion of hydrochloric acid, and secretion of the inflammatory cytokine interleukin-8. Our data show that ML 3000 inhibits pig gastric microsomal H,K-ATPase activity, inhibits histamine-stimulated acidification in rabbit gastric parietal cells, and inhibits IL-1β-stimulated IL-8 secretion by human gastric epithelial cells.
Methods
Reagents
ML 3000 and ZD-2138 were provided by Forest Laboratories, Inc., New York, NY, omeprazole was provided by AstraZeneca R & D, Molndal, Sweden, SCH 28080 was from Schering-Plough Research Institute, Kenilworth, NJ, TEDBC came from Tocris Cookson, Inc., Ballwin, MO, and NS 398 came from Cayman Chemicals, Inc., Ann Arbor, MI. Arachidonic acid, indomethacin, acetyl salicylic acid, naproxen, PGE2, leukotrienes B4 and D4, and IL-1β were all acquired from Sigma-Aldrich Corp., St. Louis, MO. Dimethyl sulfoxide (Sigma-Aldrich) was used to solubilize ML3000, SCH28080, ZD-2138, TEDBC, NS 398, and indomethacin. Acetyl salicylic acid and naproxen were dissolved in ATPase assay buffer (vide infra). Arachidonic acid and PGE2 were dissolved in absolute ethanol, and omeprazole was dissolved in methanol. Leukotrienes B4 and D4 were supplied dissolved in a 70:30 mix of methanol and 17 mM ammonium acetate. IL-1β was dissolved in phosphate-buffered saline (pH 7.4), 0.1% w/v bovine serum albumin. All assays using these compounds included appropriate solvent controls, and all solvents were present at concentrations less than 1% w/v in the final assay mixture.
Preparation of H,K-ATPase
Microsomal gastric H,K-ATPase was prepared from pig gastric mucosal homogenates by differential and sucrose/Ficoll step gradient centrifugation as described previously [17]. This preparation yields a relatively heavy (7% Ficoll/1.1 M sucrose interface) GII microsomal fraction, which showed no latency of K-ATPase activity, and which comprised ~90% H,K-ATPase, based on densitometry of a 94 kDa band by SDS-PAGE analysis. GII microsomes were stored at -80°C in 20% glycerol.
H,K-ATPase Assay
ATP hydrolytic activity of pig gastric microsomes was quantitated as orthophosphate release from substrate ATP using a colorimetric malachite green procedure [18]. ATPase assays were carried out in 96-well microplates. Briefly, 100 μl/well assay buffer (60 mM Tris-HCl, pH 7.4, 2 mM MgCl2, 1 mM EGTA, ± 7.5 mM KCl, ± 50 μM SCH28080), containing 80 ng microsomal H,K-ATPase, ML 3000 (10-9 M to 10-4 M) or other compounds, and 1 mM ATP was incubated for 30 min at 37°C. Enzymatic activity was stopped by addition (45 μl/well) of colorimetric reagent (0.07% malachite green, 3.7 NH2SO4, 2.27% ammonium molybdate tetrahydrate, 0.134% Tween-20). After 10 seconds, the color reaction was developed by addition (45 μl/well) of 15% sodium citrate. After 45 min at room temperature, absorbance of the wells at 570 nm was measured in a microplate reader.
To measure the effects of ML 3000 acidification on ATPase inhibition, ML 3000 aliquots (10 mM in 5 mM Tris-HCl) were titrated to pHs ranging from 2.0 to 7.4 for 30 min. Gastric microsomes suspended in standard ATPase assay buffer at pH 7.4 were incubated for 30 min with acidified aliquots of ML 3000 (16.6 μM final ML 3000 concentration) and then ATPase activity was measured as described above. To measure omeprazole inhibition of H,K-ATPase activity, gastric microsomes suspended in ATPase assay buffer at pH 6.1 were incubated for 30 min with varying concentrations of omeprazole. Omeprazole acidification to pH 6.1 was necessary to allow formation of the inhibitory thiol-reactive sulfoxide intermediate [19]. The gastric microsomes were then transferred to standard ATPase assay buffer at pH 7.4, and ATPase activity was measured as described above. Specific H,K-ATPase activity was calculated as the difference in microsomal ATPase activities in the presence and absence of the specific reversible gastric H,K-ATPase inhibitor SCH28080, and was expressed as μmoles Pi/mg protein/hr. H,K-ATPase activity of freshly-prepared pig gastric microsomes ranged from 140 to 170 μmoles Pi/mg protein/hr. Graphical depiction of the data shows percent inhibition of H,K-ATPase activity as a function of compound concentrations.
Primary Cell Preparation
Gastric parietal cells were isolated from New Zealand White rabbits by pronase/collagenase digestion of fundic mucosa followed by enrichment of cells on discontinuous Nycodenz gradients as described previously [20]. Parietal cell preparations contained approximately 107 cells/stomach, of which 80 ± 5% were parietal cells based on immunocytochemistry with H,K-ATPase-specific monoclonal antibody.
Acid Accumulation Assay
Aminopyrine accumulation into parietal cells was assessed in 96 well filter plates with Durapore membranes, as described previously [21]. Briefly, cells were preincubated with [14C]-aminopyrine and then 100,000 cells/200 μl per well were incubated without or with test compounds for 15 minutes prior to incubation for a further 30 min in the absence or presence of 100 μM histamine or 100 μM forskolin. All determinations were performed in quadruplicate. Basal aminopyrine accumulation was determined as aminopyrine accumulation into untreated cells subtracted from accumulation in the presence of KSCN (a reflection of non-specific isotope trapping). Graphical depiction of the data shows percent inhibition of histamine-stimulated acid accumulation by the cells as a function of compound concentrations.
Gastric Adenocarcinoma Cells
Human gastric adenocarcinoma cells (AGS cells, ATCC CRL 1739), were maintained in AGS medium (Ham's F-12, 10% fetal bovine serum, 100 units/ml penicillin G, 0.25 ug/ml amphotericin B, 100 ug/ml streptomycin) at 37°C in a 5% CO2/95% air incubator and used between passages 42 and 56. For studies of IL-8 secretion, AGS cells were plated into 96-well plates (50,000 cells/100 μl/well) and allowed to attach for 18 hr at 37°C in 5% CO2/95% air. Cells were then incubated with test compounds in the presence and absence of IL-1β for 24 hr, at which time samples of medium were removed from the wells and stored at -70°C. IL-8 concentrations of the samples were determined by enzyme-linked immunosorbent assay (Human IL-8 Duo-Set ELISA development system, R&D Systems Inc., Minneapolis, MN). Graphical depiction of the data shows percent inhibition of unstimulated or stimulated IL-8 secretion as a function of compound concentrations.
Statistical Analysis
All experiments were carried out at least three times; data points in each assay represent measurement means from triplicate samples. Data points fitting a three parameter logistic equation were graphed as sigmoidal dose-response curves using the Prism statistical package (GraphPad Software, Inc., San Diego, CA). Half-maximal inhibitory concentrations (IC50) and IC50 95% confidence intervals (CI) were also calculated using Prism. Data points not fitting a three parameter logistic were drawn as simple point-to-point curves. Standard error was the measure of variance used for error bars in graphical depiction of data
Results
Effects of ML 3000 on H,K-ATPase activity
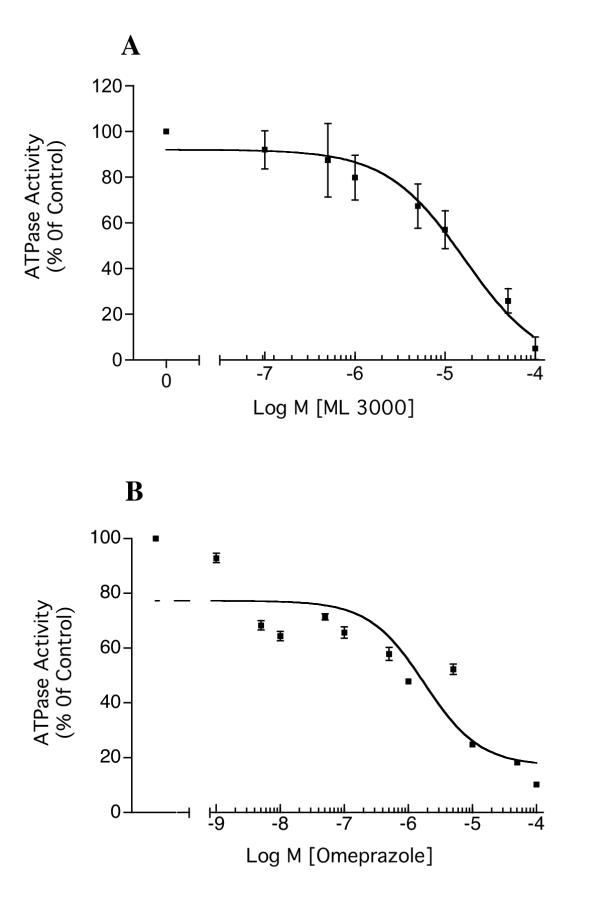
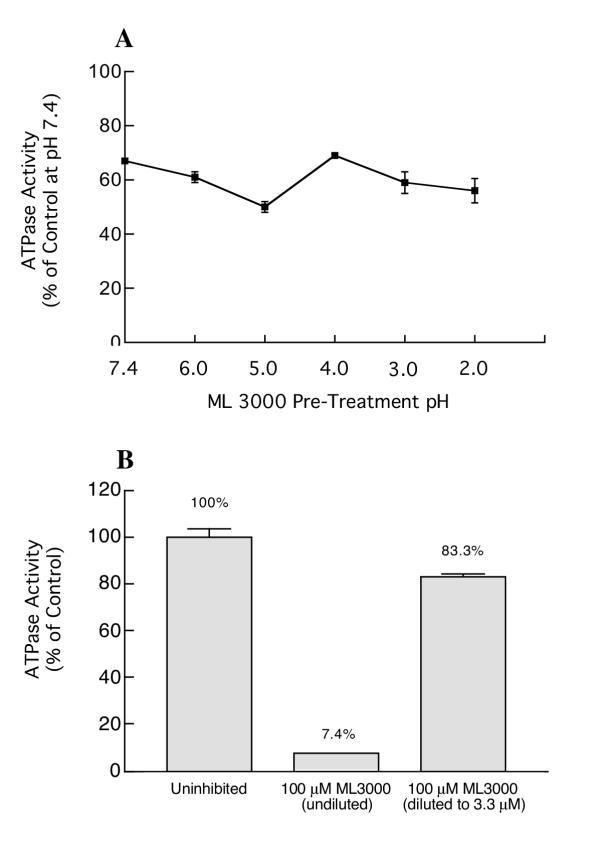
Gastric H,K-ATPase activity was dose-dependently inhibited by ML 3000, with a half-maximal inhibitory concentration (IC50) of 16.4 μM (CI = 6.84 – 39.3 μM) (Figure 1A). The inhibitory activity of ML 3000 was compared to that of a classical proton pump inhibitor (PPI), the substituted benzimidazole omeprazole. Figure 1B shows the effect of omeprazole on H,K-ATPase activity under assay conditions identical to those in Figure 1A; the calculated IC50 for omeprazole was 1.7 μM (CI = 0.26 – 11.3 μM). These data show that ML 3000 is considerably less potent than omeprazole with respect to gastric H,K-ATPase inhibitory activity, at least in the setting of this particular in vitro assay under the specified conditions. To determine whether ML 3000 displayed comparable acid-activation properties, a half-maximal inhibitory concentration of ML 3000 was titrated to different pHs and the effects on H,K-ATPase activity were measured. As shown in Figure 2A, acidification of ML 3000 had no significant effect on its H,K-ATPase inhibitory profile. These data indicate that ML 3000, in contrast to omeprazole, does not require acidification for induction of inhibitory activity. Given that PPIs are irreversible inhibitors of H,K-ATPase activity, covalently binding to the catalytic α subunit, we sought to determine whether ML 3000 inhibition of H,K-ATPase was reversible or irreversible. Gastric H,K-ATPase-enriched microsomes were treated with a maximally-inhibitory concentration of ML 3000 and then diluted with a large excess of buffer to reduce the ML 3000 concentration from 100 μM to 3.3 μM. The results, shown in Figure 2B, indicated that dilution of ML 3000 restored H,K-ATPase activity, and are consistent with ML 3000 inhibiting H,K-ATPase activity in a reversible manner, ie., ML 3000 does not covalently derivatize either subunit of the gastric H,K-ATPase, at least under the conditions of the present in vitro assay of H,K-ATPase activity. In this respect, ML 3000 is kinetically similar to SCH28080, the specific reversible inhibitor of gastric H,K-ATPase.
Figure 1.
Dose-dependent inhibition of H,K-ATPase activity by ML 3000 (A) and by omeprazole (B). (A). Gastric microsomes suspended in ATPase assay buffer at pH 7.4 were incubated for 30 min with varying concentrations of ML 3000 and ATPase activity was then measured at pH 7.4. The half-maximal inhibitory concentration (IC50) for ML 3000 was 16.4 μM (CI = 6.84–39.3 μM). (B). Gastric microsomes suspended in ATPase assay buffer at pH 6.1 were incubated for 30 min with varying concentrations of omeprazole, and then transfered to standard ATPase assay buffer at pH 7.4 for ATPase activity measurement. The IC50 for omeprazole was 1.1 μM (CI = 0.26 – 11.3 μM). The data-points show mean ± s.e. from three independent assays in each of which SCH28080-sensitive ATPase activity was measured in triplicate.
Figure 2.
Effect of ML 3000 acidification on H,K-ATPase activity (A), and ML 3000 inhibition of H,K-ATPase activity is reversible (B). (A) ML 3000 aliquots (10 mM in 5 mM Tris-HCl) were titrated to pHs ranging from 2.0 to 7.4 for 30 min before addition to standard ATPase assay buffer (pH 7.4) containing gastric microsomes. (B) Gastric microsomes were treated with a maximally-inhibitory concentration (100 μM) of ML 3000. The microsomal suspension was then diluted with a large excess of buffer to reduce the ML 3000 concentration from 100 μM to 3.3 μM. In both (A) and (B), the data-points show mean ± s.e. from three independent assays in each of which SCH28080-sensitive ATPase activity was measured in triplicate.
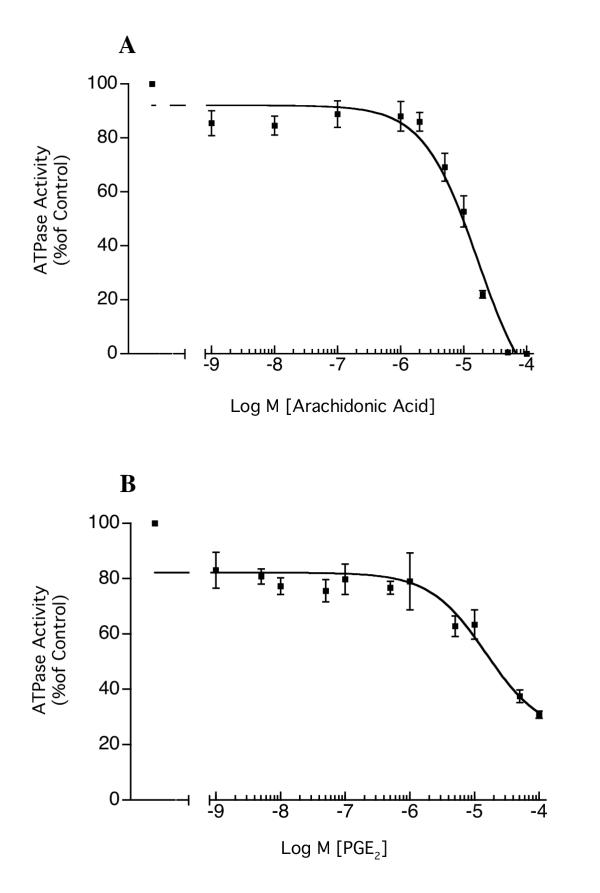
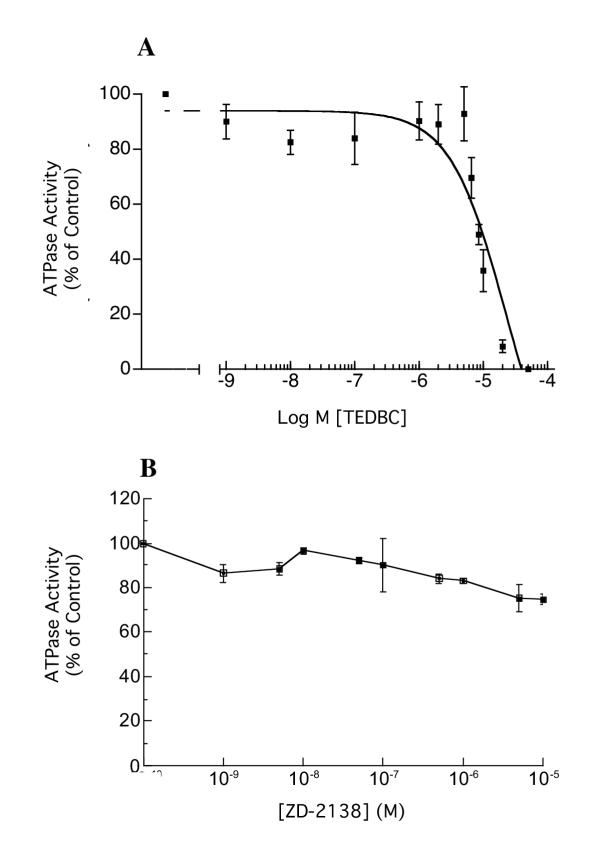
Arachidonic acid and prostaglandin E2 (PGE2) also dose-dependently inhibited H,K-ATPase activity, with IC50 of 16.7 μM (CI = 8.9 – 31.5 μM) and 15 μM (CI = 3.96 – 57.3 μM) respectively (Figure 3A and 3B). Since ML 3000 also shows 5-lipoxygenase inhibition, we studied the effects of two lipoxygenase inhibitors on microsomal H,K-ATPase activity. Figure 4A shows that 2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate (TEDBC), a powerful inhibitor of 5-, 12-, and 15-lipoxygenses, inhibited microsomal H,K-ATPase activity with an IC50 of 22.6 μM (CI = 6.3 – 81.2 μM). In contrast, the 5-lipoxygenase-specific inhibitor 6-((3-fluor-5-(methoxy-3,4,5,6-tetrahydro-2H-pyran-4-yl)phenoxy)methyl)chinolin (ZD-2138) had a minimal effect on H,K-ATPase activity (~20% inhibition at 10-5 M) (Figure 4B). These data are consistent with gastric microsomal 12- and 15-lipoxygenases playing a role in H,K-ATPase activation, or with direct interaction of TEDBC with H,K-ATPase subunits altering enzyme conformation and hence activity. Clearly, both interpretations are provocative and merit further study.
Figure 3.
Inhibition of H,K-ATPase activity by arachidonic acid (A) and prostaglandin E2 (B). Gastric microsomes suspended in ATPase assay buffer at pH 7.4 were incubated for 30 min with varying concentrations of arachidonic acid or PGE2 and ATPase activity was then measured at pH 7.4. IC50 for arachidonic acid was 16.7 μM (CI = 8.9 – 31.5 μM), and the IC50 for PGE2 was 15 μM (CI = 3.96 – 57.3 μM). The data-points show mean ± s.e. from three independent assays in each of which SCH28080-sensitive ATPase activity was measured in triplicate.
Figure 4.
Inhibition of H,K-ATPase activity by the 5-, 12-, and 15-lipoxygenase inhibitor TEDBC (A), and the 5-lipoxygenase inhibitor ZD-2138 (B). Gastric microsomes suspended in ATPase assay buffer at pH 7.4 were incubated for 30 min with varying concentrations of TEDBC or ZD-2138 and ATPase activity was then measured at pH 7.4. In (A), the data-points show mean SCH28080-sensitive ATPase activity measured in triplicate in one of three independent assays; typical data are shown. The data-points in (B) show mean ± s.e. from three independent assays in each of which SCH28080-sensitive ATPase activity was measured in triplicate.
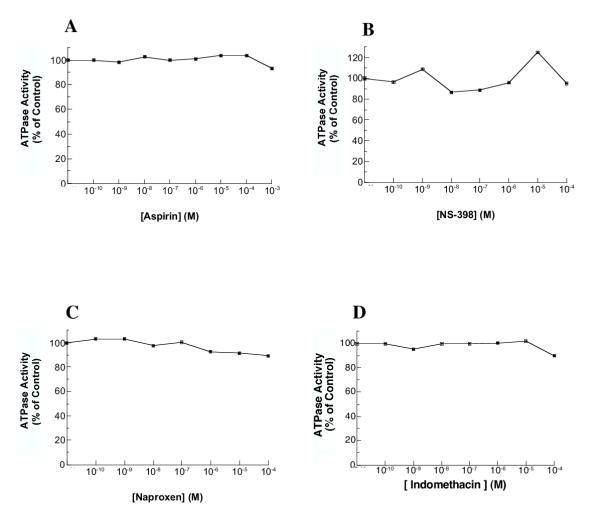
In order to compare the H,K-ATPase inhibitory effect of ML 3000 with other NSAIDS, we measured the effects of acetyl salicylic acid, NS 398, naproxen, and indomethacin on proton pump activity. All four NSAIDS were without inhibitory effects on microsomal H,K-ATPase activity at concentrations up to 10-4 M (10-3 M for acetyl salicylic acid) (Figure 5A,5B,5C and 5D). Indomethacin was previously reported to inhibit gastric H,K-ATPase at somewhat higher concentration (Ki = 0.67 × 10-3 M) [22]. Our data clearly differentiate ML 3000 from other NSAIDS in terms of inhibitory effect on gastric H,K-ATPase activity; the mechanistic basis for this difference, and the potential correlation with the observed gastric-sparing property of ML 3000, remain to be investigated.
Figure 5.
Effects of acetyl salicylic acid, NS 398, naproxen, and indomethacin on H,K-ATPase activity. Gastric microsomes suspended in ATPase assay buffer at pH 7.4 were incubated for 30 min with varying concentrations of acetyl salicylic acid, NS 398, naproxen, or indomethacin and ATPase activity was then measured at pH 7.4. The data-points show mean SCH28080-sensitive ATPase activity measured in triplicate in one of three independent assays; typical data are shown in each case.
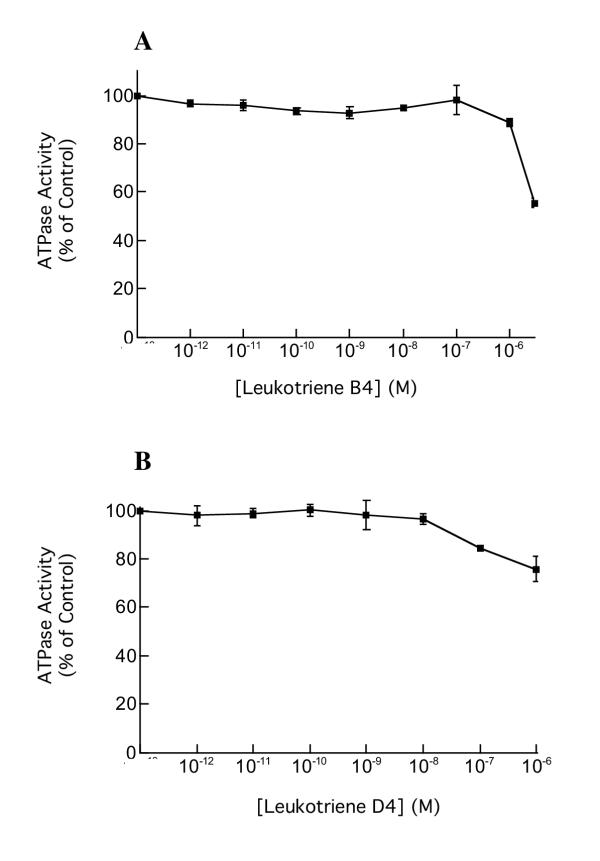
Lastly, in order to establish whether the ML 3000 inhibition of gastric H,K-ATPase reflected the compound's effects on putative functional leukotriene metabolic pathways present in pig gastric microsomes, we studied the effects of leukotriene B4 and D4 on H,K-ATPase activity. Solubility issues precluded studying LTB4 or LTD4 concentrations greater than 1 μM. As shown in Figure 6A and 6B, neither leukotriene showed any inhibitory activity against H,K-ATPase at physiological concentrations (10-9–10-8 M); only at non-physiological concentrations greater than 10-7 M was there any significant attenuation of H,K-ATPase activity.
Figure 6.
Effects of leukotrienes B4 and D4 on SCH28080-sensitive H,K-ATPase specific activity. Gastric microsomes suspended in ATPase assay buffer at pH 7.4 were incubated for 30 min with varying concentrations of leukotriene B4 or leukotriene D4 and ATPase activity was then measured at pH 7.4. The data-points show mean ± s.e. from three independent assays in each of which SCH28080-sensitive ATPase activity was measured in triplicate.
Effects of ML 3000 on gastric parietal cell histamine-stimulated acid accumulation
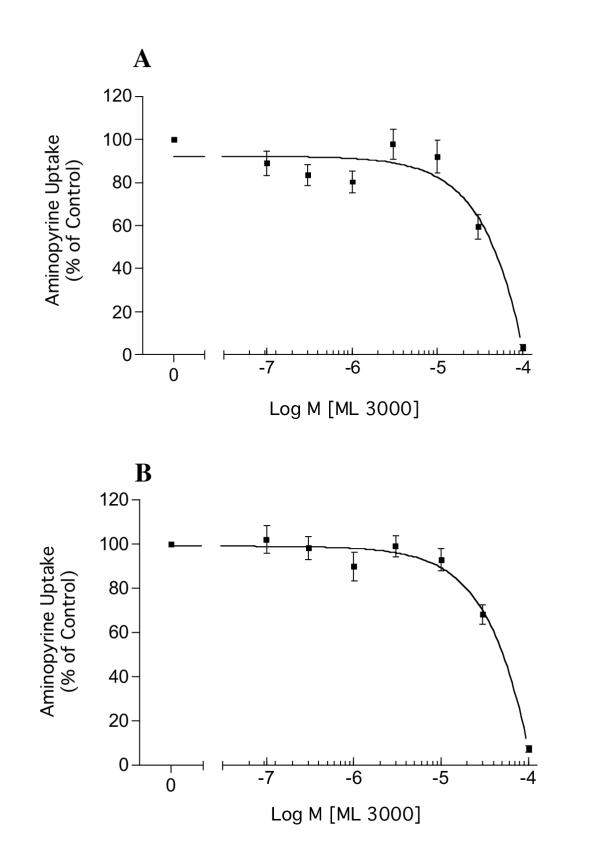
ML 3000 dose-dependently inhibited histamine-stimulated (100 μM) acid accumulation by rabbit gastric parietal cells, with a half-maximal inhibitory concentration (IC50) of 40 μM (Figure 7A). ML 3000 also dose-dependently inhibited forskolin-stimulated (100 μM) acid accumulation by rabbit gastric parietal cells, with an IC50 of ~45 μM (Figure 7B). These data indicate that ML 3000 affects parietal cell acid-secretory mechanisms downstream of cAMP mobilization induced by histamine H2 receptor activation. The data are consistent with ML 3000 inhibition of parietal cell acid secretion resulting from direct interaction of ML 3000 with the gastric H,K-ATPase. However, the discepancy between ML 3000 IC50 in microsomal vesicles (15 μM) and in isolated parietal cells (40–45 μM) suggests that ML 3000 access to the intracellular H,K-ATPase compartment in parietal cells may be slowed by permeability constraints at the plasma membrane. Alternatively, ML 3000 may be subject to cytoplasmic metabolic modifications which limit inhibitory potency of the compound.
Figure 7.
ML 3000 inhibits acid accumulation in rabbit gastric parietal cells. (A) Dose-dependent inhibition by ML 3000 of histamine-stimulated (100 μM) acid accumulation by rabbit gastric parietal cells (IC50 = 40 μM). (B) Dose-dependent inhibition by ML 3000 of forskolin-stimulated (100 μM) acid accumulation by rabbit gastric parietal cells (IC50 = 45 μM). The data-points show mean ± s.e. from four independent assays in each of which acid accumulation into parietal cells was measured in duplicate.
Finally, as was found with microsomal H,K-ATPase, other NSAIDS such as acetyl salicylic acid, naproxen, and NS 398 (up to concentrations of 10-4 M) had no effect on acid accumulation by isolated rabbit parietal cells (data not shown)
Effects of ML 3000 on IL-1β-induced IL-8 secretion in human gastric adenocarcinoma (AGS) cells
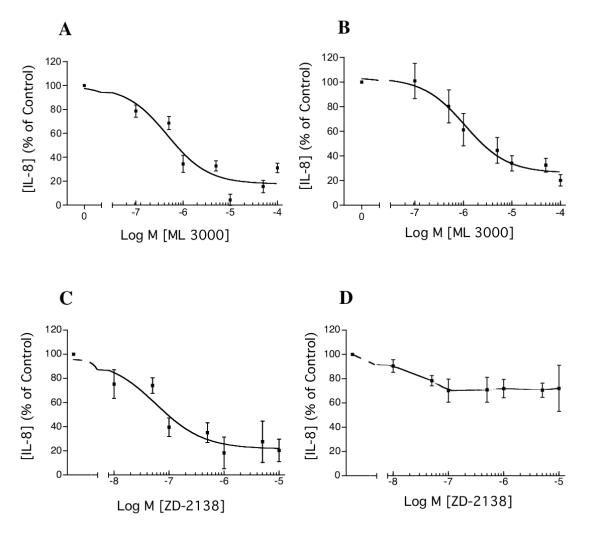
Under baseline conditions, AGS cells (5 × 104 in 100 μl culture medium) secreted IL-8 over a period of 24 hr to a concentration of ~225 pg/ml medium. When stimulated by IL-1β (1 ng/ml), AGS cell IL-8 secretion over a period of 24 hr was increased ~27-fold, to a concentration of ~6000 pg/ml. ML 3000 inhibited both baseline and IL-1β-stimulated IL-8 secretion, with IC50s of 0.46 μM (CI = 0.09 – 2.4 μM) and 1.1 μM (CI = 0.52 – 2.2 μM) respectively (Figure 8A and 8B).
Figure 8.
Effects of ML 3000 on IL-1β-induced IL-8 secretion in human gastric adenocarcinoma (AGS) cells. ML 3000 inhibited both baseline (A) and IL-1β-stimulated (B) IL-8 secretion, with IC50s of 0.46 μM (CI = 0.09 – 2.4 μM) and 1.1 μM (CI = 0.52 – 2.2 μM) respectively. The 5-lipoxygenase-specific inhibitor ZD-2138 showed dose-dependent inhibition of baseline AGS cell IL-8 secretion (C), with an IC50 of 58 nM (CI = 15.6 – 218 nM) and minimal effect on AGS cell IL-1β-stimulated IL-8 secretion (D). The data-points show mean ± s.e. from three independent assays in each of which IL-8 concentrations were measured in triplicate.
The 5-lipoxygenase-specific inhibitor (ZD-2138), which was without effect on microsomal H,K-ATPase activity, showed dose-responsive inhibition of baseline AGS cell IL-8 secretion (Figure 8C), with an IC50 of 58 nM (CI = 15.6 – 218 nM). ZD-2138 showed minimal effect on IL-1β-stimulated IL-8 secretion by AGS cells (Figure 8D). H,K-ATPase inhibition by ML 3000, which we have demonstrated in the present study, does not underlie IL-8 secretory inhibition in this model because immunocytochemistry with H,K-ATPase-specific monoclonal antibody shows no H,K-ATPase expression in AGS cells (data not shown).
Discussion
This study provides information about the effects of ML 3000 on aspects of gastric physiology related to ulcerogenesis. Three experimental models of gastric function were investigated; highly-purified functional H,K-ATPase (proton pump) isolated from the gastric mucosa of slaughterhouse hogs, isolated rabbit gastric parietal cells, and human gastric adenocarcinoma cells in culture. In the first model, ML 3000 inhibited the proton pump with an IC50 of 16.4 μM. The proton pump inhibitor (PPI) omeprazole displayed an IC50 of 1.1 μM in the same assay. Unlike PPIs, ML 3000 inhibitory activity was independent of pH and was reversible. Selective and non-selective NSAIDS showed no H,K-ATPase inhibitory activity in the same assay. In the second model, ML 3000 inhibited both histamine and forskolin-stimulated acid accumulation (IC50 = 40 and 45 μM), consistent with ML 3000 proton pump inhibition. In the third model, ML 3000 inhibited both baseline and IL-1β-stimulated interleukin-8 secretion from AGS cells (IC50 = 0.46 and 1.1 μM respectively), although the latter effect appears unrelated to the 5-lipoxygenase inhibitory activity of ML 3000 (Figure 8D).
Published IC50 for omeprazole with respect to gastric proton pump activity range from 470 nM to 36 μM depending on the conditions of the assay [23-25]. For other PPIs, picoprazole IC50 is 2 μM [26], rabeprazole IC50 is 72 nM [23], and lansoprazole IC50 is 2.1 μM [27]. The wide range of published PPI IC50 values for microsomal H,K-ATPase reflects the mechanistic necessity for compound acidification to allow formation of a thiol-reactive sulfoxide intermediate which then irreversibly derivatizes H,K-ATPase α subunit cysteine residues leading to enzyme inhibition.
These ML 3000 data also contrast dramatically with omeprazole inhibition of acid accumulation in isolated parietal cells. Acid secretory capacity IC50s (in isolated parietal cells) of proton pump inhibitors (PPIs) such as omeprazole, rabeprazole, and lansoprazole are in the range 59 nM to 360 nM [27,28]. The greater potency of PPIs in the context of acid secretory capacity compared to microsomal H,K-ATPase (IC50s ranging from 72 nM to 40 μM) reflects more complete acid activation of PPIs in isolated parietal cell canaliculi than occurs in in vitro microsomal assays at pH 6.1 or higher.
The PGE2 data are in contrast to a previous study in which no inhibitory effect of PGE2 on pig gastric H,K-ATPase [29] was reported. Differences in the specific ATPase assay used in that study may account for this discrepancy. Since ML3000 and arachidonic acid are anionic amphiphiles, their inhibitory effects could result from specific interactions with H,K-ATPase subunit binding sites, or from less-specific hydrophobic interactions with H,K-ATPase-associated microsomal membrane lipids, or a combination of both factors.
The reversibility of ML 3000 inhibition of H,K-ATPase activity in vitro, shown in Figure 2B, contrasts with the irreversibility of H,K-ATPase inhibition by substituted benzimidazoles such as omeprazole or lansoprazole. In a pharmacological context, relief of acid secretory inhibition by the latter reagents is dependent primarily on de novo synthesis and apical membrane targeting of H,K-ATPase subunits, and to some extent on reduction of the inhibitory disulfide bond by intracellular glutathione. Reversible inhibition of H,K-ATPase by ML 3000 offers the potential for less profound inhibition of acid secretion, thereby attenuating rebound acid hypersecretion which has been reported in H. pylori-negative subjects after omeprazole treatment [30]. At the same time, the potentially less-prolonged acid inhibition by ML 3000 may be insufficient to prevent NSAID-ulcers in humans. In vitro reversibility of ML 3000 inhibition of H,K-ATPase activity is also significant in a mechanistic sense, since it allows further analysis of ML 3000 inhibition using classical Michaelis-Menten kinetic assays to determine whether inhibition is competitive, non-competitive, or mixed, each of which options present differing lines of further investigation with respect to pharmaceutical applications of ML 3000.
To the extent that IL-8 is a potent inflammatory mediator in the gastric mucosa, our preliminary finding that ML 3000 profoundly inhibits baseline and IL-1β-stimulated IL-8 secretion in gastric epithelial cells is consistent with the previously-observed gastric sparing properties of ML 3000. The sensitivity of baseline IL-8 secretion to ZD-2138 indicates that ML 3000 inhibition may be mediated by ML 3000 5-lipoxygenase inhibitory activity. In contrast, the relative insensitivity of IL-1β-stimulated IL-8 secretion to ZD-2138 suggests that ML 3000 inhibition is not mediated by ML 3000 5-lipoxygenase inhibitory activity. ML 3000 cycloxygenase inhibitory activity or other as yet undetected activity may underlie the inhibition of IL-1β-stimulated IL-8 secretion in this model. Important objectives of further studies of ML 3000 are definition of the mechanisms of H,K-ATPase inhibition and AGS cell IL-8 secretory inhibition, an assessment of ML 3000 in vivo acid inhibitory efficacy in a rat model, and whole body distribution and localization of the compound.
Conclusions
This study provides evidence that the pyrrolizine derivative ML 3000 inhibits gastric H,K-ATPase activity and gastric epithelial cell IL-8 secretion. The data indicate that the anti-secretory properties of ML 3000, in terms of both acid and IL-8 secretion, may underlie previously-noted gastric-sparing properties of the drug. The apparent mechanistic differences between ML 3000 and omeprazole inhibition of H,K-ATPase identify ML 3000-based compounds as a potentially important novel class of proton pump inhibitors.
Competing interests
This study was supported by a grant to AS from Forest Laboratories, Inc. SG is an employee of Forest Laboratories, Inc.
Authors' contributions
AS conceived of the study, participated in its design and coordination, performed the statistical analysis, and drafted the manuscript. JG carried out assays of acid accumulation by gastric parietal cells. SG participated in study design and analysis. CH carried out assays of H,K-ATPase activity and IL-8 secretion. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Adam J Smolka, Email: smolkaaj@musc.edu.
James R Goldenring, Email: jim.goldenring@vanderbilt.edu.
Sandeep Gupta, Email: sandeep.gupta@frx.com.
Charles E Hammond, Email: hammondc@musc.edu.
References
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Gastric ulceration: critical events at the neutrophil-endothelium interface. Can J Physiol Pharmacol. 1993;71:98–102. doi: 10.1139/y93-014. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001;62:1433–1438. doi: 10.1016/S0006-2952(01)00747-X. [DOI] [PubMed] [Google Scholar]
- Laufer SA, Augustin J, Dannhardt G, Kiefer W. 6,7-Diaryldihydropyrrolizine-5-yl-acetic acids, a novel class of potent dual inhibitors of both cyclo-oxygenase and 5-lipoxygenase. J Med Chem. 1994;37:1894–1897. doi: 10.1021/jm00038a021. [DOI] [PubMed] [Google Scholar]
- Laufer SA, Tries S, Augustin J, Dannhardt G. Pharmacological profile of a new pyrrolizine-derivative that inhibits the enzymes cyclo-oxygenase and 5-lipoxygenase. Arzneim Forsch. 1994;44:629–636. [PubMed] [Google Scholar]
- Wallace JL, Carter L, McKnight W, Tries S, Laufer SA. ML 3000 reduces gastric prostaglandin synthesis without causing mucosal injury. Eur J Pharmacol. 1994;271:525–531. doi: 10.1016/0014-2999(94)90814-1. [DOI] [PubMed] [Google Scholar]
- Algate DR, Augustin J, Atterson PR, Beard DJ, Jobling CM, Laufer SA, Munt PL, Tries S. General pharmacology of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid in experimental animals. Arzneim Forsch. 1995;45:159–165. [PubMed] [Google Scholar]
- Heidemann A, Tries S, Laufer SA, Augustin J. Studies on the in vitro and in vivo genotoxicity of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid. Arzneim Forsch. 1995;45:486–490. [PubMed] [Google Scholar]
- Deigner HP, Freyberg CE, Laufer SA. Distribution and excretion of [14C]-labeled [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-[2'-14C]-acetic acid in rats. Arzneim Forsch. 1995;45:272–276. [PubMed] [Google Scholar]
- Laufer SA, Tries S, Augustin J, Elsasser R, Algate DR, Atterson PR, Munt PL. Gastrointestinal tolerance of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid in the rat. Arzneim Forsch. 1994;44:1329–1333. [PubMed] [Google Scholar]
- Laufer SA, Tries S, Augustin J, Elsasser R, Albrecht W, Guserle R, Algate DR, Atterson PR, Munt PL. Acute and chronic anti-inflammatory properties of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid. Arzneim Forsch. 1995;45:27–32. [PubMed] [Google Scholar]
- Hamlet A, Lindholm C, Nilsson O, Olbe L. Aspirin-induced gastritis, like Helicobacter pylori-induced gastritis, disinhibits acid secretion in humans: Relation to cytokine expression. Scand J Gastroenterol. 1998;33:346–356. doi: 10.1080/00365529850170964. [DOI] [PubMed] [Google Scholar]
- Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NFκB in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- Camorlinga-Ponce M, Aviles-Jimenez F, Cabrera L, Hernandez-Pando R, Munoz O, Soza J, Torres J. Intensity of infammation, density of colonization and interleukin-8 response in the gastric mucosa of children infected with Helicobacter pylori. Helicobacter. 2003;8:554–560. doi: 10.1046/j.1523-5378.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- Harris PR, Weber HC, CM W, RT J, PD S. Cytokine gene profile in gastric mucosa in Helicobacter pylori infection and Zollinger-Ellison syndrome. Am J Gastroenterol. 2002;97:312–318. doi: 10.1016/S0002-9270(01)04025-4. [DOI] [PubMed] [Google Scholar]
- Sharma SA, Tummuru MKR, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabon EC, Im WB, Sachs G. Preparation of gastric H,K-ATPase. Methods Enzymol. 1988;157:649–654. doi: 10.1016/0076-6879(88)57112-4. [DOI] [PubMed] [Google Scholar]
- Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- Wallmark B, Jaresten B-M, Larsson H, Ryberg B, Brandstrom A, Fellenius E. Differentiation among inhibitory actions of omeprazole, cimetidine, and SCN- on gastric acid secretion. Am J Physiol . 1983;245:G64–G71. doi: 10.1152/ajpgi.1983.245.1.G64. [DOI] [PubMed] [Google Scholar]
- Chew CS, Ljungstrom M, Smolka A, Brown MR. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol. 1989;256:G254–G263. doi: 10.1152/ajpgi.1989.256.1.G254. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Goldenring JR, Oddsdottir M, Zdon MJ, Zucker KA, Lewis JJ, Modlin IM. A micro-method for the assay of cellular secretory physiology: Application to rabbit parietal cells. Analytical Biochemistry. 1989;182:346–352. doi: 10.1016/0003-2697(89)90606-4. [DOI] [PubMed] [Google Scholar]
- Spenney JG, Mize KS. Inhibition of gastric K-ATPase by phenylbutazone and indomethacin. Biochem Pharmacol. 1977;26:1241–1245. doi: 10.1016/0006-2952(77)90112-5. [DOI] [PubMed] [Google Scholar]
- Morii M, Takata H, Fujisaki H, Takeguchi N. The potency of substituted benzimidazoles such as E38101, omeprazole, Ro 18-5364 to inhibit gastric H,K-ATPase is correlated with the rate of acid-inactivation of the inhibitor. Biochem Pharmacol. 1990;39:661–667. doi: 10.1016/0006-2952(90)90143-9. [DOI] [PubMed] [Google Scholar]
- Beil W, Sewing KF. Inhibition of partially-purified H,K-ATPase from guinea-pig isolated and enriched parietal cells by substituted benzimidazoles. Br J Pharmacol. 1984;82:651–657. doi: 10.1111/j.1476-5381.1984.tb10803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling DJ, Fallowfield C, Milliner KJ, Tingley SK, Ife RJ, Underwood AH. Studies on the mechanism of action of omeprazole. Biochem Pharmacol. 1985;34:2967–2973. doi: 10.1016/0006-2952(85)90023-1. [DOI] [PubMed] [Google Scholar]
- Wallmark B, Sachs G, Mardh S, Fellenius E. Inhibition of gastric H,K-ATPase by the substituted benzimidazole, picoprazole. Biochim Biophys Acta. 1983;728:31–38. doi: 10.1016/0005-2736(83)90433-9. [DOI] [PubMed] [Google Scholar]
- Nagaya H, Inatomi N, Nohara A, Satoh H. Effects of the enantiomers of lansoprazole (AG-1749) on H, K-ATPase activity in canine gastric microsomes and acid formation in isolated canine parietal cells. Biochem Pharmacol. 1991;42:1875–1878. doi: 10.1016/0006-2952(91)90584-R. [DOI] [PubMed] [Google Scholar]
- Fujisaki H, Shibata H, Oketani A, Murakami M, Fujimoto M, Wakabayashi T, Yamatsu I, Yamaguchi M, Sakai H, Takeguchi N. Inhibitions of acid secretion by E3810 and omeprazole, and their reversal by glutathione. Biochem Pharmacol. 1991;42:321–328. doi: 10.1016/0006-2952(91)90719-L. [DOI] [PubMed] [Google Scholar]
- Im WB, Blakeman DP. Inhibition of gastric H,K-ATPase by unsaturated long-chain fatty acids. Biochim Biophys Acta. 1982;692:355–360. doi: 10.1016/0005-2736(82)90384-4. [DOI] [PubMed] [Google Scholar]
- Gillen D, Wirz AA, JE A, McColl KEL. Rebound hypersecretion after omeprazole and its relation to on-treatment acid suppression and Helicobacter pylori status. Gastroenterology. 1999;116:239–247. doi: 10.1016/s0016-5085(99)70118-6. [DOI] [PubMed] [Google Scholar]