Abstract
Gene-environment interaction effects in predicting antisocial behavior in late childhood were investigated among maltreated and nonmaltreated low-income children (N = 627, M age = 11.27). Variants in three genes, TPH1, 5-HTTLPR, and MAOA uVNTR, were examined. In addition to child maltreatment status, we also considered the impact of maltreatment subtypes, developmental timing of maltreatment, and chronicity. Indicators of antisocial behavior were obtained from self-, peer-, and adult counselor-reports. In a series of ANCOVAs, child maltreatment and its parameters demonstrated strong main effects on early antisocial behavior as assessed by all forms of report. Genetic effects operated primarily in the context of gene-environment interactions, moderating the impact of child maltreatment on outcomes. Across the three genes, among nonmaltreated children no differences in antisocial behavior were found based on genetic variation. In contrast, among maltreated children specific polymorphisms of TPH1, 5-HTTLPR, and MAOA were each related to heightened self-report of antisocial behavior; the interaction of 5-HTTLPR and developmental timing of maltreatment also indicated more severe antisocial outcomes for children with early onset and recurrent maltreatment based on genotype. TPH1 and 5-HTTLPR interacted with maltreatment subtype to predict peer-report of antisocial behavior; genetic variation contributed to larger differences in antisocial behavior among abused children. TPH1 and 5-HTTLPR polymorphisms also moderated the effects of maltreatment subtype on adult report of antisocial behavior; again genetic effects were strongest for children who were abused. Additionally, TPH1 moderated the effect of developmental timing of maltreatment and chronicity on adult report of antisocial behavior. The findings elucidate how genetic variation contributes to identifying which maltreated children are most vulnerable to antisocial development.
Antisocial behavior exerts deleterious biological, psychological, and economic costs on individuals, relationships, the broader community, and society across the life course (Dishion & Patterson, 2006; Frick & Viding, 2009; Loeber & Farrington, 2001; Richters & Cicchetti, 1993). Consistent with the dynamic systems concepts of equifinality and multifinality (Cicchetti & Rogosch, 1996), multiple developmental pathways, as well as varied outcomes, are possible for maltreated children. Without adequate familial supports, the probabilistic course of development for maltreated children is characterized by an increased risk for unsuccessful resolution of many stage-salient developmental issues (Cicchetti & Lynch, 1995). Failure at any stage-salient task increases the risk for compromised resolution of subsequent developmental challenges. Thus, maltreated children are at high risk for developing a profile of relatively enduring vulnerability factors, thereby increasing the likelihood that they will develop future maladaptation and psychopathology (Cicchetti & Lynch, 1993; Cicchetti & Toth, 2005; Trickett & McBride-Change, 1995).
Maltreated children experience maladaptive parenting, often characterized by serious distortions and disruptions in the parent-child relationship. Empathic difficulties and problems with nurturing and protecting their offspring are some of the aspects of dysfunctional parenting provided by maltreating caregivers (Azar, 2002; Rogosch, Cicchetti, Shields, & Toth, 1995). Maltreated children manifest deficits in emotion recognition and regulation, develop insecure disorganized attachments, exhibit self-system difficulties, typically do not have effective peer relations, and have problems successfully adapting to school (Carlson, Cicchetti, Barnett, & Braunwald, 1989; Cicchetti & Toth, 1995; Eckenrode, Laird, & Doris, 1993; Pollak, Cicchetti, Hornung, & Reed, 2000; Shields & Cicchetti, 1997; Shonk & Cicchetti, 2001). Further, maltreated children evince deficits in social information processing (Dodge, Pettit, & Bates, 1997; Teisl & Cicchetti, 2008), engage in bullying behavior toward their peers, and are often the victims of bullying (Banny, Cicchetti, Rogosch, Oshri, & Crick, in press; Shields & Cicchetti, 2001).
Maltreated children have been shown to be at risk for developing antisocial behavior (Cicchetti & Rogosch, 2001; Jaffee, Caspi, Moffitt, & Taylor, 2004; Lansford, Dodge, Pettit, Bates, Crozier, & Kaplow, 2002; Manly, Kim, Rogosch, & Cicchetti, 2001; Widom, 1989). This result has motivated researchers to generate hypotheses about the mechanisms whereby antisocial behavior develops in maltreating family environments (Jaffee et al., 2004). The extant literature on the sequelae of child maltreatment, briefly reviewed above, clearly suggests that environmental factors may be mediating processes in the relation between maltreatment and antisocial behavior.
In a landmark investigation, Caspi and colleagues (2002) found that the monoamine oxidase A-uVNTR (MAOA) polymorphism moderated the impact of child maltreatment on the development of antisocial behavior in male participants (N=539) in the Dunedin Multidisciplinary Health and Development Study. Caspi et al. (2002) discovered that the adverse effects of child maltreatment on four indices of violent behavior (i.e., conduct disorder diagnosis; percentage of males convicted for violent crimes; mean scores on a disposition to violence scale; and mean scores on an antisocial personality disorder symptom scale) were significantly lower among males with high MAOA activity than among those with low MAOA activity. The findings of Caspi et al. (2002) suggest that the probability that child maltreatment will eventuate in antisocial behavior in males is increased among children whose MAOA activity is not sufficient to counteract maltreatment-induced changes in norepinephrine, serotonin, and dopamine neurotransmitter systems.
The compelling nature of these results helped to usher in a renascence of research interest on gene-environment interaction (GxE) and antisocial behavior. In this study, we investigate gene-environment interaction and early antisocial behavior in a large sample of maltreated and nonmaltreated children. Maltreatment is a strong environmental pathogen (Karg, Burmeister, Shedden, & Sen, 2011; Moffitt, Caspi, & Rutter, 2005) that also is a clearly operationalized stressor that has been shown to exert negative impacts upon brain structure and function (Cicchetti, 2002; DeBellis, 2001, 2005; Hart & Rubia, 2012; McCrory & Viding, 2010). Three candidates genes that have been demonstrated to be associated with aggression, violence, and other antisocial behaviors were chosen for inclusion in this study: tryptophan hydroxylase 1 (TPH1); the serotonin transporter (5-HTT); and monoamine oxidase A (MAOA). Each of these genes is involved in the regulation of serotonin. We next selectively review research that examines associations of these genes with antisocial behaviors.
Tryptophan Hydroxylase 1 (TPH1)
TPH1 is involved in the synthesis of the neurotransmitter serotonin. The TPH1 gene encodes the rate limiting biosynthetic enzyme in the serotonin pathway, and regulates serotonin levels (Mann, 1999; Rujescu, Giegling, Bondy, Gietl, Zill, & Moller, 2002). Thus, variations in the TPH1 gene may contribute to the predisposition to low serotonergic transmission. Central nervous system (CNS) serotonergic activity correlates in an inverse fashion with aggressive behavior in humans (Coccaro, Kavoussi, Cooper, & Hauger, 1997; Kruesi, Rapoport, Hamburger, Hibbs, Potter et al., 1990). Specifically, low serotonin activity is associated with high levels of aggression and high serotonin activity is associated with low levels of aggression.
Manuck, Flory, Ferrell, Dent, Mann, and Muldoon (1999) conducted a study with a heterogeneous community sample of men and women and discovered that individual differences in aggressive disposition were associated with a polymorphism (A218C) located in intron 7 of the TPH gene. Specifically, individuals who possessed any TPH1 U allele were found to score significantly higher on measures of aggression. Moreover, they had a tendency to experience unprovoked anger and were significantly more likely to report expressing their anger outwardly than individuals who were homozygous for the L allele of TPH1 (Manuck et al., 1999).
To the best of our knowledge, GxE findings currently are not available in the literature for TPH1 and childhood adversity. Genetic variation in TPH1 has been associated with individual differences in levels of aggression (Manuck et al., 1999; Rujescu, Bondy, Gietl, Zill, & Moller, 2002). Moreover, TPH1 is associated with the functioning of the serotonin system. Accordingly, we think that genotypic variation in TPH1 is potentially important to investigate in the context of early abuse and neglect. Thus, we decided to include TPH1 as one of the candidate genes in this study, the first investigation of the relations between child maltreatment and TPH1 with antisocial behavior in childhood.
Serotonin Transporter
The serotonin transporter (5-HTTLPR) gene is one of the major genes involved with serotonergic transmission. 5-HTTLPR is of particular interest to the study of antisocial behavior because it transcribes proteins which regulate the availability of serotonin in the brain. CNS serotonergic function exerts impacts on a broad array of biological and behavioral functions (Williams, Marchuk, Gadde, Barefoot, Grichnik et al., 2003). When these functions become dysregulated, they affect the developmental course of an equally wide range of mental and physical disorders (Williams et al., 2003). For example, 5-HTTLPR has been shown to be involved in brain development and in individual differences in mood and emotion regulation (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010). Low serotonergic function also is associated with impulsive and aggressive behavior. The diversity in behavioral outcomes associated with the serotonin transporter linked polymorphic region (5-HTTLPR) suggests the likely plausibility of its genetic influences operating in concert with environmental pathogens to affect psychopathological development.
Several studies investigating the interaction between 5-HTTLPR and adverse childhood experiences on the development of antisocial behavior have been conducted recently. Using data from Wave 1 of the National Longitudinal Study of Adolescent Health, Li and Lee (2010) investigated the association of 5-HTTLPR and child maltreatment on antisocial behavior. At wave 1, participants were approximately 15 ½ years of age. DNA was obtained from 2,488 individuals and both boys and girls participated. Antisocial behavior in boys was defined by membership in one of three latent classes: Exclusive Covert, Mixed Covert and Overt, and No Problems. In contrast, two latent classes defined antisocial behavior in girls: Exclusive Covert and No Problems. Li and Lee (2010) found that maltreatment was significantly related to the Exclusive Covert antisocial behavior class membership for girls, but not for boys. Moreover, within the group of girls, the influence of maltreatment and Exclusive Covert antisocial behavior group membership was moderated by 5-HTTLPR genotype. Specifically, whereas no significant GxE interaction was obtained for boys, maltreated girls who were homozygous for the SS gene were twelve times more likely to have membership in the Exclusive Covert than in the No Problems group. Furthermore, among nonmaltreated girls no genotype effects were observed.
Douglas and colleagues (2011) used the triallelic polymorphism of 5-HTTLPR and examined genetic and environmental risks for developing Antisocial Personality Disorder (ASPD) in a heterogeneous sample of substance-dependent individuals (N=1381; mean age = approximately 39 years). Nearly fifteen percent of the sample (14.7%, N=203) were diagnosed with ASPD, exceeding the base rate of this disorder for the general population. Information on adverse childhood events (e.g., child maltreatment) was gleaned from the semi-structured psychiatric disorder interview utilized in the study (i.e., Semi-Structured Assessment for Drug Dependence and Alcoholism; Pierucci-Lagha, Gelernter, Chan, Arias, Cubells et al., 2007). Among European-Americans, there were no GxE interactions obtained between 5-HTTLPR and adverse childhood events on ASPD for either males or females. Among African American men, each additional adverse childhood event significantly increased the odds of ASPD regardless of 5-HTTLPR genotype. In contrast, for African American women, there was evidence of moderation by 5-HTTLPR genotype and adverse childhood events on ASPD outcome. Specifically, African American women who possessed the homozygous SS genotype of 5-HTTLPR and who had experienced adverse childhood events evidenced a higher percentage of ASPD. However, this result is in need of replication due to the small sample size of African American women with the SS genotype of 5-HTTLPR (N=7).
Monoamine Oxidase-A (MAOA)
MAOA is a mitochondrial enzyme that is expressed predominantly in catecholaminergic neurons (Youdim, Edmondson, & Tipton, 2006). It is responsible for the degradation of a variety of biogenic amines, including the neurotransmitters dopamine, norepinephrine, and serotonin (Youdim, Finberg, & Tipton, 1988). There exists a well-characterized upstream variable number tandem repeat (uVNTR) polymorphism in the promoter region of the MAOA gene that is known to affect gene expression. The number of tandem repeats of this polymorphism (i.e., 2, 3, 5 vs. 3.5 or 4) determines the efficiency with which MAOA is transcribed and ultimately produced within individuals (Caspi et al., 2002).
Following the publication of the seminal paper by Caspi and colleagues (2002), research to replicate and to extend these findings has burgeoned. Kim-Cohen et al. (2006) conducted a meta-analysis that demonstrated that, across studies, the association between child maltreatment and mental health problems was significantly stronger in males who had the low activity MAOA genotype. Moreover, Kim-Cohen et al. (2006) also reported on the results of a new investigation of a sample of 975 7-year-old boys. These investigators replicated and extended the Caspi et al. (2002) study through the finding that the MAOA polymorphism moderates the effect of the exposure to physical abuse on the development of psychopathology as assessed close in time to the experience of maltreatment. Despite the fact that all of the studies conducted on MAOA, childhood maltreatment, and mental health do not replicate the original Caspi et al. (2002) results, aggregation of extant studies into a meta-analysis provided strong evidence suggesting that genetic variation in MAOA influences vulnerability to adverse childhood experiences. Additionally, findings obtained from the study of 7-year-old physically abused children suggest that this biological process may be initiated during early development (Kim-Cohen et al., 2006).
Since the publication of the Kim-Cohen et al. (2006) meta-analysis, a number of investigations of the interaction of genetic variation in MAOA and childhood maltreatment on the development of adversity have been conducted. Once again, not all published papers replicate the Caspi et al. (2002) results; however, the majority have produced findings similar to those obtained in the original study. A number of investigations have confirmed the interaction between child maltreatment and MAOA genotype on aggressive/antisocial behavior. This finding has been demonstrated in studies of maltreated children (e.g., Weder et al., 2009), adolescents (e.g., Aslund, Nordquist, Comasco, Lepert, Oreland, & Nilsson, 2011), and adults (e.g., Beach, Brody, Gunter, Packer, Wernett, & Philibert, 2010; Ducci et al., 2008; Fergusson, Boden, Horwood, Miller, & Kennedy, 2011; Widom & Brzustowicz, 2006).
The Current Study
The potential for genetic variation to contribute to multifinality in outcomes and to antisocial behavior in particular requires further investigation. In this study, we take a developmental lens, honing in on early indicators of antisocial behavior in order to discover which maltreated children are most vulnerable to antisocial behavior. Similar to Caspi et al.’s (2002) original study, we utilized multiple measures and indicators of antisocial behavior, as well as multiple informants. In addition to child self-report of antisocial behavior, we also thought it was important to assess what others’ experiences were in relation to these children. To this end, we included the assessments of adult raters who had observed the children for 7 hours per day over the course of a week in a day camp context. Furthermore, peer ratings of the children were obtained from youngsters who had been in their peer group for a week’s duration. These multiple assessments enabled us to assess a variety of antisocial behaviors from the perspective of each child’s peers, including physical victimization, bullying, and relational victimization.
In contrast to the existing literature, our focus on maltreated children, rather than on adolescents or adults, addresses a significant gap in the literature. Additionally, having a large group of maltreated children whose experiences were coded prospectively allowed us to examine GxE effects in more depth because of adequate group sizes of maltreated children with different genotypes and variation in maltreatment experiences.
This multi-genic GxE investigation of early antisocial indicators in maltreated and nonmaltreated children was guided by the following hypotheses and research questions:
Maltreated children will evince higher levels of antisocial symptoms nonmaltreated children, irrespective of informant – self, peer, or adult, each of whom provides different perspectives on early antisocial behavior.
More extreme maltreatment will be related to increased levels of antisocial behavior.
We do not expect to obtain evidence for a gene-environment correlation (rGE). Thus, we predict that maltreatment per se will not elicit antisocial behavior.
We expect that polymorphisms of TPH1, 5-HTTLPR, and MAOA (u-VNTR) will moderate the effects of maltreatment experiences on antisocial behavior.
Method
Participants
This investigation recruited age 10- to 12-year-old children (N = 627; M age = 11.27, SD = .97) for participation in a summer camp research program designed for school-aged low-income children. The sample included both maltreated children (n = 348) and nonmaltreated children (n = 279). Among the participants, 315 were girls and 312 were boys. The sample was racially and ethically diverse. The Add Health system for coding race and ethnicity was used (http://www.cpc.unc.edu/projects/addhealth/data/code/race) (DeYoung, Cicchetti, Rogosch, Gray, Eastman, & Grigorenko, 2011); 67.1% was African American, 10.7 % was white, 18.2% was Hispanic, and 4.0% was other racial/ethnic groups.
Recruitment Procedures
Parents of all maltreated and nonmaltreated children provided informed consent for their child’s participation, as well as consent for examination of any Department of Human Services (DHS) records pertaining to the family. Children in the maltreated group had been identified by the county DHS as having experienced child abuse and/or neglect, and the sample was representative of the children in families receiving services from the DHS. A recruitment liaison from DHS contacted eligible maltreating families, explained the study, and if parents were interested, then their names were released to the project team for recruitment. Families were free to choose whether or not to participate. Comprehensive searches of DHS records were completed, and maltreatment information was coded utilizing operational criteria from maltreatment nosology specified in the Maltreatment Classification System (MCS: Barnett, Manly, & Cicchetti, 1993), as discussed below.
Consistent with national demographic characteristics of maltreating families (National Incidence Study – NIS-4; Sedlak et al., 2010), the maltreated children were predominantly from low socioeconomic status families. Consequently, demographically comparable nonmaltreated children were recruited from families receiving Temporary Assistance for Needy Families (TANF). A DHS recruitment liaison contacted eligible nonmaltreating families, described the project, and if interested, parents signed a release for their names to be given to the project for recruitment. DHS record searches were completed for these families to verify the absence of any record of child maltreatment. Trained research assistants also interviewed mothers of children recruited for the nonmaltreatment group to confirm a lack of DHS involvement and prior maltreatment experiences utilizing the Maternal Maltreatment Classification Interview (Cicchetti, Toth, & Manly, 2003). Subsequently, record searches were conducted in the year following camp attendance to verify that all available information had been accessed. Only children from families without any history of documented abuse or neglect were retained in the nonmaltreatment group. In addition, families who had received preventive services through DHS due to concerns over risk for maltreatment were excluded from the sample to reduce the potential for unidentified maltreatment existing within this group.
The demographic characteristics of the maltreated and nonmaltreated groups of children were comparable. (See Table 1). The two groups did not differ on child age or gender distribution. In terms of race/ethnicity, a difference was observed, χ2(3, N = 626) = 8.10, p = .04. Contrasts indicated that the proportion of Hispanic children was higher in the nonmaltreated group than the maltreated group, χ2(1, N = 626) = 5.62, p = .02; however, the proportions of African-American and Caucasian children did not differ significantly. No differences in maternal marital status were observed between groups. In the sample, over 90% of the families had a history of receiving public assistance. Nevertheless, 93.0% of the nonmaltreated group and 99.0% of the maltreated group had received assistance and this difference was χ2(1, N = 523) = 12.95, p = .001, although not substantively meaningful, given the high overall rate in the sample.
Table 1.
Demographic characteristics
| Nonmalreated M (SD) or % |
Maltreated M (SD) or % |
P-Value t or χ2 |
|
|---|---|---|---|
| Age | 11.23 (.99) | 11.29 (.96) | .92 |
| Gender (% male) | 48.7% | 50.6% | .67 |
| Race/Ethnicity | .04 | ||
| African-American | 66.3 | 67.8 | |
| Caucasian | 8.2 | 12.6 | |
| Hispanic | 22.2 | 14.9 | |
| Other | 3.2 | 4.6 | |
| Maternal Marital Status | .97 | ||
| Single | 38.3 | 38.2 | |
| Married, Living together | 32.6 | 31.7 | |
| No longer married | 29.1 | 30.0 | |
| Family History of Public assistance | 93.0 | 99.0 | .001 |
Maltreatment Classification
The MCS is a reliable and valid method for classifying maltreatment (Bolger, Patterson, & Kupersmidt, 1998; English, Upadhyaya, Litrownik, Marshall, Runyan et al., 2005; Manly, 2005) that utilizes DHS records detailing investigations and findings involving maltreatment in identified families over time. Rather than relying on official designations and case dispositions, the MCS codes all available information from DHS records, making independent determinations of maltreatment experiences. Based on operational criteria, the MCS designates all of the subtypes of maltreatment children have experienced (i.e., neglect, emotional maltreatment, physical abuse, sexual abuse). Coding of the DHS records was conducted by trained research assistants, doctoral students, and clinical psychologists. Coders were required to meet acceptable reliability with criterion standards before coding actual records for the study. Coders demonstrated acceptable reliability with the criterion (weighted κ’s with the criterion ranging from .86 to .98. Reliabilities (κ’s) for the presence vs. absence of maltreatment subtypes ranged from .90 to 1.00.
In terms of the subtypes of maltreatment, neglect involves failure to provide for the child’s basic physical needs for adequate food, clothing, shelter, and medical treatment. In addition to inadequate attention to physical needs, forms of this subtype include lack of supervision, moral-legal neglect, and education neglect. Emotional maltreatment involves extreme thwarting of children’s basic emotional needs for psychological safety and security, acceptance and self-esteem, and age-appropriate autonomy. Examples of emotional maltreatment of increasing severity include belittling and ridiculing the child, extreme negativity and hostility, exposure to severe marital violence, abandoning the child, and suicidal or homicidal threats. Physical abuse involves the non-accidental infliction of physical injury on the child (e.g., bruises, welts, burns, choking, broken bones). Injuries range from minor and temporary to permanently disfiguring. Finally, sexual abuse involves attempted or actual sexual contact between the child and caregiver for purposes of the caregiver’s sexual satisfaction or financial benefit. Events range from exposure to pornography or adult sexual activity, to sexual touching and fondling, to forced intercourse with the child.
Children in the maltreatment group all had documented histories of abuse and/or neglect occurring in their families according to DHS records. However, DHS record information was not complete enough to code maltreatment subtype information for 23 (6.6%) of the maltreated children. Among the remaining maltreated children, 84.3% had experienced neglect, 55.7% had experienced emotional maltreatment, 30.5% had experienced physical abuse, and 9.2% had experienced sexual abuse. As is typical in maltreated populations (Bolger et al., 1998; Manly et al., 1994; 2001), the majority of children had experienced multiple subtypes of maltreatment. Specifically, 59.3% of the maltreated children had experienced two or more maltreatment subtypes. Among maltreated children, we derived two indices to characterize maltreatment subtype experiences. Given the overlap among subtypes and the relatively lower rates of physical and sexual abuse as compared to neglect and emotional maltreatment, we identified children who had experience neglect and/or emotional maltreatment (PNEM; 64.9%) without physical or sexual abuse versus children who had experience physical and/or sexual abuse (PASA; 35.1%). The PASA group also may have experienced neglect or emotional maltreatment.
The MCS also determines when in the course of development maltreatment events occurred, providing indices of developmental timing. Events were coded as occurring during five developmental periods, including infancy (0-12 months), toddlerhood (13-36 months), preschool (36 to 60 months), early school age (age 5 to 7), and later school age (age 8 to 12). The timing information allows for the determination of whether maltreatment occurred within each of the developmental periods. The number of developmental periods in which maltreatment occurred was used as an index of chronicity. Additionally, onset of maltreatment was identified as the earliest developmental period in which maltreatment occurred, whereas recency was based on the last period in which maltreatment occurred. We used the onset and recency variables to identify onset/recency groups. Children with onset prior to early school age were designated as early onset, whereas onset in early or later school age was categorized as later onset. Similarly, recency that was prior to early school age was designated as not recent, whereas recency in the early or late school age periods was categorized as recent. Combining these variables resulted in three onset/recency groups among the maltreated children: early onset, not recent; early onset, recent; and later onset, recent.
Procedure
Children attended a week-long day camp program and participated in research assessments. At the camp, children were assigned to groups of eight same-age and same-sex peers; half of the children assigned to each group were maltreated. Each group was conducted by three trained camp counselors, who were unaware of the maltreatment status of children and the hypotheses of the study. Camp lasted 7 hrs/day for five days, providing 35 hours of interaction between children and counselors. In addition to the recreational activities, after providing assent, children participated in various research assessments (see Cicchetti & Manly, 1990, for detailed descriptions of camp procedures) and provided DNA samples. Trained research assistants, who also were unaware of research hypotheses and maltreatment status, conducted individual research sessions with children, in which questionnaires and other research measures were administered. Clinical consultation and intervention occurred if any concerns over danger to self or others emerged during research sessions. At the end of the week, children in each group completed sociometric ratings of their peers. The counselors, who had been trained extensively for two weeks prior to the camp, also completed assessment measures on individual children, based on their observations and interactions with children in their respective groups.
Measures
The measures described below constitute a subset of assessments conducted during the research camp. The camp context and associated measurement battery provide a multi-informant, multi-perspective view of child functioning, including indicators of antisocial behavior. Measures include child self-report, peer evaluations, and counselor-report assessments of individual children.
Assessment of Antisocial Behaviors
Pittsburgh Youth Survey (PYS: Loeber et al., 1998) is a child self-report measure of delinquent behavior and substance use. The format is appropriate for use with school-aged. The interview protocol is constructed in a developmentally sensitive manner, and questions are carefully framed to insure the child’s understanding of content. Children’s involvement in range of antisocial behavior is queried, including aggressive behavior, cheating, stealing, running away, skipping school, damaging property, setting fires, as well as use of tobacco, alcohol, marijuana, and glue sniffing. Children report if they have every engage in the behaviors and if the behaviors occurred within the past six month. Total scores for lifetime behaviors and those in the past six months were determined.
Peer Ratings
After children had interacted with each other during the week of summer camp, children evaluated the characteristics of their peers in their respective camp groups using a sociometric peer ratings method on the last day of camp (cf., Coie & Dodge, 1983; Bukowski, Sippola, Hoza, & Newcomb, 2000). Counselors conducted the sociometric assessment with individual children. For each peer in the camp group, children were given six behavioral descriptors characterizing different types of social behavior. Children then rated each peer on how characteristic the behavioral descriptor was for that peer on a three-point scale. Of interest in the current study were ratings from peers for physically aggressive behavior, disruptiveness, and relational aggression. All ratings from peers on each child for each of the social behavioral descriptors were averaged. The correlations among the three antisocial behavior categories were highly intercorrelated, ranging from .76 to .84. The average scores of the peer ratings were standardized, and the average of these standardized scores was used a peer composite rating of child antisocial behavior. Cronbach’s alpha for the scale was .92.
Teacher Report Form (TRF; Achenbach, 1991)
Behavioral symptomatology was evaluated at the end of each week by counselors’ completion of the TRF. The TRF is a widely used and validated instrument to assess behavioral disturbance from the perspective of teachers, and the measure was used in the present study, because camp counselors are able to observe similar behaviors to that of teachers. The TRF, containing 118 items rated for frequency, assesses two broadband dimensions of child symptomatology, externalizing and internalizing, as well as total behavior problems. In the present study, interrater reliability for the externalizing and internalizing dimensions based on average intraclass correlations among pairs of raters ranged from .78 to .88 (M = .83) for externalizing and from .56 to .84 (M = .68) for internalizing. In the current study focusing on antisocial behaviors, in addition to the externalizing broadband dimension we also examined the Rule Breaking or Delinquent Behavior Problems subscale and the aggressive behavior problem subscale. The counselors’ scores for each child were averaged to obtain individual child scores for the externalizing dimension and the two subscales.
DNA collection, extraction, and genotyping
Using an established protocol, trained research assistants obtained DNA samples from participants by collecting buccal cells with the Epicentre Catch-All Collection Swabs. Subsequently, using the conventional method, DNA was extracted with the Epicentre BuccalAmp DNA Extraction Kit, in order to prepare DNA for PCR amplification. Genotyping was conducted following previously published protocols.
DNA was whole-genome amplified using the Repli-g kit (Qiagen, Catalogue No. 150043) per the kit instructions to ensure the availability of data over the long term for this valuable sample. Amplified samples were then diluted to a working concentration.
For the TPH1, two single nucleotide polymorphisms (SNPs), rs18000532 and rs1799913, were genotyped, resulting in two alleles, G and T. Genotype distributions for the two SNPs were identical, with 100% agreement for the GG, GT, and TT genotypes. Thus, we used the common distribution exhibited by these two SNPs for classifying the TPH1 genotypes in our analysis. Individual allele determinations for TPH1 were made using TaqMan Genotyping Master Mix (Applied Biosystems, Catalog 4371357) with amplification on an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200 using JMP 8.0 (SAS, Inc.). The call rate for TPH1 was 99.8%.
The 5-HTT gene has a polymorphism in the linked polymorphic region (5-HTTLPR) in the 5’ regulatory region due to a 44-base pair deletion that eventuates in either the short (s) or long (l) allele (Lesch et al., 1996). 5-HTTLPR samples were genotyped for fragment length polymorphisms of 5-HTTLPR with Hot Star Taq PCR Mix (Qiagen, Catalog No. 203205) and previously described primers (Gelernter, Kranzler, & Cubells, 1997), followed by fragment analysis using a CEQ 8000 (Beckman-Coulter, Inc.). The call rate for 5-HTTLPR was 99.8%.
For MAOA, genotyping was conducted following previously published protocols. The procedures of Sabol, Hu, and Hamer (1998) for genotyping and the classification of alleles into high and low activity were utilized. Representative genotypes were identified and sequenced with a Beckman-Coulter CEQ8000 semiautomated fluorescent sequencing system, utilizing the Fragment Analysis Application and associated software. The call rate for MAOA was 100%.
If a genotype for any gene or SNP could not be determined after the first run, then it was repeated up to four times. If the null result persisted, then a genotype was not assigned to that individual.
All DNA samples were genotyped in duplicate for quality control. Additionally, human DNA from cell lines was purchased from Coriell Cell Repositories for all representative genotypes in duplicate and genotypes confirmed by sequencing using DTC& chemistry on an ABI 3130x1. These and a no template control were run alongside study samples representing 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same conditions.
Results
Genotype distributions for the TPH1 and 5-HTTLPR genes did not differ significantly from Hardy-Weinberg equilibrium, χ2 (1, N = 626) = 2.53, p = .11 and χ2 (1, N = 626) = .62, p = .43, respectively. The genotype frequencies for TPH1 were GG: 398, GT: 194, and TT: 34. Because of the low frequency of the TT genotype, children with a T allele, either the GT or TT genotypes, were combined into a single group in statistical analyses and compared with children having the GG genotype. For 5-HTTLPR, the genotype distribution was as follows: LL: n = 312, SL: n = 254; and SS: n = 60.
Because the MAOA gene is sex-linked and located on the X chromosome, males have one allele on the X chromosome. In contrast, females have an MAOA allele on each X chromosome; however, only one of the alleles is active. Consequently, ambiguity results in determining which allele is active among females who are heterozygous for MAOA activity level alleles. Consequently, analyses involving MAOA were conducted only among boys. Because males have only one MAOA allele, Hardy-Weinberg equilibrium cannot be determined. Male participants with 3.5 or 4 repeats were classified into a high activity MAOA group (n = 154), and boys with the remaining repeat lengths were combined into a low activity MAOA group (n = 158).
Initially, the maltreated and nonmaltreated groups of children were compared on their distributions of genotypes for each of the genes. No significant maltreatment group differences were obtained for TPH1, χ2 (2, N = 626) = .21, p = .90, 5-HTTLPR, χ2 (2, N = 626) = .1.70, p = .43, or MAOA activity level among boys, χ2 (1, N= 312) = 2.65, p = .11. Thus, no support for gene-environment correlations involving child maltreatment and the respective genes was found.
Next, we conducted a series of ANCOVAs to examine the influence of child maltreatment and genotypic variation in predicting childhood indicators of early antisocial behavior. In these ANCOVAs, gender (for TPH1 and 5-HTTLPR) and age were included as covariates, and race/ethnicity group was included as a main effect to control for potential population stratification. The main effects of child maltreatment and genotype and their interaction for respective genes were then considered. We also evaluated whether different maltreatment parameters, including subtype group, number of developmental periods of maltreatment, and onset/recency groups, provided a more detailed depiction of genetic and environmental effects. For each gene, we present findings related to self-, peer, and adult-report outcomes, in that order. The dependent variables in these analyses included child self-report of antisocial problems on the PYS, both in the last six months and ever having engaged in the behaviors, the composite peer ratings variable including physical aggression, disruptiveness, and relational aggression, and adult reports from the camp counselors on the TRF scales of delinquent behavior problems, aggressive behavior problems, and externalizing behavior symptoms.
Overall, the findings demonstrate that child maltreatment and its various parameters always have a main effect in predicting outcomes, and this main effect is retained in the context of GxE interactions. In none of the analyses involving TPH1, 5-HTTLPR, and MAOA did child maltreatment and genotype both contribute independent main effects on outcomes in the absence of GxE interaction. Rather, the influence of genotype typically occurred only in interaction with maltreatment parameter variables.
Tryptophan Hydroxylase
In the first MANCOVA to evaluate the child self-report antisocial behavior, age, gender, and race/ethnicity all had significant effects on the outcome. More importantly, maltreatment demonstrated a significant main effect, F(1, 584) = 14.262, p < .001, partial ή2 = .024, with maltreated children reporting higher antisocial behaviors than nonmaltreated children, whereas the TPH1 genotype did not have a significant effect, F(1, 584) = .532, p = .47, partial ή2 = .001. These results are clarified by a significant interaction effect, F(1, 584) = 4.14, p = .04, partial ή2 = .007, as shown in Figure 1. Post hoc probing of the interaction with Bonferroni corrected comparisons indicated that among children with the GG genotype, maltreated and nonmaltreated children were not significantly different in symptom level, p = .14, whereas among children with the GT/TT genotype maltreated children had significantly higher antisocial scores than nonmaltreated children, p < .001. Among the nonmaltreated children no difference in antisocial behavior was observed between the two genotype groups, p = .54; however, in maltreated children the GT/TT group had marginally significantly higher scores, p = .057, than maltreated children in the GG group. Thus, maltreated children with the GG genotype were at lower risk for exhibiting antisocial behavior.
Figure 1.

TPH1 and Maltreatment Status to Predict Self Report of Antisocial Behavior in past six months.
When peer ratings of antisocial behavior were examined, a similar pattern of relations was observed. In the ANCOVA model for maltreatment subtype groups, a main effect for maltreatment subtype was obtained, F(1, 591) = 11.39, p < .001, partial ή2 = .038, whereas the main effect of TPH1 genotype was not significant, F(1, 591) = .13, p =.72, partial ή2 = .000. A significant interaction also was found, F(2, 591) = 3.28, p = .038, partial ή2 = .011. This interaction effect is depicted in Figure 2. Bonferroni corrected contrasts indicated that in the GT/TT genotype group, children with physical and/or sexual abuse (PASA) had significantly higher peer-rated antisocial behavior than both the nonmaltreated group, p = .001, and the emotional maltreatment/neglect only group (EMPN), p = .005. In contrast, within the GG group, only the EMPN group had significantly higher peer antisocial behavior than the nonmaltreated group (p < .001), whereas the PASA group did not differ significantly from the other groups. Within each of the subtype groups, differences between genotypes were not significant. Thus, the children who experienced PASA were at lower risk for antisocial behavior with peers when they had the GG genotype.
Figure 2.
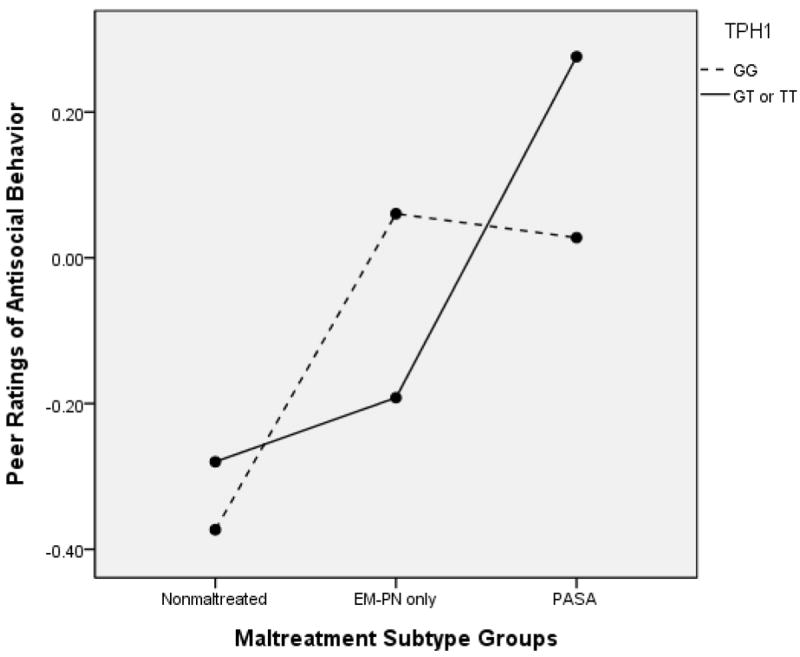
TPH1 and Maltreatment Subtype Group to Predict Peer Ratings of Antisocial Behavior
In terms of adult report of antisocial behavior, we obtained a number of similar GxE effects. First, we examined maltreatment subtype groups in relation to delinquent behavior problems and, comparable to the findings for peer ratings, observed a significant main effect for subtype group, F(1, 591) = 9.66, p < .001, partial ή2 = .032, no effect for TPH1 genotype, F(1, 591) = 1.21, p = .27, partial ή2 = .002, and a significant GxE interaction, F(2, 591) = 3.03, p < .0376, partial ή2 = .011. (See Figure 3). Follow-up probes of the interaction revealed the same pattern of effects as for peer ratings. Specifically, with the GT/TT group, the PASA group had significantly higher delinquent behavior scores than the nonmaltreated group, p = .001, and the EMPN group, p = .02, whereas in the GG genotype group only the EMPN group had higher scores than the nonmaltreated group, p < .001. Within subtype groups, differences between children with different genotypes were not significant. These results are consistent with the prior results in showing that the children in the PASA group with GG genotypes were at relatively lower risk for delinquent behavior problems than those in the GT/TT group.
Figure 3.

TPH1 and Maltreatment Subtype Group to Predict TRF Delinquent Behavior Problems
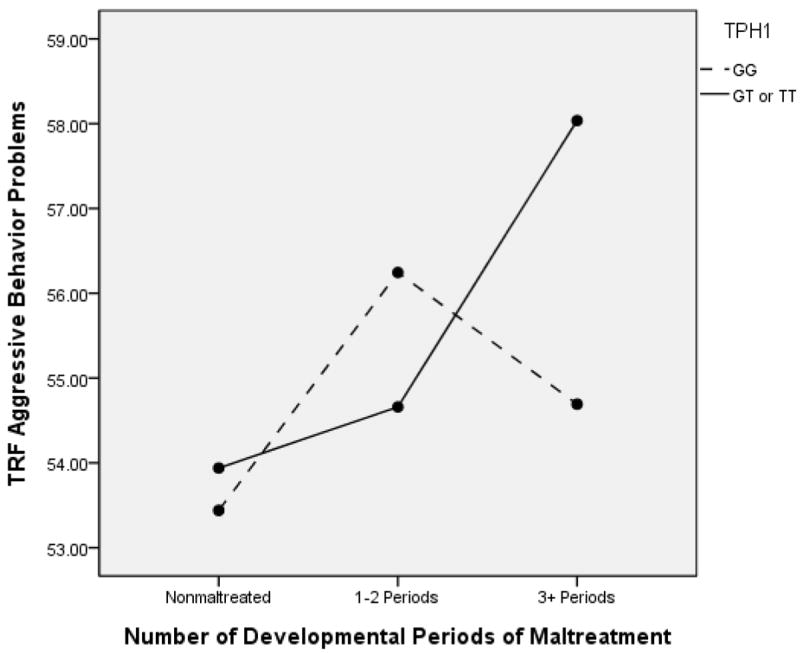
We also observed this pattern when the maltreatment parameter was chronicity, or number of developmental periods of maltreatment. In this analysis examining the TRF delinquent behavior subscale, significant main effects were observed for number of developmental periods, F(1, 592) = 10.66, p < .001, partial ή2 = .035, as well as TPH1 genotype, F(1, 592) = 4.16, p < .04, partial ή2 = .004. However, these main effects were clarified by a significant GxE interaction, F(1, 592) = 4.61, p = .01, partial ή2 = .016, as shown in Figure 4. Follow-up Bonferroni comparisons indicated that within the GT/TT group, children with three or more developmental periods of maltreatment had significantly higher delinquent behavior problems than nonmaltreated children, p < .001, and children with 1 or 2 developmental periods of maltreatment, p = .007. Within the GG group, children with 1-2 developmental periods of maltreatment were significantly higher on delinquent behavior problems than were nonmaltreated children, p < .001, whereas children with 3-4 periods did not differ. Within the chronicity groups, only among children with three or more developmental periods of maltreatment did the difference between genotype groups approach significance, p = .09, with children in the GT/TT group tending to show higher symptoms than children with the GG genotype. Thus, more chronically maltreated children were less susceptible to exhibiting delinquent behavior when they carried the GG genotype.
Figure 4.

TPH1 and Number of Developmental Periods of Maltreatment to Predict TRF Delinquent Behavior Problems
Figures 5 and 6 portray the same above analysis, with different outcome variables. The pattern of effects is very similar. For TRF aggressive behavior problems, the main effect for number of developmental periods was significant, F(1, 592) = 6.28, p = .002, partial ή2 = .021, whereas the TPH1 genotype effect was not, F(1, 592) = 1.03, p = .30 partial ή2 = .002. However, the GxE interaction was significant, F(1, 592) = 3.65, p = .027, partial ή2 = .012. Similarly, for the dependent variable of TRF externalizing behavior problems, a significant main effect was obtained for number of developmental periods, F(1, 592) = 6.62, p < .001, partial ή2 = .022 but not for genotype group, F(1, 592) = 1.93, p = .17 partial ή2 = .003. The interaction effect was significant, p = .02, partial ή2 = .013.
Figure 5.
TPH1 and Number of Developmental Periods of Maltreatment to Predict TRF Aggressive Behavior Problems
Figure 6.

TPH1 and Number of Developmental Periods of Maltreatment to Predict TRF Externalizing Behavior Problems
Finally, for TPH1, we examined developmental onset/recency groups to predict delinquent behavior problems. The ANCOVA revealed a significant main effect for onset/recency, F(1, 591) = 8.54, p < .001, partial ή2 = .047, but not for TPH1 genotype group, F(1, 591) = .27, p = .60, partial ή2 = .000. These results are clarified by a significant interaction effect, F(1, 591) = 4.13, p = .007, partial ή2 = .021. Figure 7 depicts a notable elevation in symptom level among children with the GT/TT genotype and early onset and recent maltreatment. Within the GT/TT genotype group, children with early onset and recent maltreatment had higher delinquent behavior problems than all other groups, including the nonmaltreated children, p < .001, children with early onset/not recent maltreatment, p = .001, and children with late onset/recent maltreatment, p = .02. For children with the GG genotype, children with early onset/not recent maltreatment had higher symptoms of delinquent behavior than nonmaltreated children, p = .002; no other Bonferroni contrasts were significant. Notably, all three of the onset/recency groups among maltreated children did not differ from each other. When we examined contrasts within the onset/recency groups, we found that among children with early onset and recent maltreatment that they had significantly higher delinquent behavior problems, p = .02, if they had the GT/TT genotype as compared to those with the GG genotype. In contrast, among children with early onset/not recent maltreatment, those with the GG genotype had higher delinquent behavior than those with the GT/TT genotype, p = .03. Other contrasts were not significant. Overall, the results indicate the higher risk of children with early onset and recent maltreatment, but demonstrate the reduction in that risk for children with the GG genotype.
Figure 7.

TPH1 and Onset/Recency Group to Predict TRF Delinquent Behavior Problems
Serotonin transporter
We first examined how maltreatment status and the genotypes of 5-HTTLPR contributed to children’s self-report of lifetime involvement in antisocial behavior. In the ANCOVA model, maltreatment status had a significant main effect, F(1, 586) = 33.01, p < .001, partial ή2 = .054, whereas 5-HTTLPR did not, F(2, 586) = .07, p = .93, partial ή2 = .000. Additionally, a significant interaction effect, F(2, 586) = 3.91, p = .02, partial ή2 = .013, also was obtained, as depicted in Figure 8. Bonferroni contrasts to probe the interaction indicated that maltreated children reported significantly higher levels of antisocial behavior in each of the genotype groups. However, it is important to note that the strength of these differences varied. Specifically, the partial ή2 effect size for the SS genotype was .18, contrasting with .06 for the SL and .025 for the LL group. Contrasts within the maltreated and nonmaltreated groups for the effects of genotype were not significant. Thus, differences between maltreated and nonmaltreated children on self-reported antisocial behavior were greatest among children with the SS genotype, with less differentiation occurring based on maltreatment for children with the LL genotype.
Figure 8.
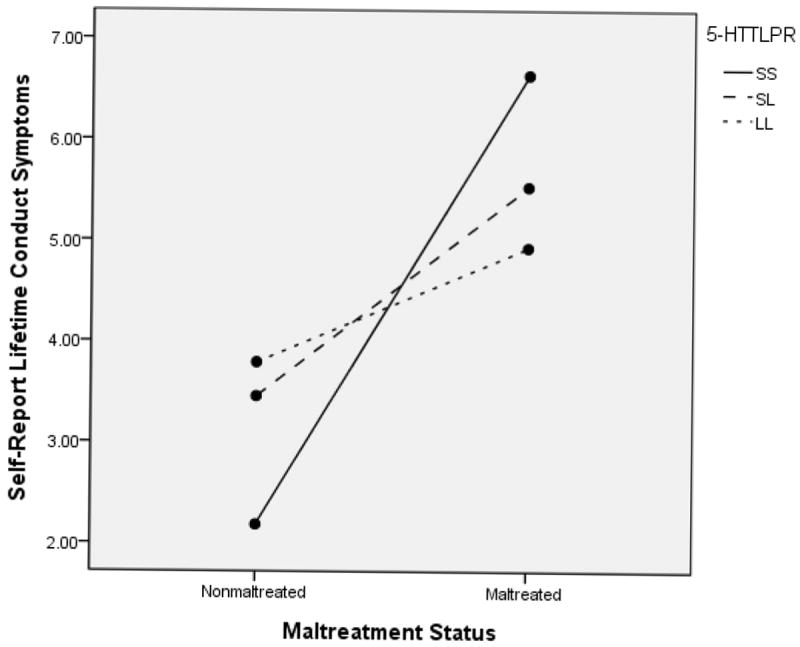
5-HTTLPR and Maltreatment Status to Predict Self Report of Lifetime Antisocial Behaviors
These results were further elaborated when onset/recency groups were considered as the maltreatment parameter. This ANCOVA model resulted in a significant main effect for onset/recency groups on antisocial behaviors, F(3, 563) = 15.06, p < .001, partial ή2 = .076, a nonsignificant 5-HTTLPR main effect, F(2, 563) = .88, p =.42, partial ή2 = .003, and a significant interaction effect, F(6, 563) = 2.08, p = .05, partial ή2 = .022. (See Figure 9). Follow-up Bonferroni contrasts within genotype groups indicated that among children with the LL genotype, no significant onset/recency group differences were present. In contrast, among children with the SS genotype, children with late onset and recent maltreatment had significantly higher antisocial behaviors than nonmaltreated children, p = .03, whereas the contrast between early onset and recent maltreatment and nonmaltreated children was marginally significant, p = .075. Group differences were also found among children with the SL genotype. Children with late onset and recent maltreatment had significantly higher self-report of antisocial behavior than the nonmaltreated children, p < .001, and the early onset/not recent children, p = .03. Early onset and recent maltreatment also was significantly higher than nonmaltreatment, p = .01. Important to note is that children with early onset and not recent maltreatment were not statistically different from nonmaltreated children in any of the genotype groups. When differences among genotype groups were examined within the onset/recency groups, significant genotype differences were not obtained. In general, the findings suggest that maltreated children with the LL genotype are at less risk for antisocial behavior across variation in onset and recency of maltreatment experiences in development.
Figure 9.

5-HTTLPR and Onset/Recency Groups to Predict Self Report of Lifetime Antisocial Behaviors
The moderating influence of 5-HTTLPR also was observed based for peer ratings of antisocial behavior. In an ANCOVA examining maltreatment subtype groups and 5-HTTLPR, significant main effects for subtype, F(2, 591) = 9.15, p < .001, partial ή2 = .031, 5-HTTLPR, F(2, 591) = 1.73, p = .18, partial ή2 = .006, and the interaction effect, F(4, 591) = 2.60, p =.035, partial ή2 = .018, were obtained. This GxE interaction effect is depicted in Figure 10. Notable in this figure is the high elevation in peer-rated antisocial behavior in the PASA group for children with the SS genotype. However, significant genotype group differences within the maltreatment subtype groups were not obtained. We also examined differences within each of the genotype groups. Among children with the SS genotype, the PASA group had significantly higher antisocial ratings than the EMPN group, p = .008. In the SL genotype group, children in the EMPN group had higher scores than nonmaltreated children, p = .001, and in the LL group, both children in the EMPN group, p = .02, and the PASA group, p = .007, received higher antisocial ratings from peers than nonmaltreated children. Overall, the results illustrated that the greatest risk for antisocial behavior as perceived by peers was for children who have been abused and have the SS genotype.
Figure 10.
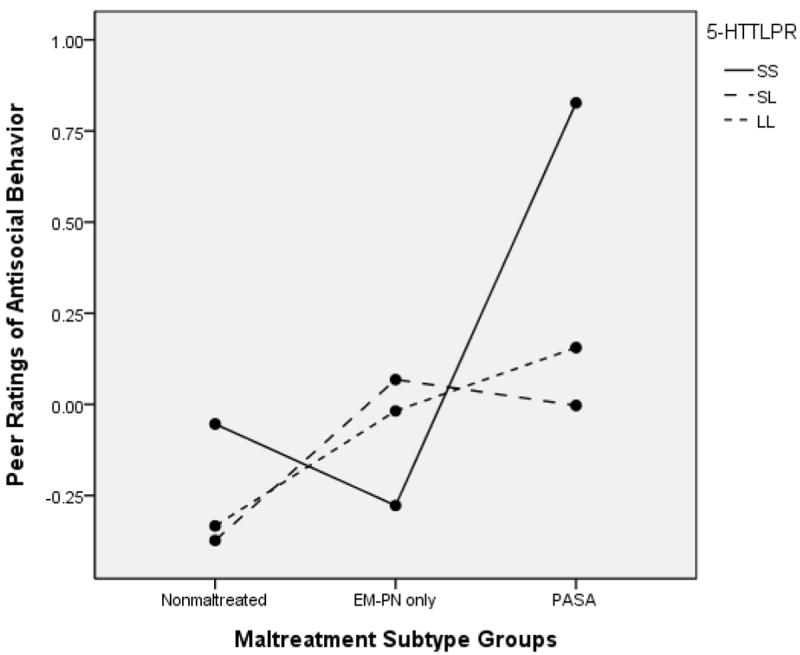
5-HTTLPR and Maltreatment Subtype Group to Predict Peer Ratings of Antisocial Behavior
The appraisals of children’s antisocial behavior by counselors also provided support for moderation by 5-HTTLPR of the effects of maltreatment experiences. In examining maltreatment status in the ANCOVA model to predict TRF delinquent behavior problems, we found a significant effect for maltreatment status, F(1, 614) = 21.29, p < .001, partial ή2 = .034, but no main effect for 5-HTTLPR, F(2, 614) = .57, p = .57, partial ή2 = .002. Again, the GxE interaction effect was significant, F(2, 614) = 2.93, p = .05, partial ή2 = .010. (See Figure 11). Follow-up Bonferroni contrasts to probe the interaction indicated that maltreated children evinced higher delinquent behavior problems than nonmaltreated children in both the SS group, p = .002, and the SL group, p = .001, but not the LL group, p = .11. Within the maltreated and nonmaltreated groups, significant differences among genotype groups were not obtained. Our findings suggest an attenuation in risk for delinquent behavior associated with maltreatment for children with the LL genotype.
Figure 11.

5-HTTLPR and Maltreatment Status to Predict TRF Delinquent Behavior Problems
These results were further clarified through consideration of variation due to the experience of maltreatment subtype on delinquent behavior as reported by the adult counselors. The ANCOVA model resulted in a significant effect for maltreatment subtype, F(2, 591) = 12.32, p < .001, partial ή2 = .041, 5-HTTLPR, F(2, 591) = 2.71, p = .11, partial ή2 = .008, and the GxE interaction, F(4, 591) = 3.19, p = .013, partial ή2 = .022. The interaction effect is shown in Figure 12. Within the PASA group, higher delinquent behavior was observed among children with the SS genotype as compared to the LL genotype, p = .01. Genotype differences were not found in the EMPN or nonmaltreated groups. Within genotype groups, for children with the SS variant, children in the PASA group had higher symptoms than those in the nonmaltreated group, p = .002. This was also the case in the SL genotype group, where children in the PASA group had higher delinquent behavior that those in the nonmaltreated group, p = .001. In contrast, for children with the LL genotype, no significant differences were found among the maltreatment subtype groups. These results further indicate the reduction in risk for abused children who have the LL genotype.
Figure 12.
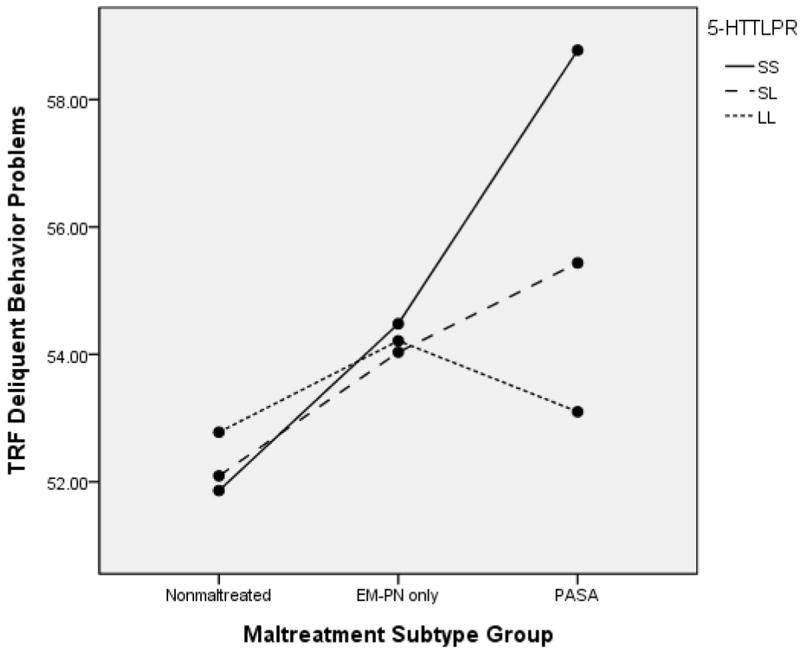
5-HTTLPR and Maltreatment Subtype Group to Predict TRF Delinquent Behavior Problems
Monoamine Oxidase-A
Our final set of analyses examined the potential genetic influence on antisocial behavior among boys and GxE interaction. We did not find MAOA to contribute to models of antisocial behavior as reported by peers or adults, beyond the influence of maltreatment effects. However, informative GxE effects were observed based on child self-report of antisocial behavior.
For lifetime antisocial behaviors, the ANCOVA model resulted in a significant effect for maltreatment status, F(1, 288) = 24.64, p < .001, partial ή2 = .081, but no main effect for MAOA, F(1, 288) = 1.55, p = .21, partial ή2 = .00. The GxE interaction effect was significant, F(1, 288) = 4.30, p = .04, partial ή2 = .015. This interaction effect is depicted in Figure 13. Follow-up contrasts indicated that among nonmaltreated children, no differences were observed between high and low MAOA activity groups, p = .53; however, among maltreated children, those with low activity genotypes had significantly higher symptoms than those with high activity genotypes, p = .02. Maltreated children in the low activity MAOA group had higher self-reported antisocial behavior than nonmaltreated children, p < .000; this was also true to a lesser extent among children in the high activity MAOA group, p = .05. A very similar pattern of findings was observed for child self-report of antisocial behavior in the past six months. (See Figure 14). The main effect of maltreatment was significant, F(1, 288) = 10.53, p < .001, partial ή2 = .036, whereas MAOA genotype was not, F(1, 288) = 2.25, p = .14, partial ή2 = .008. Additionally, the GxE interaction was significant, F(1, 288) = 5.00, p = .03, partial ή2 = .017. This interaction is shown in Figure 14. Probing the interaction indicated that among maltreated children, those with low MAOA activity genotypes had higher recent antisocial behavior than those with high activity genotypes, p = .01; no significant differences were found among nonmaltreated children based on MAOA genotype group. Among children with low MAOA activity genotypes, maltreated children had higher level self-reported antisocial symptoms than nonmaltreated children, p < .001; no significant differences among maltreated and nonmaltreated children were observed for children in the high activity MAOA genotype group. Across these two analyses, the risk for antisocial behavior associated with maltreatment is reduced among children with high MAOA activity genotypes.
Figure 13.

MAOA and Maltreatment Status to Predict Self Report of Lifetime Antisocial Behavior. Boys only
Figure 14.
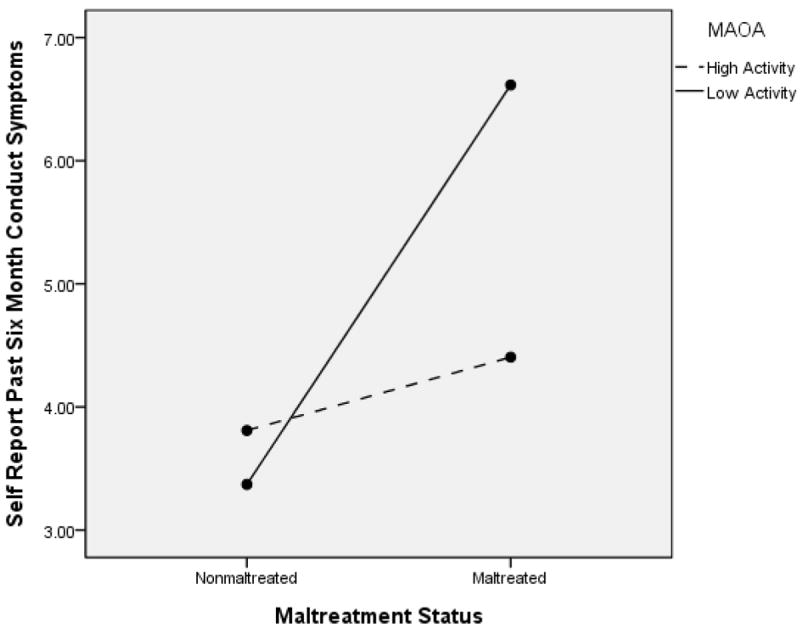
MAOA and Maltreatment Status to Predict Self Report of Antisocial Behavior in the Past 6 Months. Boys only.
Discussion
Consistent with our hypotheses, no matter how antisocial behavior was assessed (i.e., self-, peer-, or adult counselor-report), there was a substantial significant difference between maltreated and nonmaltreated comparison children drawn from comparable low-socioeconomic status backgrounds in terms of heightened early indicators of antisocial behavior. Thus, strong evidence was obtained for child maltreatment as a potent environmental pathogen for the development of antisocial behavior. Because not all maltreated children develop antisocial behavior, the examination of potential genetic contributions to variation in adverse outcomes is important. The investigation of the genetic moderation of the linkage between child maltreatment and antisocial behavior helps to elucidate the understanding of which maltreated children are most vulnerable to traversing the pathways to antisocial behavior.
We found no evidence that gene variants were related to being maltreated. The absence of an evocative gene-environment correlation reveals that children are not likely to be maltreated because they have a particular genotype. Main effects of genes on antisocial behavior outcomes were rarely found in this investigation. Moreover, when we discovered that a particular gene did exert a main effect on an antisocial outcome, we also found that this main effect occurred in the context of a gene x maltreatment interaction. We did not find even one instance whereby genetic variation predicted significant differences in antisocial behavior within the nonmaltreatment group
Child maltreatment and its various parameters were found to have main effects in predicting antisocial outcomes. Importantly, maltreatment experience main effects also were retained in the context of significant gene-environment interactions. Depending on the nature of the reporter (i.e., self, peers, adult counselor), different GxE interaction findings were obtained for the TPH1, 5-HTTLPR, and MAOA genes. However, significant GxE effects on antisocial outcomes were discovered for each of the genes investigated in this study.
Maltreatment and its differentiation in terms of other parameters (e.g., PASA vs. EMPN, developmental timing of maltreatment, number of different developmental periods in which maltreatment occurred) in the context of genetic variation had a very strong effect on antisocial behavior. More extreme experiences of maltreatment were related to higher levels of antisocial behavior, both in maltreatment parameter main effects and in gene-environment interactions. For example, children who experienced physical and sexual abuse had a higher risk for developing antisocial behavior than did children who experienced physical neglect and emotional maltreatment. Moreover, children who experienced maltreatment across a greater number of developmental periods were at greater risk for having higher levels of antisocial behavior. Additionally, children whose onset of maltreatment occurred early in their lives and continued over time were more at risk for developing higher levels of antisocial behavior.
The finding that GxE interaction effects were stronger with more differentiated maltreatment parameters is in keeping with the extant GxE interaction literature on antisocial behavior. For example, in their investigation of MAOA genotype, maltreatment, and antisocial behavior, Caspi and colleagues (2002) found their most significant low activity MAOA interactions with the severely maltreated group of adults. Likewise, Weder and colleagues (2009), in their prospective examination of MAOA genotype, maltreatment, and aggressive behavior in children (M age =9.7 years), discovered that problems in aggressive behavior in maltreated children residing in out-of-home care were moderated by low activity MAOA genotype, but only up to moderate levels of exposure to trauma (cf. Cicchetti, Rogosch, Sturge-Apple, & Toth, 2010). More extreme levels of maltreatment were show to override the effect of MAOA on trauma exposure. The maltreated children in the Weder et al. (2009) study were all residing in foster care. Hence, a higher percentage of these youngsters may have been more severely and extensively maltreated.
The present investigation is among a very few studies that have examined GxE interactions on antisocial behavior in maltreated children. Both Kim-Cohen et al. (2006) and Weder et al. (2009) have conducted research on the role of the MAOA genotype in moderating the relation between child maltreatment and violence/aggression in children. Kim-Cohen and colleagues (2006) utilized the Environmental Risk (E-Risk) Longitudinal Twin Study and focused on physical abuse. 975 seven year old children, all twins, participated in Kim-Cohen et al.’s (2006) study. Sixty-two of the children were assessed as physically abused based on interviews with mothers and project determination of abuse status. Children did not have confirmed maltreatment through the Department of Human Services investigations. In addition, Weder et al. (2009) studied 114 children (M age = 9.7), 73 of whom had physical abuse, neglect, or both. The remaining 41 children served as comparisons from the community. As noted above, the maltreated children were residing in foster care and had indicated child maltreatment as determined by the State Department of Children and Families. Aggression ratings were based on a one-time assessment by a single informant (i.e., teacher). Each of these studies found that MAOA moderated the relation between child maltreatment and antisocial behavior.
The present investigation possesses many strengths. It contains a large number of maltreated children (N = 348), 99 of whom were physically abused. Additionally, there was a comparison sample from a similar SES background (N = 279). Importantly, a comprehensive assessment of maltreatment was made, including all maltreatment subtypes and a variety of maltreatment parameters (Barnett et al., 1993; Manly, 2005). Maltreatment and its parameters were determined prospectively and cumulatively using a well-defined, reliable and valid nosological system. The comprehensive assessment enabled us to compare GxE interactions on antisocial behavior between maltreated and nonmaltreated children and to investigate GxE interactions on antisocial behavior across maltreatment subtypes and parameters.
The current study also was the first multi-genic investigation to examine GxE interaction on antisocial behavior in maltreated children. In fact, to the best of our knowledge, there has been no other investigation of the genetic moderation of TPH1 on the relation between maltreatment and antisocial behavior. As with the Kim-Cohen et al. (2006) and the Weder et al. (2009) studies, our early characterization of antisocial behavior in late childhood and our ascertainment of the developmental timing and cumulative course of maltreatment puts us in the position to determine whether maltreated children exhibiting early indicators of antisocial behavior manifest increased levels of antisocial behavior in further longitudinal assessments into adulthood. Perhaps a sizeable proportion of these children will be candidates for inclusion in the life course persistent group described by Moffitt (1993, 2006).
An additional strength of our investigation was the use of three sources of evaluation of children’s antisocial behavior, including self-report, peer-report, and adult counselor-report. Each of the means of assessment provided a complementary perspective on the individual child’s presentation, and we chose not to consolidate the different sources of information into one global factor of child antisocial behavior to retain these individual perspectives. Peers and counselors were able to observe individual children in the context of the supportive camp environment and base their ratings on actual experiences of interacting and relating to the target child. However, given a week’s observational period, the peers and counselors were more restricted in their ability to be aware of individual children’s more covert antisocial behavior. In contrast, the self-report of antisocial behavior obtained from children themselves allowed us to access involvement in antisocial behavior outside of the camp setting. All sources of information provided consistent support for the greater risk of antisocial behavior observed among maltreated children relative to nonmaltreated children.
Furthermore, the inclusion of a large group of maltreated children allowed us to examine GxE interaction effects more fully because there were sufficient group sizes of maltreated individuals with different genotypes. Moreover, the use of Bonferroni corrections in our post-hoc probes of significant GxE interaction effects mitigates against the likelihood of the type 1, false position errors often found in contemporary GxE analyses (Duncan & Keller, 2011), thereby increasing the replicability and generalized validity of our findings. Finally, the accuracy of our genotyping procedures is strongly supported by the numerous quality controls that were employed.
Despite the strengths of this investigation, there exist limitations. For example, although we controlled gender as a covariate in our analyses we would need to recruit an even larger sample in order to examine gender as a moderator and the potential for differential processes operating among boys and girls in the development of antisocial behavior. In addition, we were not able to include ancestral proportion scores as covariates in our analyses. Because we had a heterogeneous sample, it is conceivable that ancestral proportion scores may have been significantly related to antisocial behavior symptoms. Rather than regarding population stratification as a critical methodological flaw in candidate gene studies, Hutchison, Stalling, McGeary, and Bryan (2004) contended that increased reliability in GxE interaction research requires improved specification and measurement of the behavioral phenotypes in question, increased focus on internal validity, and the testing of GxE interactions in the context of multivariate longitudinal research. Although we have not yet conducted a longitudinal follow-up of our sample, we satisfy the first two suggestions of Hutchison et al. (2004), who believe that attention to these topics may help to increase the replication across GxE studies and reduce the threat of population stratification as a cause of the nonreplications found in candidate gene studies.
Finally, future research that elucidates the neural mechanisms of genetic risk is needed. In the context of trauma, abuse, and neglect, susceptibility alleles may bias neurobiological development toward alterations in brain structure, function, and connectivity that can be investigated through neuroimaging. These neurobiological alterations, in concert with compromised resolution of stage-salient developmental tasks, may promote the development of antisocial behavior through enhancing the negative effects of adverse childhood experiences.
In summary, the results of the current investigation suggest a number of implications. First, given the numerous GxE findings that involve maltreatment and its parameters, as a society we must continue to strive to prevent the occurrence of child maltreatment. The developmental timing results regarding the deleterious effects of early onset and continued maltreatment underscore the need to implement intervention as close to the experience of the traumatic event as possible (Cicchetti, Rogosch, & Toth, 2006; Cicchetti, Toth, Nilsen, & Manly (in press).
Relatedly, because children who manifest early onset antisocial behavior may traverse a life-course persistent trajectory (Moffitt, 1993, 2006), early identification and intervention with such children is strongly indicated. This is especially warranted for the abused and neglected children who have experienced extreme maltreatment. Although there were no genetic effects for antisocial behavior in nonmaltreated children, the GxE findings in maltreated children may indicate which children will require the most intensive forms of intervention.
Finally, as is shown in the current investigation, different genetic variants can increase or decrease the likelihood of antisocial behavior in maltreated children. Even though particular genotypes were associated with the reduction of risk for antisocial behavior in maltreated children, we have not labeled these effects as resilient in nature. Resilience is a dynamic process that encompasses the attainment of positive adaptation within the context of exposure to significant adversity (Cicchetti, 2010; Luthar, Cicchetti, & Becker, 2000; Masten, 2001; Rutter, 2012). Resilience is multidimensional in nature and thus resilience should be assessed through the achievement of competent functioning across multiple domains. As such, we do not consider the finding that particular genotypic variations were associated with a lower likelihood of antisocial behavior to be signs of resilient functioning, per se. These variants can be conceived as conferring protection against the development of antisocial behavior; however, given that we have conducted a cross-sectional study, our preference is to refer to these genotypic variants as being associated with a decrease in antisocial symptoms among maltreated children.
Acknowledgments
This research was supported by grants received from the National Institute on Drug Abuse (R01DA17741) and the Spunk Fund, Inc.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Aslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW. Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behavior Genetics. 2011;41:262–272. doi: 10.1007/s10519-010-9356-y. [DOI] [PubMed] [Google Scholar]
- Azar ST. Parenting and child maltreatment. In: Bornstein MH, editor. Handbook of parenting: Social conditions and applied parenting. 2. Vol. 4. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 361–388. [Google Scholar]
- Banny A, Cicchetti D, Rogosch FA, Crick NR, Oshri A. Vulnerability to depression: A moderated mediation model of the roles of child maltreatment, peer victimization, and 5-HTTLPR genetic variation among children from low-SES backgrounds. Development and Psychopathology. doi: 10.1017/S0954579413000047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex; 1993. pp. 7–74. [Google Scholar]
- Beach SR, Brody GH, Gunter TD, Packer H, Wernett P, Philibert RA. Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. Journal of Family Psychology. 2010;24:12–20. doi: 10.1037/a0018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger KE, Patterson CJ, Kupersmidt JB. Peer relationships and self-esteem among children who have been maltreated. Child Development. 1998;69:1171–1197. [PubMed] [Google Scholar]
- Bukowski WM, Sippola L, Hoza B, Newcomb AF. Pages from a sociometric notebook: An analysis of nomination and rating scale measures of acceptance, rejection, and social preference. In: Cillessen AHN, Bukowski WM, editors. New directions for child and adolescent development: Recent advances in the measurement of acceptance and rejection in the peer system. Vol. 88. San Francisco: Jossey-Bass; 2000. pp. 11–26. [DOI] [PubMed] [Google Scholar]
- Carlson V, Cicchetti D, Barnett D, Braunwald K. Disorganized/disoriented attachment relationships in maltreated infants. Developmental Psychology. 1989;25:525–531. [Google Scholar]
- Caspi A, Hariri A, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene (5-HTT) and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt T, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. The impact of social experience on neurobiological systems: Illustration from a constructivist view of child maltreatment. Cognitive Development. 2002;17:1407–1428. [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective. World Psychiatry. 2010;9:1–10. doi: 10.1002/j.2051-5545.2010.tb00297.x. Special Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Toward an ecological/transactional model of community violence and child maltreatment: Consequences for children’s development. Psychiatry. 1993;56:96–118. doi: 10.1080/00332747.1993.11024624. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. Vol. 2. New York: John Wiley & Sons, Inc; 1995. pp. 32–71. [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of family research: Families at risk. Vol. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology upon neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple M, Toth SL. Interaction of child maltreatment and 5-HTT polymorphisms: suicidal ideation among children from low-SES backgrounds. Journal of Pediatric Psychology. 2010;35:536–546. doi: 10.1093/jpepsy/jsp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18:623–650. doi: 10.1017/s0954579406060329. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A developmental psychopathology perspective on child abuse and neglect. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal Maltreatment Interview. Rochester, NY: 2003. Unpublished manuscript. [Google Scholar]
- Cicchetti D, Toth SL, Nilsen WJ, Manly JT. What do we know and why does it matter? The dissemination of evidence-based interventions for child maltreatment. In: Schaffer HR, Durkin K, editors. Blackwell Handbook of Developmental Psychology in Action. Oxford: Blackwell; in press. [Google Scholar]
- Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. The American Journal of Psychiatry. 1997;154:1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- Coie JD, Dodge KA. Continuities and changes in children’s social status: A five-year longitudinal study. Merrill-Palmer Quarterly. 1983;29:261–282. [Google Scholar]
- DeBellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- DeBellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- DeYoung C, Cicchetti D, Rogosch FA, Gray J, Eastman M, Grigorenko E. Sources of cognitive exploration: Genetic variation in the prefrontal dopamine system predicts Openness/Intellect. Journal of Research in Personality. 2011;45:364–371. doi: 10.1016/j.jrp.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Patterson GR. The development and ecology of antisocial behavior. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. 2. Vol. 3. Hoboken, NJ: Wiley; 2006. pp. 503–541. [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. How the experience of early physical abuse leads children to become chronically aggressive. In: Cicchetti D, Toth SL, editors. Rochester symposium on developmental psychopathology: Trauma: Perspectives on theory, research, and intervention. Vol. 8. Rochester, NY: University of Rochester Press; 1997. pp. 263–288. [Google Scholar]
- Douglas K, Chan G, Gelernter J, Arias AJ, Anton RF, Poling J, et al. 5-HTTLPR as a potential moderator of the effects of adverse childhood experiences on risk of antisocial personality disorder. Psychiatric Genetics. 2011;21:240–248. doi: 10.1097/YPG.0b013e3283457c15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenrode J, Laird M, Doris J. School performance and disciplinary problems among abused and neglected children. Developmental Psychology. 1993;29:53–62. [Google Scholar]
- English DJ, Upadhyaya MP, Litrownik AJ, Marshall JM, Runyan DK, Graham JC, et al. Maltreatment’s wake: The relationship of maltreatment dimensions to child outcomes. Child Abuse & Neglect. 2005;29(5):597–619. doi: 10.1016/j.chiabu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ, Miller AL, Kennedy MA. MAOA, abuse exposure and antisocial behaviour: 30-year longitudinal study. British Journal of Psychiatry. 2011;198:457–463. doi: 10.1192/bjp.bp.110.086991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Viding EM. Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology. 2009;21:1111–1131. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: Evidence of an environmentally mediated process. Journal of Abnormal Psychology. 2004;113:44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kruesi MJP, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, et al. Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Archives of General Psychiatry. 1990;47:419–426. doi: 10.1001/archpsyc.1990.01810170019003. [DOI] [PubMed] [Google Scholar]
- Lansford J, Dodge K, Pettit G, Bates J, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics and Adolescent Medicine. 2002;156:824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Latent class analysis of antisocial behavior: Interaction of serotonin transporter genotype and maltreatment. Journal of Abnormal Child Psychology. 2010;38:789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Farrington DP. Child delinquents: Development, intervention, and service needs. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Multiple risk factors for multiproblem boys: Co-occurrence of delinquency, substance use, attention deficit, conduct problems, physical aggression, covert behavior, depressed mood, and shy/withdrawn behavior. In: Jessor R, editor. New perspectives on adolescent risk behavior. New York: Cambridge University Press; 1998. pp. 90–149. [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JT. Advances in research definitions of child maltreatment. Child Abuse & Neglect. 2005;29(5):425–439. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Manly JT, Cicchetti D, Barnett D. The impact of subtype, frequency, chronicity, and severity of child maltreatment on social competence and behavior problems. Development and Psychopathology. 1994;6:121–143. [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biological Psychiatry. 1999;45:603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- McCrory E, Viding E. The neurobiology of maltreatment and adolescent violence. Lancet. 2010;375:1856–1857. doi: 10.1016/S0140-6736(10)60842-2. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent anti-social behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. Life-course-persistent versus adolescence-limited antisocial behavior. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology Risk, Disorder, and Adaptation. 2. Vol. 3. New York: Wiley; 2006. pp. 570–598. [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Perspectives in Psychological Science. 2005;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, et al. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug and Alcohol Dependence. 2007;91:85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology. 2000;36:679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Richters JE, Cicchetti D. Mark Twain meets DSM-III-R: Conduct disorder, development, and the concept of harmful dysfunction. Development and Psychopathology. 1993;5:5–29. [Google Scholar]
- Rogosch FA, Cicchetti D, Shields A, Toth SL. Parenting dysfunction in child maltreatment. In: Bornstein MH, editor. Handbook of parenting. Vol. 4. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 127–159. [Google Scholar]
- Rujescu D, Giegling I, Bondy B, Gietl A, Zill P, Moller HJ. Association of anger-related traits with SNPs in the TPH gene. Molecular Psychiatry. 2002;7:1023–1029. doi: 10.1038/sj.mp.4001128. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience as a dynamic concept. Development and Psychopathology. 2012;24:335–344. doi: 10.1017/S0954579412000028. [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, Li S. Fourth National Incidence Study of Child Abuse and Neglect (NIS–4): Report to Congress, Executive Summary. Washington, DC: U.S Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- Shields A, Cicchetti D. Emotion regulation among school-age children: The development and validation of a new criterion Q-sort scale. Developmental Psychology. 1997;33:906–916. doi: 10.1037//0012-1649.33.6.906. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology. 2001;30:349–363. doi: 10.1207/S15374424JCCP3003_7. [DOI] [PubMed] [Google Scholar]
- Shonk SM, Cicchetti D. Maltreatment, competency deficits, and risk for academic and behavioral maladjustment. Developmental Psychology. 2001;37:3–14. [PubMed] [Google Scholar]
- Teisl M, Cicchetti D. Physical abuse, cognitive and emotional processes, and aggressive/disruptive behavior problems. Social Development. 2008;17:1–23. [Google Scholar]
- Trickett PK, McBride-Chang C. The developmental impact of different types of child abuse and neglect. Developmental Review. 1995;15:311–337. [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biological Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS. The cycle of violence. Science. 1989;244:160–166. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence:” childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biological Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. National Reviews of Neuroscience. 2006;7:295–209. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Finberg JPM, Tipton KF. Monoamine oxidase. In: Trendelemburg U, Weiner U, editors. Catechloamine II. Berlin: Springer Verlag; 1988. pp. 192–199. [Google Scholar]


