Abstract
BACKGROUND:
The high recurrence rate of hepatocellular carcinoma (HCC) after potentially curative treatment determines the long-term prognosis.
OBJECTIVE:
To evaluate the efficacy and safety of adjuvant therapies in patients with HCC who have undergone hepatic resection, transplantation or locoregional ablation therapy.
METHODS:
Several databases were searched to identify randomized controlled trials (RCTs) fulfilling the predefined selection criteria. Meta-analyses were performed to estimate the effects of adjuvant therapies of any modality on recurrence-free survival (RFS) and overall survival (OS).
RESULTS:
Eight adjuvant modalities were identified from 27 eligible RCTs conducted predominantly in Asian populations comparing adjuvant with no adjuvant therapy. Adjuvant chemotherapy, internal radiation and heparanase inhibitor PI-88 therapy failed to improve RFS or OS, while interferon (IFN) therapy yielded significant survival results. The findings of adjuvant vitamin analogue therapy required further examination. Adjuvant adoptive immunotherapy conferred significant benefit for RFS but not for OS. Although cancer vaccine therapy and radioimmunotherapy may improve survival after radical surgery, the results were from single, small-scale trials. Severe side effects were observed in the studies of adjuvant chemotherapy and of IFN therapy.
CONCLUSIONS:
Adjuvant IFN therapy can improve both RFS and OS; however, the benefits of using this agent should be weighed against its side effects. Combination of systemic and transhepatic arterial chemotherapy is not recommended for HCC after potentially curative treatment. Other adjuvant therapies produce limited success for survival. Additional RCTs with proper design are required to establish the role of adjuvant therapies for HCC.
Keywords: Adjuvant therapy, Hepatocellular carcinoma, Recurrence, Survival
Abstract
HISTORIQUE :
Le taux élevé de récurrence de carcinome hépatocellulaire (CCH) après un traitement au potentiel curatif en détermine le pronostic à long terme.
OBJECTIF :
Évaluer l’efficacité et l’innocuité des thérapies adjuvantes chez des patients atteints d’une CCH qui ont subi une résection hépatique, une transplantation ou une ablation locorégionale.
MÉTHODOLOGIE :
Les auteurs ont fait des recherches dans plusieurs bases de données pour en extraire les essais aléatoires et contrôlés (EAC) qui respectaient les critères de sélection prédéfinis. Ils ont procédé à des méta-analyses pour évaluer les effets des thérapies adjuvantes, quelles que soient leurs modalités, sur la survie sans récurrence (SSR) et la survie globale (SG).
RÉSULTATS :
Les chercheurs ont établi huit modalités tirées des 27 EAC admissibles menés surtout auprès de populations asiatiques, qui comparaient la thérapie adjuvante à l’absence de thérapie adjuvante. La chimiothérapie adjuvante, la radiation interne et la thérapie par inhibiteur de l’héparanase PI-88 n’amélioraient pas la SSR et la SG, mais la thérapie à l’interféron (IFN) donnait des résultats significatifs sur le plan de la survie. L’immunothérapie adjuvante adoptive avait d’importants avantages pour la SSR, mais par pour la SG. Même si la thérapie du cancer par la vaccination et la radio-immunothérapie peuvent accroître la survie après une chirurgie radicale, les résultats provenaient d’essais uniques à petite échelle. Les chercheurs ont observé des effets secondaires marqués dans les études sur la chimiothérapie adjuvante et la thérapie à l’IFN.
CONCLUSIONS :
La thérapie à l’IFN adjuvante peut améliorer la SSR et la SG, mais il faudrait soupeser les avantages de cet agent par rapport à ses effets secondaires. Il n’est pas recommandé de dispenser une association de chimiothérapie systémique et artérielle transhépatique du CCH après un traitement au potentiel curatif. Les autres thérapies adjuvantes avaient peu de succès sur le plan de la survie. D’autres EAC bien conçus s’imposent pour établir le rôle des thérapies-adjuvantes du CCH.
Worldwide, hepatocellular carcinoma (HCC), the most common primary cancer of the liver, ranks sixth among malignant tumours in incidence and is the third leading cause of cancer-related death (1). In China, owing to the high prevalence of hepatitis B viral infection and associated liver cirrhosis, HCC accounts for >54% of the world annual incidence, with an estimated 372,079 mortalities in 2008 (1–3).
For many years, surgery (hepatic resection and transplantation) has been considered the only curative option for HCC. Locoregional ablation therapy, particularly percutaneous radiofrequency ablation (RFA), was recently demonstrated to have similar efficacy to surgical resection for small HCC nodules (<3 cm in diameter) (4). Although these treatments offer a possibility of cure for HCC, the long-term outcomes after surgery or ablation therapy are disappointing because of the high frequency of recurrence. HCC often recurs as a result of intrahepatic dissemination of primary cancer cells or the development of a de novo tumour in the cirrhotic liver, which, in total, complicates 70% of cases at five years (5–7). However, because it is difficult to distinguish the two types of HCC recurrence in routine clinical practice, the presence of tumour originating from either primary or metachronous multicentric carcinogenesis is regarded as a recurrence (8). Therefore, adjuvant therapy that aims to reduce or delay the incidence of recurrence by eradicating the growth of pre-existing minute tumour foci not detected before initiation of adjuvant therapy, or by inhibiting the occurrence of metachronous multicentric tumours, is essential to improve the efficacy of curative treatment of HCC.
Several adjuvant modalities have been developed in the past decades; nevertheless, the clinical use of these therapies is either controversial or requires further evaluation (9). To date, there have been five reviews or meta-analytic studies (10–14) published between 2002 and 2009 assessing the role of neoadjuvant and/or adjuvant therapy for HCC. While the early analyses (10–12) had achieved modest survival benefits from some adjuvant therapies, two recent studies with updated evidence (13,14) only applied qualitative descriptive approaches for therapeutic evaluation. Furthermore, these studies (10–14) neither considered modalities other than surgical resection as potentially curative treatment for HCC, nor quantitatively measured the effects of adjuvant therapies on survival using time-to-event outcomes. On the other hand, although previous individual meta-analyses have also shown encouraging results with adjuvant chemotherapy (15,16), adjuvant immunotherapy (17–23) and vitamin analogue chemoprevention (24), some of these may be inconclusive or of doubtful accuracy because of bias due to limited sample size and statistical methodological flaws (15,16,24). Nevertheless, emerging evidence from randomized controlled trials (RCTs) of novel adjuvant modalities and from trials with updated information is currently available. Given that no therapies after potentially curative treatments have been accepted as standard of care in HCC to date, the effects of adjuvant therapies on recurrence and survival are even less clear. Using currently available RCT evidence and knowledge of survival analysis (25–27), the aim of the present study was to evaluate the efficacy of adjuvant therapies of any modality in terms of the hazard ratio (HR) of recurrence-free survival (RFS) and overall survival (OS), and the safety of these adjuvant therapies after potentially curative treatment with surgical resection, liver transplantation or ablation therapy for HCC.
METHODS
Literature search
The Cochrane Library, MEDLINE (via PubMed) and Embase were searched using the keywords “hepatocellular carcinoma” and “recurrence”. Both medical subject and text terms were used and combined, and the search strategy was not restricted to languages or publication date. However, only RCTs were considered in the present meta-analysis, for which searches in MEDLINE and Embase were limited by study type. The searches were performed mainly in June 2011, and the result was updated in November 2011. In addition, published meta-analyses and reviews of relevance were scrutinized for other potential studies, and reference lists of included trials were manually searched.
Inclusion and exclusion criteria
RCTs published as full text assessing adjuvant therapy in patients with HCC who had undergone potentially curative treatment with surgical resection, hepatic transplantation or locoregional ablation therapy were eligible if recurrence-related outcomes were analyzed using survival analysis. However, to guarantee that adjuvant therapy was truly adjuvant, only trials in which randomization was performed after patients had been treated with initially curative treatment were considered. Trials concurrently comparing sequential combinations of curative and adjuvant therapies versus curative treatment alone were excluded because adjuvant effects in such trials could not be separately evaluated due to the presence of initially curative treatment. Similarly, given the nature of neoadjuvant trials for which patient allocation is achieved before curative treatment, neoadjuvant therapy was not included in the present study. Furthermore, because no well-accepted adjuvant therapeutic modality has been currently established, the meta-analysis only compared adjuvant therapy with no active adjuvant therapy (no treatment or concurrent placebo). Other exclusions were trials with non-randomized design, studies involving noncurative or palliative treatment for HCC, and trials comparing different adjuvant therapies or different schedules of one adjuvant therapy.
Study selection and outcomes measurement
All references records retrieved from the searches were stored in an EndNote (Thomson Reuters, USA) file and duplicates were removed. Two authors independently assessed eligibility against the inclusion criteria by scanning the title and abstract of each record, with disagreement resolved by discussion. Where studies had multiple publications, the most recent report was included and secondary articles were also considered. Trials containing three or more study groups were retained if at least two groups addressed an eligible comparison.
The primary outcome was RFS, which is also referred to as disease-free survival (DFS). However, for studies in which neither of the two outcomes was reported, time to recurrence (TTR) was used as a surrogate outcome. The secondary outcomes were OS and side effects.
Data extraction and analysis
Information regarding adjuvant therapy protocols of each trial was extracted and tabulated, along with tumour histological factors (size, number and vascular invasion) and staging of HCC, and underlying liver disease, which are the variables that are the most important predictors of recurrence and survival (28–30). Details of methodological quality assessment of the included RCTs were also abstracted. The number of patients developing events (recurrence or death) during follow-up was recorded. Locations and types of recurrence, and reasons for death were also presented. Furthermore, to determine the risk of patients developing events, recurrence and survival rates at various time points (one, two, three, four and five years) for patients in the control group (did not receive an active adjuvant therapy) were reported.
The HR of time-to-event outcomes (ie, RFS, TTR and OS) was directly extracted from trial publication, if available, or was estimated indirectly using the reported number of events and the corresponding P value for the log-rank statistics, or by reading survival curves, as described by Parmar et al (25). An Excel spreadsheet (Microsoft, USA) developed by the Meta-analysis Group of the MRC Clinical Trials was used for the calculations (26).
A pooled analysis was performed for RCTs testing a similar modality of adjuvant therapy, for which all trials included were analyzed in several subgroups to assess their effects on RFS and OS. Sensitivity analyses were performed to examine effects of excluding extraordinary studies with either quality concerns or confounding data. In the safety analyses, however, because the criteria for assessment varied across trials, only side effects requiring a discontinuation of adjuvant therapy were reported. All outcomes data were extracted on worksheets and were cross-checked for accuracy by two authors before combining for analysis.
Quality assessment
The methodological quality of RCTs was assessed independently by two authors, using the criteria outlined in Cochrane Handbook for Systematic Reviews of Interventions (Version 5) (31). Any disagreement was resolved by discussion or by consulting a third author. Six items assessing components of internal validity of RCTs were applied: generation of random sequence, allocation concealment, completeness of outcomes data reporting, whether free of selective reporting, and other bias including whether balanced in baseline characteristics and whether there was a priori sample size calculation. Blinding was, however, removed in the assessment of risk of bias, given the impracticability to mask adjuvant therapy in most trials using no treatment as a comparator. Each item was scored as ‘yes’ for low risk of bias, ‘unclear’ for either lack of information or uncertainty over the potential for bias, and ‘no’ for high risk of bias.
Statistical analysis
Time-to-event outcomes were combined using the inverse variance method in Review Manager Software (Version 5.1.1, The Nordic Cochrane Centre, The Cochrane Collaboration) with a fixed-effect model. A pooled HR represents the overall risk of an event on adjuvant therapy over control, in which HR <1.0 favoured adjuvant therapy and HR ≥1.0 favoured control. Respective 95% CIs were calculated for each estimate. Statistical heterogeneity of the results of the trials was assessed using the χ2 statistic and the proportion of variation due to heterogeneity was expressed as I2, where I2<25% is considered to be low-level heterogeneity, 25% to 50% as moderate-level, and I2>50% as high-level heterogeneity (32). Recurrence and survival rates were expressed as median values with the minimum to maximum range.
RESULTS
Search results and characteristics of the included RCTs
The databases searches indentified 527 records, and another two were obtained from references lists. Of these, 474 were initially judged as irrelevant, yielding 55 potential study reports that were assessed for eligibility at full-text level, resulting in retrieval of another prospective trial. Finally, 32 publications corresponding to 28 RCTs met the inclusion criteria, from which 31 publications (33–63) of 27 trials, involving a total of 2614 randomized patients, were eligible for analysis (one study [64] was excluded due to insufficient reporting of survival outcomes data). Figure 1 summarizes the study identification flow according to the recommendations of the PRISMA statement (65).
Figure 1).
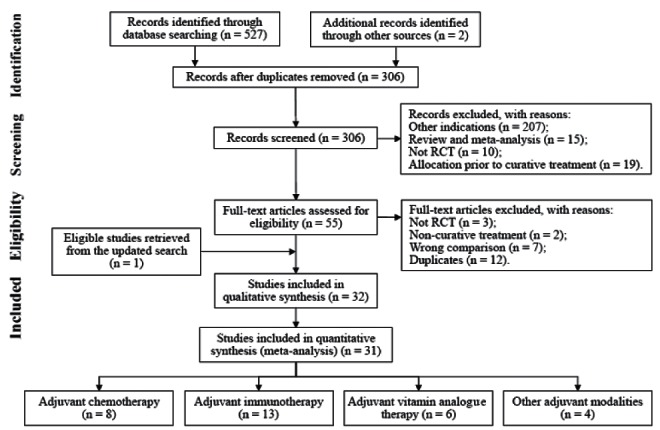
PRISMA flow diagram for randomized controlled trials (RCT)
From the 31 trial publications assessing adjuvant therapy, eight modalities were indentified, including chemotherapy in eight (three [33–35] involved oral, three [36–38] transhepatic arterial and two [39,40] a combination of systemic and transhepatic arterial approaches), interferon (IFN) therapy in nine (eight [42–49] used IFN-α and one [41] IFN-β), vitamin analogue therapy in six (two [50,51] tested polyprenoic acid, a vitamin A [VA] analogue; and four [52–55] menatetrenone, a vitamin K2 [VK2] analogue), adoptive immunotherapy in three (one [56] involved lymphokine-activated killer [LAK] and two [57,58] cytokine-induced killer [CIK] cells), cancer vaccine therapy with autologous HCC fragments in one (59), internal radiation therapy using transhepatic arterial infusion of iodine 131-labelled lipiodol (131I-lipiodol) in two (60,61), antibody-targeted radioimmunotherapy with 131I-lipiodol metuximab (Licartin, Chengdu Hoist Hitech Co. Ltd, China; and the Fourth Military Medical University, Xi’an, China) in one (62), and molecular targeted therapy with heparanase inhibitor PI-88 in one study (63) (Table 1). Most of the trials were conducted or presented in the past decade, and all except one (47) were performed among Asian populations (Japan and China). The sample size of the RCTs varied from 15 (38) to 548 (55), and the range of median follow-up duration was between 12.3 (62) and 66 (61) months. Randomization was done after curative treatment for HCC in all of these adjuvant trials, and the delay between curative and the first adjuvant therapy was usually scheduled to commence after one month. Of the RCTs included, 24 trials compared adjuvant therapy with no active adjuvant therapy, while the other three (50,55,62) used a concurrent control group involving placebo.
TABLE 1.
General information regarding the included studies
| Item | RCTs (publications), n (n) |
|---|---|
| Total | 27 (31) |
| Years of publication | |
| 1990–2000 | 9 (11*) |
| 2001–2010 | 17 (19) |
| 2011–present | 1 (1) |
| Countries involved (investigator affiliations) | |
| Japan | 15 (18) |
| China | 11 (12) |
| Italy | 1 (1) |
| Adjuvant therapeutic modality | |
| Chemotherapy | 8 (8) |
| Interferon therapy | 7 (9) |
| Adoptive immunotherapy | 3 (3) |
| Cancer vaccine therapy | 1 (1) |
| Vitamin analogue therapy | 5 (6) |
| Internal radiation therapy | 1 (2) |
| Antibody targeted radioimmunotherapy | 1 (1) |
| Heparanase inhibitor PI-88 therapy | 1 (1) |
| Curative treatment modality for hepatocellular carcinoma | |
| Hepatic resection | 17 (20) |
| Locoregional ablation therapy† | 3 (3) |
| Hepatic resection or locoregional ablation therapy† | 6 (7) |
| Liver transplantation | 1 (1) |
| Sample size‡ | |
| <30 patients per study group | 13 (16) |
| ≥30 patients per study group | 14 (15) |
| Duration of follow-up, years§ | |
| <2 | 8 (8) |
| ≥2 | 19 (23) |
| Outcomes of interest | |
| Recurrence-free survival or disease-free survival | 18 (21) |
| Time to recurrence | 9¶ (10) |
| Overall survival | 22 (26) |
Two reports from one trial were presented in 1999 and in 2008, respectively;
Includes ablation therapy with or without transhepatic arterial therapy;
In terms of patients analyzed;
One study reported time to second primary hepatocellular carcinoma;
According to presented recurrence curves. RCT Randomized controlled trial
With regard to modes of initially curative treatment, 26 RCTs used hepatic resection, or locoregional ablation therapy (percutaneous ethanol/acetic acid injection or RFA) given with or without transarterial chemoembolization, while the remaining RCT (62) involved liver transplantation. Nevertheless, among the individual trials in which surgical resection or ablation therapy was used as initial treatment for HCC (41,50,52–55), the proportion of patients receiving either of the treatments was balanced between the compared groups. Curative treatment was performed for primary HCC in all trials except for one (55), in which 21% of the enrolled patients had undergone hepatic resection or ablation therapy for their first intrahepatic recurrence of HCC. Of the 27 RCTs, 22 (33,35–37,39,40,44–50,52–54,56–59,61,63) specified curativity of initial treatment, which was usually on the basis of defining a completed tumour-eliminating surgical or ablation procedure and/or identifying a postprocedural imaging findings indicative of no demonstrable residual tumour.
The searches identified three publications of one trial by Kubo et al and others (42–44). Although the latest report (44) provided final outcomes data, the previous two publications (42,43) were also considered for analysis because they reported other important information of the trial accrual. The polyprenoic acid study by Muto et al (50,51) and internal radiation study by Lau et al (60,61) also presented two unduplicated articles; hence, data from both reports of each of the studies were used, separately (50,51) or in combination (60,61), in the outcomes analysis. Six RCTs (33,46,49,55,58,59) involved three or more study groups. In two of these (46,55), because no difference in recurrence rates and in RFS was found between two adjuvant groups, respectively, data from both adjuvant groups were combined for analysis. However, for the other four trials (33,49,59,63), due to the lack of combined outcomes data, only selected adjuvant groups were compared with control. The HR of RFS, TTR or OS was estimated using indirect methods (25,26) for most of the RCTs, because only a few studies (34,35,55,59) directly provided data for these time-to-event outcomes.
Overall, 13 trials (33,35–37,46,49,50,52,54,56–59) found adjuvant therapy could confer a significant advantage in RFS or TTR, and six (38,44,48,51,59,62) indentified a significant OS gain with adjuvant therapy. In four other trials (34,39,40,55), however, adjuvant therapy demonstrated worse outcomes compared with no adjuvant therapy, although differences in RFS or OS were insignificant.
Summary of study results on recurrence and survival
A total of 1151 patients from both adjuvant and control groups of 24 trials (33–36,39–41,44,46–50,52–59,61–63) reported recurrence of HCC during follow-up, and the proportion of intrahepatic recurrence among 14 studies (33,35,36,39,40,44,48,49,55–59,61) was in the range of 79% (49) to 100% (44) (Table 2). In the only trial involving liver transplantation as initial curative treatment (62), more patients developing extrahepatic recurrence were observed (68%). With regard to types of recurrence, although most of the studies failed to specify the distinction between intrahepatic metastasis and de novo tumour for recurrent disease, it was noteworthy the incidence of second primary HCC characterized by multicentric carcinogenesis in the only study (50,51) was 79% (27 of 34 recurrences). Of 525 deaths noted from 18 RCTs (33,35,36,39,40,44–49,52,54,56,58,59,61,62), 67% (47) to 100% (44,45,59) of cases were the result of recurrent HCC that occurred predominantly in residual liver.
TABLE 2.
Summary of study results regarding recurrence and survival
| Study* (reference) | Recurrence | Survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Overall recurrence, n | Intrahepatic recurrence, % | Recurrence rates for patients without adjuvant therapy, % | Overall death, n | Death due to recurrence, % | Survival rates for patients without adjuvant therapy, % | |||||||||
|
|
|
|||||||||||||
| 1-year | 2-year | 3-year | 4-year | 5-year | 1-year | 2-year | 3-year | 4-year | 5-year | |||||
| Izumi et al (36) | 40 | 93 | 57 | 78 | 88 | 94 | 94 | 31 | 87 | 81 | 65 | 53 | 37 | 29 |
| Yamamoto et al (33) | 35 | 83 | 28 | 48 | 66 | 71 | 82 | 22 | 68 | 82 | 78 | 67 | 52 | 48 |
| Ono et al (40) | 38 | 97 | 19 | 30 | 59 | 71 | 84 | 23 | 83 | 92 | 81 | 77 | 56 | 56 |
| Lai et al (39) | 40 | 80 | 31 | 47 | 52 | 52 | NA | 20 | 95 | 94 | 88 | 64 | 64 | NA |
| Muto et al (50,51) | 34 | NA | 28† | 36† | 50† | NA | NA | NA | NA | 98 | 86 | 77 | 67 | 60 |
| Ueno et al (37) | NA | NA | 56 | 78 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lau et al (60,61) | 24 | 88 | 41 | 64 | 64 | 68 | 68 | 26 | 81 | 86 | 62 | 46 | 36 | 36 |
| Ikeda et al (41) | 8 | NA | 63 | 100 | NA | NA | NA | 0‡ | NA | NA | NA | NA | NA | NA |
| Takayama et al (56) | 102 | 90 | 40 | 55 | 67 | 73 | 78 | 52 | 92 | 95 | 85 | 74 | 68 | 62 |
| Kubo et al (42–44) | 22 | 100 | 19 | 47 | 74 | 80 | 87 | 12 | 100 | 94 | 87 | 80 | 58 | 47 |
| Shiratori et al (45) | NA | NA | 24 | 70 | 76 | 85 | 92 | 41 | 100 | 96 | 88 | 84 | 62 | 48 |
| Lin et al (46) | 18 | NA | 40 | 70 | 90 | 90 | NA | 9 | 78 | NA | NA | NA | NA | NA |
| Kuang et al (59) | 16 | 94 | 53 | 60 | NA | NA | NA | 9 | 100 | 72 | 50 | NA | NA | NA |
| Tanaka et al (38) | NA | NA | 63 | 88 | NA | NA | NA | NA | NA | 75 | 25 | 25 | NA | NA |
| Hasegawa et al (34) | 115 | NA | 29 | 38 | 63 | 66 | 71 | NA | NA | 99 | 98 | 92 | 83 | 73 |
| Sun et al (48) | 138 | 87 | 42 | 54 | 60 | 65 | 65 | 98 | 92 | 78 | 62 | 52 | 44 | 44 |
| Mazzaferro et al (47) | 100 | NA | 34 | 51 | 63 | 77 | 94 | 61 | 67 | NA | NA | NA | NA | NA |
| Mizuta et al (52) | 40 | NA | 55 | 83 | 92 | NA | NA | 11 | 73 | 96 | 81 | 64 | NA | NA |
| Lo et al (49) | 43 | 79 | 30 | 45 | 50 | 53 | 55 | 21 | 76 | 85 | 72 | 70 | 70 | 61 |
| Hotta et al (53) | 19 | NA | 33 | 47 | 73 | NA | NA | NA | NA | 88 | 82 | 82 | NA | NA |
| Kakizaki et al (54) | 34 | NA | 28 | 64 | 90 | NA | NA | 11 | 82 | 96 | 90 | 66 | NA | NA |
| Xu et al (62) | 25 | 32 | 57 | NA | NA | NA | NA | 15 | NA | 62 | NA | NA | NA | NA |
| Weng et al (57) | 46 | 93 | 30 | NA | NA | NA | NA | 0‡ | NA | NA | NA | NA | NA | NA |
| Hui et al (58) | 77 | 83 | 17 | 69 | 79 | 82 | 89 | 63 | 73 | 87 | 85 | 65 | 54 | 37 |
| Liu et al (63) | 42 | NA | 68 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Xia et al (35) | 39 | 90 | 37 | 50 | 70 | 77 | 77 | 27 | NA | 84 | 70 | 57 | 50 | 40 |
| Yoshida et al (55) | 56 | 98 | 30 | NA | NA | NA | NA | NA | NA | 97 | NA | NA | NA | NA |
Listed according to year of publication;
Recurrence rate of second primary hepatocellular carcinoma;
No patients died during follow-up. NA Not available.
As illustrated in Table 2, for patients receiving no active adjuvant therapy after potentially curative treatment for HCC, the median one-, two-, three-, four- and five-year recurrence rates were 36% (range 17% to 68%), 58% (range 30% to 100%), 69% (range 50% to 92%), 73% (range 52% to 94%) and 82% (range 55% to 94%), respectively; and that for survival rates at the corresponding time point were 88% (range 62% to 99%), 81% (range 25% to 98%), 67% (range 25% to 92%), 57% (range 36% to 83%) and 48% (range 29% to 73%), respectively.
Efficacy and safety evaluation of adjuvant therapy
For assessment and analysis of adjuvant therapies for which there was potential for clinical benefit, these 27 RCTs were classified into five categories: chemotherapy, IFN therapy, vitamin analogue therapy, adoptive immunotherapy and other therapies (each therapy was tested in a single trial) including cancer vaccine therapy, internal radiation therapy, radioimmunotherapy and heparanase inhibitor PI-88 therapy. A detailed description of treatment protocols, study characteristics and methodological quality assessment of the RCTs are presented in Tables 3 to 12.
TABLE 3.
Characteristics of the randomized controlled trials evaluating adjuvant chemotherapy
| Study (reference) | Adjuvant protocol and number of patients |
Patients’ baseline characteristics
|
||
|---|---|---|---|---|
| Tumour characteristics | Liver disease | Follow-up | ||
| Oral chemotherapy | ||||
| Yamamoto et al (33) | Study arm: oral HCFC 200 mg twice daily until recurrence or severe side effects developed; n=35 Control arm: no adjuvant therapy; n=32 |
UICC stage II | LCSGJ stage I–II; cirrhosis 65% | NR |
| Hasegawa et al (34) | Study arm: oral UFT 300 mg/day for 1 year; n=79 Control arm: no adjuvant therapy; n=80 |
Single nodule 70%; median tumour size 33 mm for study arm and 34 mm for control arm; vascular invasion 22% | C-P A 87%; cirrhosis 50% | Median 4.8 years |
| Xia et al (35) | Study arm: oral CAP 1000 mg/m2 twice daily for 4–6 courses (1 course consisted of 2 weeks of CAP followed by a 1-week interval); n=30 Control arm: no adjuvant therapy; n=30 |
pTNM stage I–III; single nodule 70%; tumour size >5 cm 57%; microvessels invasion 63% | C-P A 100%; cirrhosis 33% | Median 47.5 months |
| Transhepatic arterial chemotherapy | ||||
| Izumi et al (36) | Study arm: L-TAC (ADM 20 mg/m2 + MMC 10 mg/m2) with or without embolization once; n=23 Control arm: no adjuvant therapy; n=27 |
Tumour size >5 cm 76%; vascular invasion and/or intrahepatic metastasis 100% | Cirrhosis 82% | NR |
| Ueno et al (37) | Study arm: TAC (CDDP 50–80 mg/body + MMC 10 mg/body) 2–3 times (1-month intervals); n=10 Control arm: no adjuvant therapy; n=11 |
NR | NR | NR |
| Tanaka et al (38) | Study arm: TAC (CDDP 10 mg + 5-FU 250 mg) for 4 courses (one course consisted of daily TAC for 5 consecutive days and a 2-day interval); n=7 Control arm: no adjuvant therapy; n=8 |
Portal vein invasion and/or intrahepatic metastasis 100% | NR | NR |
| Systemic and transhepatic arterial chemotherapy | ||||
| Lai et al (39) | Study arm: L-TAC (CDDP 10 mg) 3 times (2-month intervals), and iv EPI 40 mg/m2, 8 doses (6-week intervals); n=30 Control arm: no adjuvant therapy; n=36 |
LCSGJ stage I–III; single nodule 60%; tumour size >5 cm 65%; vascular invasion 45% | Cirrhosis 55% | Median 28.3 months |
| Ono et al (40) | Study arm: TAC (EPI 40 mg/m2) once, followed by iv EPI 40 mg/m2 (3-month interval) and daily oral HCFC 300 mg/day for 2 years; n=29 Control arm: no adjuvant therapy; n=27 |
Mean tumour number 4.2 for study arm and 3.7 for control arm; Mean tumour size 1.4 cm for study arm and 1.2 cm for control arm vascular invasion 34% | C-P A 75%; cirrhosis 68% | NR |
5-FU 5-Fluorouracil; ADM Adramycin (doxorubicin); CAP Capecitabine; CDDP Cisplatin; C-P Child-Pugh class; EPI Epirubicin; HCFC 1-hexylcarbamoyl-5-fluoroura-cil (carmofur); iv Intravenous; LCSGJ Liver Cancer Study Group of Japan; L-TAC Transarterial chemotherapy and lipiodolization; MMC Mitomycin c; NR Not reported; pTNM Pathological tumour-node metastasis; TAC Transarterial chemotherapy; UFT Uracil-tegafur; UICC Union for International Cancer Control
TABLE 12.
Methodological quality assessment of the randomized evaluating other adjuvant therapies
| Study (reference) | Random sequence generation | Allocation concealment | Incomplete outcome data | Selective reporting |
Other bias
|
|
|---|---|---|---|---|---|---|
| Balanced in baseline characteristics | Sample size calculation | |||||
| Kuang et al (59) | Unclear: unspecified block randomization | Unclear: by an independent investigator | Unclear: ITT analysis excluded 2 randomized patients. | Unclear: the study protocol was unavailable | Yes | Yes |
| Lau et al (60,61) | Yes: computer-generated random number | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Xu et al (62) | Unclear: randomization by doctors | Yes: by sequentially numbered drug containers of identical appearance | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Liu et al (63) | Unclear: unspecified block randomization | Yes: pharmacy-controlled randomization | Unclear: ITT analysis excluded 1 patient randomized to the treatment group | Unclear: the study protocol was unavailable | Yes | Yes |
ITT Intention-to-treat
RFS
The outcome of RFS or DFS was reported in 18 trials (33–40,44,47–49, 55–59,61), four (35,56,57,59) of which also included TTR as a primary outcome. However, for the other nine trials (41,45,46,50,52–54,62,63) that reported recurrence or recurrence-free rates, TTR was used as surrogate outcome. Eight subgroup analyses were performed for RFS (Figure 2).
Figure 2).
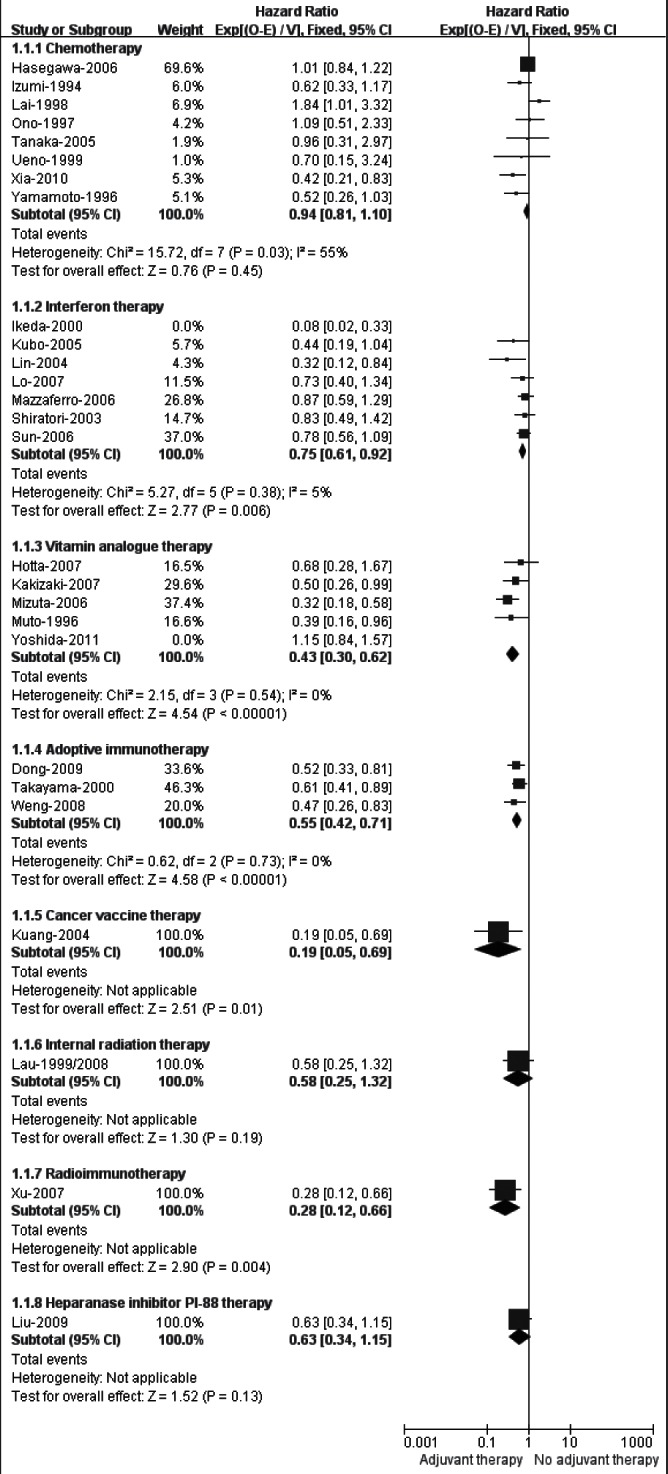
Meta-analysis (sensitivity analysis) of recurrence-free survival in randomized controlled trials evaluating adjuvant therapy
Chemotherapy:
The meta-analysis for eight trials (33–40) demonstrated that adjuvant chemotherapy failed to improve RFS compared with no treatment (HR 0.94 [95% CI 0.81 to 1.10]). Nevertheless, a significant statistical heterogeneity among the trials was found (I2=55%). After excluding any of these eight trials by sensitivity analysis, heterogeneity was consistently high (data not shown). Therefore, the eight trials were further subdivided according to the different administration routes of chemotherapy (Figure 3), and the results showed that adjuvant oral chemotherapy (HR 0.91 [95% CI 0.77 to 1.09]; I2=77%) and transhepatic arterial chemotherapy (HR 0.69 [95% CI 0.41 to 1.16]; I2=0%) did not improve RFS, while the pooled HR for two trials (39,40) testing the combination of systemic and transhepatic arterial chemotherapy marginally favoured no adjuvant therapy (HR 1.51 [95% CI 0.94 to 2.40]; I2=11%).
Figure 3).
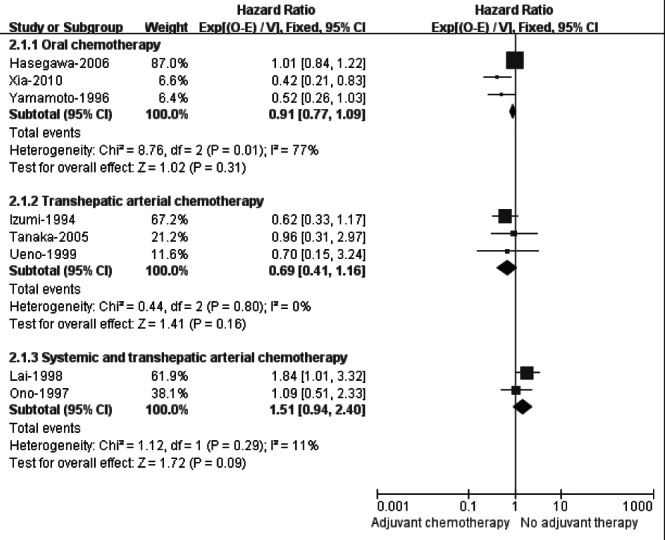
Subgroup analysis of recurrence-free survival in randomized controlled trials evaluating adjuvant chemotherapy
IFN therapy:
Seven RCTs (41–49) of adjuvant IFN therapy were pooled for RFS, and the result favoured IFN therapy (HR 0.72 [95% CI 0.59 to 0.88]; data not shown) but with an obvious heterogeneity (I2=60%). After carefully examining the characteristics of each trial, the RCT by Ikeda et al (41) appeared to be the source of the heterogeneity. This was the only trial identified that used IFN-β, and the sample sizes were small (10 patients each in IFN and control groups); it was also restricted by methodological limitations. The sensitivity analysis excluding the study (41) showed that pooled HR was 0.75 (95% CI 0.61 to 0.92; Figure 2), significantly favouring adjuvant IFN therapy with acceptable heterogeneity (I2=5%).
Vitamin analogue therapy:
The pooled analysis for five trials testing polyprenoic acid (50,51) or menatetrenone (52–55) indentified a significant RFS gain with these vitamin analogues (HR 0.76 [95% CI 0.60 to 0.96]; data not shown). However, an elevated heterogeneity was observed across the studies (I2=78%), which was likely due to the inclusion of the VK2 study by Yoshida et al (55). Despite having the largest sample size (n=548), this trial (55) enrolled 21% patients with first intrahepatic recurrence of HCC and was terminated after a maximum follow-up of only 36 months, all of which may confound the combined result. Nevertheless, restricting the analysis to the other four RCTs (50–54) did not change the effects of adjuvant vitamin analogue therapy on RFS (HR 0.43 [95% CI 0.30 to 0.62]; Figure 2), while heterogeneity was eliminated (I2=0%).
Adoptive immunotherapy:
The meta-analysis of RFS for the three RCTs (56–58) showed a significant difference favouring adoptive immunotherapy, compared with no adjuvant therapy (HR 0.55 [95% CI 0.42 to 0.71]). There was no heterogeneity among these studies (I2=0%).
Other therapeutic modalities:
Cancer vaccine therapy (59) and radioimmunotherapy (62) achieved significantly higher RFS (HR 0.19 [95% CI 0.05 to 0.69]) and longer TTR (HR 0.28 [95% CI 0.12 to 0.66]) compared with control, respectively; adjuvant therapy with 131I-lipiodol (60,61) or PI-88 (63) tended to decrease the risk of recurrence, although difference in RFS (HR 0.58 [95% CI 0.25 to 1.32]) or TTR (HR 0.63 [95% CI 0.34 to 1.15]) between adjuvant and no adjuvant therapy groups failed to reach statistical significance.
OS
Twenty-two trials (33–36,38–40,44,45,47–50,52–56,58,59,61,62) provided OS data. Nevertheless, due to the insufficient data reporting for the calculation of HR, three (47,53,55) of these 22 studies were further excluded from the analysis of the outcome. Seven subgroup analyses were performed for OS (Figure 4).
Figure 4).
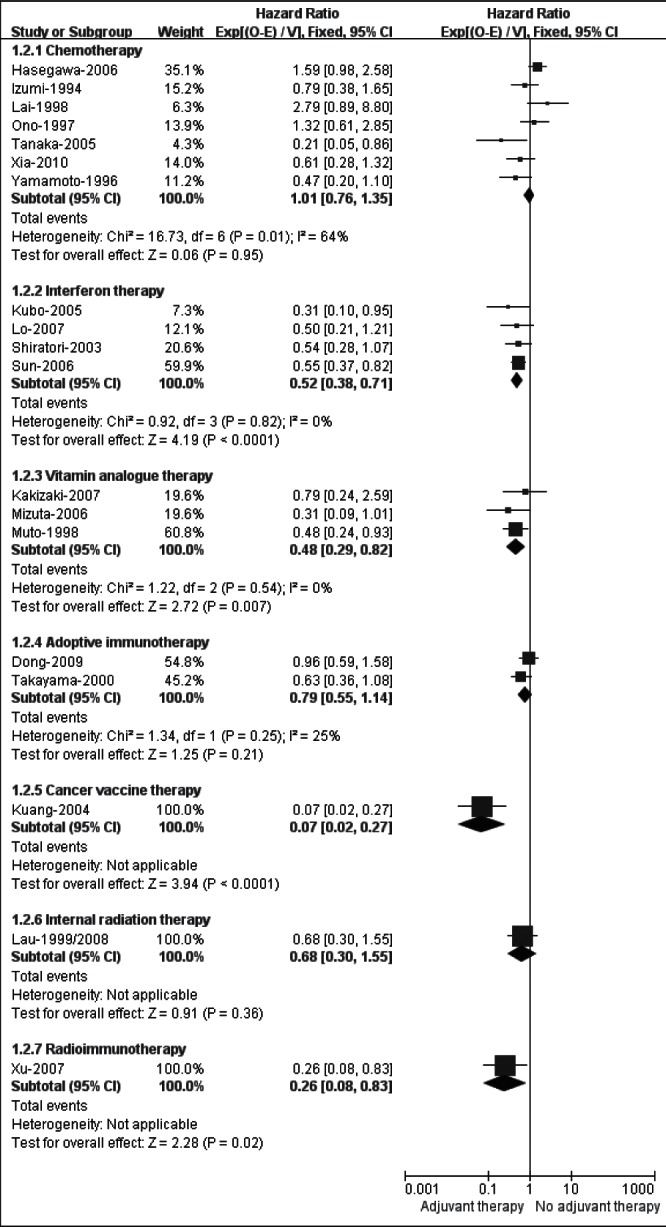
Meta-analysis of overall survival in randomized controlled trials evaluating adjuvant therapy
Chemotherapy:
The meta-analysis for seven (33–36,38–40) trials assessing adjuvant chemotherapy yielded a nonsignificant but heterogeneous result (HR 1.01 [95% CI 0.76 to 1.35]; I2=64%). The separate subgroup analysis (Figure 5) in terms of different routes of administration demonstrated that adjuvant oral (HR 1.01 [95% CI 0.70 to 1.47]; I2=75%) or transhepatic arterial chemotherapy (HR 0.59 [95% CI 0.31 to 1.14]; I2=62%) did not indentify an OS gain, while the combination of systemic and transhepatic arterial chemotherapy had a trend favouring no adjuvant therapy (HR 1.67 [95% CI 0.88 to 3.16]; I2=12%).
Figure 5).
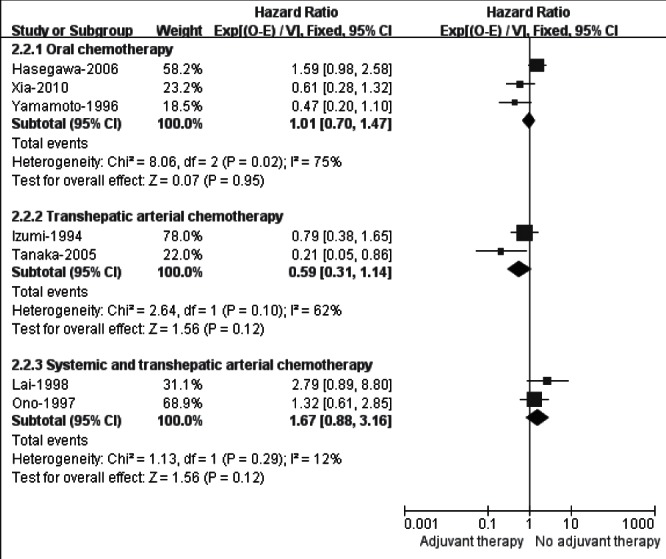
Subgroup analysis of overall survival in randomized controlled trials evaluating adjuvant chemotherapy
IFN therapy:
The pooled HR of 0.52 (95% CI 0.38 to 0.71) among four (44,45,48,49) trials in favour of adjuvant group demonstrated a convincing benefit of IFN therapy over no adjuvant therapy in OS. There was no heterogeneity among trials (I2=0%).
Vitamin analogue therapy:
The meta-analysis for three trials (51,52,54) showed that adjuvant therapy with VK2 analogue significantly improved OS compared with control, with no heterogeneity across the trials (HR 0.48 [95% CI 0.29 to 0.82]; I2=0%).
Adoptive immunotherapy:
The pooled HRs for two trials (56,58) testing adoptive immunotherapy demonstrated there was no significant difference between adjuvant and no adjuvant groups in terms of OS (HR 0.79 [95% CI 0.55 to 1.14]). An acceptable heterogeneity was observed between the trials (I2=25%).
Other therapeutic modalities:
The HR of OS for the trial of cancer vaccine therapy (59) was 0.07 (95% CI 0.02 to 0.27), and that for the radioimmunotherapy study (62) was 0.26 (95% CI 0.08 to 0.83), each of which significantly favoured adjuvant therapy. However, OS in the internal radiation trial (60,61) did not differ significantly between adjuvant and the control groups (HR 0.68 [95% CI 0.30 to 1.55]).
Side effects
All except one trial (37) reported data on side effects. In the studies of chemotherapy (33–36,38–40) and of IFN therapy (41–49), 24% (50 of 212) and 11% (35 of 328) patients in adjuvant group, respectively, developed severe toxicity or treatment-related side effects requiring a discontinuation of adjuvant therapy, while cancer vaccine (59), adoptive immunotherapy (56–58) and heparanase inhibitor PI-88 therapy (63) caused frequent but mild adverse effects. Other adjuvant modalities such as vitamin analogue therapy (50–55), internal radiation therapy (60,61) and radioimmunotherapy (62) appeared to be safe, because side effects of these therapies were barely reported in the publications.
DISCUSSION
HCC is a major health problem in hepatitis-prevalent countries such as China (1–3). Despite great improvement in diagnostic and therapeutic techniques, the long-term outcomes of HCC remain unsatisfactory, even after treatment with cure intent. From our analysis for patients receiving no active adjuvant therapy, the median five-year recurrence rate after surgical resection, liver transplantation or ablation therapy was up to 82% (range 55% to 94%), and that for survival rate at five years was 48% (range 29% to 75%). The high recurrence rate was explained by intrahepatic metastasis of primary HCC, which accounted for >79% of recurrence cases; the main cause of death after these potentially curative treatments was recurrent HCC (67% to 100%) that occurred mostly in residual liver.
The current meta-analysis included a broad spectrum of adjuvant therapies with either antitumour or chemopreventive effects to be evaluated. Adjuvant chemotherapy failed to confer any benefit to RFS and OS, regardless of what agents or administration modes were used. On the contrary, the combination of systemic and transarterial chemotherapy may have a deleterious effect on patient prognosis. Although the efficacy results of the adjuvant IFN studies were encouraging, one caveat is that the survival benefits from IFN therapy should be weighed against the risks of adverse effects. The findings of chemo-prevention with vitamin analogues require further examination because the meta-analysis of RFS and of OS was performed only in a limited number of small RCTs with methodological weaknesses. Adoptive immunotherapy with either LAK or CIK cells may be as promising a strategy as adjuvant therapy for HCC because it increased the RFS by 45% and resulted in few adverse effects, although the approaches are too cumbersome and costly for use in large clinical trials. Nevertheless, follow-up periods in these trials (56–58) were too short to confer a statistically significant benefit in OS. Postoperative adjuvant therapy using either transhepatic arterial 131I-lipiodol infusion or subcutaneous injection of heparanase inhibitor PI-88 did not appear able to decrease recurrence and to improve survival, while cancer vaccine therapy and Licartin radioimmunotherapy showed some promise after radical surgery for HCC. However, each of these modalities was examined in single trial with small-scale and preliminary settings.
The results of the current meta-analysis suggest that the effectiveness of an adjuvant modality depends largely on front-line therapy for HCC. In fact, because the cancer is insensitive to chemotherapy and radiotherapy (66), adjuvant use of any chemotherapeutic agent or radioactive material may not be effective. Furthermore, the beneficial effects of adjuvant IFN therapy on recurrence and survival may contribute to its efficacy in preventing hepatitis from developing into HCC (67,68). On the other hand, the lack of effective therapeutic agents remains the main challenge in the provision of adjuvant therapy for HCC (9) because the presence of underlying cirrhosis limits the capability of remnant liver insulted by initially curative treatment to tolerate any adjuvant cytotoxic therapy. Nevertheless, because intrahepatic recurrence can either represent metastasis from primary HCC or de novo tumour formation in a cirrhotic liver, an agent or regimen with both tumouricidal and chemopreventive effects, but less toxicity, may be effective to prevent recurrence after curative treatment for HCC (7,61,69). In the current analysis, however, the effect of adjuvant therapy on metachronous de novo carcinogenesis may have been obscured because of the presence of recurrence resulting from intrahepatic metastases of HCC, and because most of the trials lacked a precise description of the difference between primary metastasis and de novo HCC. For future trials, therefore, measures for metastatic recurrence in the residual liver may be necessary, and molecular diagnostic techniques, such as comparative genomic hybridization, integration pattern of HBV, or DNA fingerprinting using loss of heterozygosity assays or microarray analysis (70–72) are recommended, if feasible, to differentiate the two types of recurrence.
Theoretically, reduced recurrence is expected to improve survival. Nevertheless, several trials (38,44,48) found an improvement in OS but not in RFS. These were studies that had relatively small number of participants and insufficient follow-up, and there was little consideration of statistical power in the design. These limitations, however, may prevent or delay recognition of potentially beneficial therapies. Furthermore, effects of adjuvant therapy may differ over time. This was apparent in studies involving polyprenoic acid (50,51) and internal radiation therapy (60,61), in which the benefits in decreasing recurrence became more evident or were lost after expending follow-up visits. Therefore, larger sample sizes and longer periods of observation are emphasized for future trials.
For trials testing adjuvant therapy, any adjuvant effect should be evaluated independently from other potential effects. The database searches initially identified 19 reports on adjuvant therapies in which randomization was performed before patients underwent mainly curative resection for HCC (Figure 1). Although postoperative recurrence was addressed, these studies were not considered for inclusion because adjuvant effects in treatment group may have been confounded by the introduction of initially curative treatment. Also, it is crucial to distinguish recurrent disease after a curative treatment from residual tumour after a palliative therapy when testing an adjuvant therapy that aims primarily to decrease the recurrence of HCC. Therefore, proper documentation of curability of initial treatment for HCC is necessary. The curability in most of the trials included was defined as the complete elimination or necrosis of all macroscopically detectable tumours, with no demonstrable evidence of residual or recurrent tumours on image studies before initiation of adjuvant therapy. However, it was either not described at all, or insufficiently described by other trials (34,38,41,55,62), which may have resulted in inadvertent enrollment of patients with residual HCC before randomization. Nevertheless, given that there have been no established assessment criteria of curability after surgical resection or ablation therapy, a more stringent definition of curability is required.
One of the major weakness of the present meta-analysis is that the generalizability of the results was limited by predominantly including RCTs conducted among Japanese and Chinese patients. Conceivably, there could be differences in the natural history of HCC among geographical regions, although these potential differences have not been well understood. Therefore, it is possible that the findings of the current meta-analysis may not be extrapolated to the non-Asian population.
Another limitation is that the study failed to detect the impact of underlying liver disease, particularly cirrhosis, on survival outcomes. A meta-regression analysis could be helpful to capture the effect of liver cirrhosis on survival. Nevertheless, it is hardly believable that a meta-regression analysis using cirrhosis as a covariate could affect the overall results of the current study because the majority of deaths were due to HCC recurrence rather than to liver cirrhosis (Figure 2). On the other hand, the use of RFS as primary outcome could potentially confound effects of adjuvant therapies on survival by involving death from liver failure. In the current meta-analysis, we initially planned to include TTR as the primary outcome. However, because only a few studies reported TTR (35,56,57,59) or presented data for estimating the HR of the outcome (41,45,46,52–54,62), RFS was used as a surrogate. Furthermore, we found the difference in the estimates of the HR between TTR and RFS in the trials (35,56,57,59) that reported either of the outcomes was minor (data not shown). Again, RFS or DFS may be preferable to TTR as a correlate of OS, because it is able to capture fatal toxicity in trials where the majority of deaths are expected to be related to cancer (73). Nevertheless, given the confounding composite nature of RFS that concerns not only recurrence but also death from any cause, death resulting from the natural history of cirrhosis may confound detection of potential benefits from adjuvant therapy (8,74). Therefore, the overall results of RFS in the meta-analysis should be interpreted with care, and TTR is recommended for further adjuvant trials in HCC.
Third, the present study has the typical weakness of an aggregated data meta-analysis of time-to-event outcomes. Of the 31 study reports included, only four (34,35,55,59) directly presented HR and associated 95% CIs for RFS or OS, while results of these outcomes for most of the trials were obtained by performing calculations using other statistics or data extracted from published survival curves (25,26). Bias may have been produced in estimating the HR with the use of these indirect methods, which may, however, partially explain the statistical heterogeneity among individual comparisons. Nevertheless, in the absence of necessary statistics, a meta-analysis based on data from published curves can be the only practical alternative (25). Future publications reporting time-to-event outcomes should, therefore, provide more detailed statistical information, preferably in the form of the results of log HRs and their variances, or their estimators (75).
The current meta-analysis assessed methodological quality of the included RCTs using rigorous criteria proposed by the Cochrane systematic review (31). As a result, only nine RCTs included were assessed as having adequate quality (34,44,47–50,55,61,62), while nearly one-half of the trials were subject to methodological weakness. Hence, the overall results of the present meta-analysis were prone to bias that was incurred from the bias of these original studies. Therefore, more prospective studies of good methodological quality are needed for adjuvant HCC clinical trials.
CONCLUSIONS
The prognosis of patients with HCC after potentially curative treatment is poor. Adjuvant IFN therapy can improve both RFS and OS, but the benefits of using this agent should be weighed against side effects. Combination of systemic and transhepatic arterial chemotherapy is not currently recommended for patients with HCC after potentially curative treatment. Other adjuvant therapies produce limited success for survival. Further well-designed RCTs with sufficient sample size and follow-up are required to establish the role of adjuvant therapies for HCC, and new agents or regimens with both antitumor and chemopreventive effects are expected.
TABLE 4.
Methodological quality assessment of the randomized controlled trials evaluating adjuvant chemotherapy
| Study (reference) | Random sequence generation | Allocation concealment | Incomplete outcome data | Selective reporting |
Other bias
|
|
|---|---|---|---|---|---|---|
| Balanced in baseline characteristics | Sample size calculation | |||||
| Yamamoto et al (33) | Unclear: only stated as random | Yes: by central office telephone | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Hasegawa et al (34) | Yes: minimization randomization | Unclear: by an independent investigator | Unclear: ITT analysis excluded 1 patient randomly assigned to the treatment arm | Yes: all expected outcomes were reported in accordance with the study protocol | Yes | Yes |
| Xia et al (35) | Unclear: only stated as random | Unclear: unspecified sealed envelope procedure | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Izumi et al (36) | Unclear: only stated as random | Unclear: only stated as random | Unclear: 2 patients dropped out of the treatment arm were reported, but not resolved | Unclear: the study protocol was unavailable | Yes | No |
| Ueno et al (37) | Unclear: unspecified block randomization | Unclear: unspecified sealed envelope procedure | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Tanaka et al (38) | Unclear: only stated as random | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Lai et al (39) | Unclear: only stated as random | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Ono et al (40) | Unclear: only stated as random | Unclear: unspecified sealed envelope procedure | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
ITT Intention-to-treat
TABLE 5.
Characteristics of the randomized controlled trials evaluating adjuvant interferon therapy
| Study (reference) | Adjuvant protocol and number of patients |
Patients’ baseline characteristics
|
||
|---|---|---|---|---|
| Tumour characteristics | Liver disease | Follow-up | ||
| Ikeda et al (41) | Study arm: IFN-β 6 MU twice a week for 36 months; n=10 Control arm: no adjuvant therapy; n = 10 |
Single nodule 90%; median tumour size 22 mm for study arm and 20 mm for control arm; vascular invasion 0% | HCV 100%; cirrhosis 85% | Median 25 month |
| Kubo et al (42–44) | Study arm: daily IFN-α 6 MU for 2 weeks, then three times per week for 14 weeks, followed by twice weekly for 88 weeks; n=15 Control arm: no adjuvant therapy; n=15 |
Single tumour 100%; tumour size ≤5 cm 100% | HCV 100%; C-P A 77%; cirrhosis 50% | 5.0 years for study arm and 4.1 years for control arm |
| Shiratori et al (45) | Study arm: IFN-α 6 MU three times a week for 48 months; n=49 Control arm: no adjuvant therapy; n=25 |
Single tumour 65%; tumour size ≤30 mm 100% | HCV 100%; C-P A 100%; cirrhosis 100% | Mean 7.1 years |
| Lin et al (46) | Study arm 1: IFN-α 3 MU thrice a week for 24 months; n=11 Study arm 2: IFN-α 3 MU 10 times a month for 6 months, followed by 3 MU 10 times every 3 months for 18 months; n=9 Control arm: no adjuvant therapy; n=10 |
Single tumour 83%; median tumour size 20–25 mm | HBV 53%; C-P A 87%; cirrhosis 93% | Median 27 months |
| Mazzaferro et al (47) | Study arm: IFN-α-2b 3 MU thrice a week for 48 weeks; n=76 Control arm: no adjuvant therapy; n=74 |
pTNM stage III–IV 39%; single tumour 76%; median tumour size 35 mm; vascular invasion 21% | HCV 100%; C-P A 93%; cirrhosis 100% | Median 45 months |
| Sun et al (48) | Study arm: IFN-α-1b 3 MU twice a week for two weeks, followed by 5 MU thrice a week for 18 months; n=118 Control arm: no adjuvant therapy; n=118 |
Single tumour 87%; tumor size ≤5 cm 69%; microvessel invasion 76% | HBV 100%; cirrhosis 86% | Median 36.5 months |
| Lo et al (49) | Study arm 1: IFN-α-2b 10 MU three times per week for 16 months; n=40 Study arm 2: IFN-α-2b 30 MU three times per week for 16 months; n=6 Control arm: no adjuvant therapy; n=40 |
pTNM stage III–IVA 51%; single tumour 79%; Tumour size >5 cm 51%; vascular invasion 44% | HBV 91%; cirrhosis 50% | 30–71 months |
C-P Child Child-Pugh class; HBV Hepatitis B virus; HCV Hepatitis C virus; IFN Interferon; MU Million units; pTNM Pathological tumour-node-metastasis
TABLE 6.
Methodological quality assessment of the randomized controlled trials evaluating adjuvant interferon therapy
| Study (reference) | Random sequence generation | Allocation concealment | Incomplete outcome data | Selective reporting |
Other bias
|
|
|---|---|---|---|---|---|---|
| Balanced in base-line characteristics | Sample size calculation | |||||
| Ikeda et al (41) | Unclear: only stated as random | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Kubo et al (42–44) | Yes: from a random-numbers table | Unclear: assignments were withheld from the investigators | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Shiratori et al (45) | Unclear: from an unspecified random list (2:1 assignment ratio) | Unclear: by an independent investigator | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Lin et al (46) | Unclear: only stated as random | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Mazzaferro et al (47) | Yes: computer-generated random number | Yes: by central office telephone | Unclear: ITT analysis excluded 1 patient randomized to the treatment arm | Yes: all expected outcomes were reported in accordance with the study protocol | Yes | Yes |
| Sun et al (48) | Yes: computer-generated random number | Unclear: unspecified sealed envelope procedure | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Lo et al (49) | Unclear: only stated as random | Yes: sealed envelope procedure, ensured by a research assistant | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
ITT Intention-to-treat
TABLE 7.
Characteristics of the randomized controlled trials evaluating adjuvant vitamin analogue therapy
| Study (reference) | Adjuvant protocol and number of patients |
Patients’ baseline characteristics
|
||
|---|---|---|---|---|
| Tumour characteristics | Liver disease | Follow-up | ||
| Vitamin A analogue | ||||
| Muto et al (50,51) | Study arm: oral polyprenoic acid 600 mg/day for 12 months; n=44 Control arm: the same dosage of placebo; n=45 |
LCSGJ stage I–III; mean tumour number 1.5 for study arm and 1.4 for control arm; mean tumour size 2.9 cm for study arm and 3.0 cm for control arm | HCV 75% | Median 38 or 62 months |
| Vitamin K2 analogue | ||||
| Mizuta et al (52) | Study arm: oral menatetrenone 45 mg/day until recurrence; n=32 Control arm: no adjuvant therapy; n=29 |
LCSGJ stage I–III; mean tumour number 1.5; mean tumour size 18 mm2 for study arm and 19 mm2 for control arm; vascular invasion 0% | C-P A 79%; HCV 89% | Median 28.9 months for study arm and 27.7 months for control arm |
| Hotta et al (53) | Study arm: oral menatetrenone 45 mg/day until recurrence; n=21 Control arm: no adjuvant therapy; n=24 |
Single tumour 44%; mean tumour size ≤3 cm 80% | C-P A 60%; HC V 73% | Median 19.5 months for study arm and 16.5 months for control arm |
| Kakizaki et al (54) | Study arm: oral menatetrenone 45 mg/day until recurrence; n=30 Control arm: no adjuvant therapy; n=30 |
LCSGJ stage I–III; single tumour 68%; mean tumour size 20 mm for study arm and 25 mm for control arm; vascular invasion 0% | C-P A 73%; HCV 100% | NR |
| Yoshida et al (55) | Study arm 1: oral menatetrenone 45 mg/day until recurrence; n=182 Study arm 2: oral menatetrenone 90 mg/day until recurrence; n=185 Control arm: the same dosage of placebo; n=181 |
Single tumour 71%; median tumour size 19 mm; vascular invasion 0% | C-P A 87%; cirrhosis 78%; HCV 83% | NR |
C-P Child Child-Pugh class; HCV Hepatitis C virus; LCSGJ Liver Cancer Study Group of Japan; NR Not reported
TABLE 8.
Methodological quality assessment of the randomized controlled trials evaluating adjuvant vitamin analogue therapy
| Study (reference) | Random sequence generation | Allocation concealment | Incomplete outcome data | Selective reporting |
Other bias
|
|
|---|---|---|---|---|---|---|
| Balanced in baseline characteristics | Sample size calculation | |||||
| Muto et al (50,51) | Unclear: unspecified block randomization | Yes: by sequentially numbered drug containers of identical appearance | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Mizuta et al (52) | Yes: from a table of random permutations | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | No: patients in the treatment arm had significantly lower serum DCP level | No |
| Hotta et al (53) | Unclear: from an unspecified random list | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
| Kakizaki et al (54) | Unclear: only stated as random | Unclear: only stated as random | Unclear: ITT analysis for patients treated rather than randomized | Unclear: the study protocol was unavailable | Yes | No |
| Yoshida et al (55) | Yes: minimization randomization | Yes: by sequentially numbered drug containers of identical appearance | Yes: efficacy analysis by ITT | Yes: all expected outcomes were reported in accordance with the study protocol | No: patients in the two treatment arms had significantly higher serum TBIL and AST levels | Yes |
AST Aspartate aminotransferase; DCP Des-gamma-carboxy prothrombin; ITT Intention-to-treat; TBIL Total bilirubin
TABLE 9.
Characteristics of the randomized controlled trials evaluating adjuvant adoptive immunotherapy
| Study (reference) | Adjuvant protocol and number of patients |
Patients’ baseline characteristics
|
||
|---|---|---|---|---|
| Tumor characteristics | Liver disease | Follow-up | ||
| Takayama et al (56) | Study arm: iv LAK cell-based immunotherapy for 5 times (at weeks 2, 3, 4, 12, and 24 after curative treatment); n=76 Control arm: no adjuvant therapy; n=74 |
Single tumour 69%; tumour size ≥3 cm 53%; intrahepatic metastasis or vascular invasion 56% | C-P A 69%; cirrhosis 51%; HCV 66% | Median 4.4 years |
| Weng et al (57) | Study arm: Transhepatic arterial CIK cell-based immunotherapy for 8–10 times (2-week intervals); n=45 Control arm: no adjuvant therapy; n=40 |
Tumour size ≥5 cm 56%; vascular invasion 0% | C-P A 81% | 18 months |
| Hui et al (58) | Study arm 1: iv CIK cell-based immunotherapy for 3 times (2-week intervals); n=41 Study arm 2: iv CIK cell-based immunotherapy for 6 times (2-week intervals); n=43 Control arm: no adjuvant therapy; n=43 |
Single tumour 100%; tumour size ≥5 cm 55%; vascular invasion 46% | C-P A 80%; cirrhosis 80%; HBV 76% | 5–7 years |
C-P Child Child-Pugh class; CIK Cytokine-induced killer; HBV Hepatitis B virus; HCV Hepatitis C virus; iv Intravenous; LAK Lymphokine activated killer;
TABLE 10.
Methodological quality assessment of the randomized controlled trials evaluating adjuvant adoptive immunotherapy
| Study (reference) | Random sequence generation | Allocation concealment | Incomplete outcome data | Selective reporting |
Other bias
|
|
|---|---|---|---|---|---|---|
| Balanced in base-line characteristics | Sample size calculation | |||||
| Takayama et al (56) | Unclear: unspecified block randomization | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | Yes |
| Weng et al (57) | Unclear: only stated as random | Unclear: only stated as random | Yes: analysis by ITT | Unclear: result of TTR was not presented | Yes | Yes |
| Hui et al (58) | Yes: by drawing of lots | Unclear: only stated as random | Yes: analysis by ITT | Unclear: the study protocol was unavailable | Yes | No |
ITT Intention-to-treat; TTR Time to recurrence
TABLE 11.
Characteristics of the randomized controlled trials evaluating other adjuvant therapies
| Study (reference) | Adjuvant protocol and number of patients |
Patients’ baseline characteristics
|
|||
|---|---|---|---|---|---|
| Tumour characteristics | Liver disease | Follow-up | |||
| Cancer vaccine therapy | |||||
| Kuang et al (59) | Study arm: intradermal injection of autologous formalin-fixed tumour vaccine for 3 courses (one course consisted of 5 injections of the tumour vaccine followed by a two-week interval); n=18 Control arm: no adjuvant therapy; n=21 |
TNM stage I–IIIA; single tumour 95%; tumour size ≥5 cm 51%; intrahepatic metastasis or vascular invasion 33% | C-P A 92%; cirrhosis 54%; HBV 90% | Median 15 months | |
| Internal radiation therapy | |||||
| Lau et al (60, 61) | Study arm: transhepatic arterial infusion of iodine 131-labelled lipiodol (1850 MBq) for one time; n=21 Control arm: no adjuvant therapy; n=22 |
Median tumour size 4.4 cm for study arm and 3.8 cm for control arm; vascular invasion 5% | Okuda stage I–II; HCV 88% | Median 34.6 or 66 months | |
| Antibody targeted radioimmunotherapy | |||||
| Xu et al (62) | Study arm: iv hLicartin*† 15.4 MBq/kg for 3 times (28-day intervals); n=30 Control arm: the same dosage of placebo; n=30 |
TNM stage III–IV; single lesion with tumour size >5 cm 72%; multiple lesion with tumour size >3 cm 28%; portal vein cancer thrombi 45% | C-P A 43%; cirrhosis 100%; HBV 88% | Median 12.3 months | |
| Heparanase inhibitor PI-88 therapy | |||||
| Liu et al (63) | Study arm 1: subcutaneous injection of PI-88 160 mg/day for 9 courses (one course consisted of consecutive 4 days of daily PI-88 for 3 weeks and a one-week interval); n=56 Study arm 2: PI-88 250 mg/day given with the same protocol as arm 1; n=54 Control arm: no adjuvant therapy; n=58 |
Single tumour 85%; mean tumour size 4.9 cm for study arm 1, 5.1 cm for study arm 2 and 5.5 cm for control arm; vascular invasion 23% | C-P A 96%; cirrhosis 56%; HBV 73% | 379–381 days | |
Iodine 131-labelled metuximab (HAb18 F[ab′]2).
Chengdu Hoist Hitech Co Ltd, China; and the Fourth Military Medical University, Xi’an, China. C-P Child Child-Pugh class; iv Intravenous.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest ot declare.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582–3. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 3.Tang ZY, Ye SL, Liu YK, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: Univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–43. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: Consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz JD, Schwartz M, Mandeli J, et al. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: Review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun HC, Tang ZY. Preventive treatments for recurrence after curative resection of hepatocellular carcinoma – a literature review of randomized control trials. World J Gastroenterol. 2003;9:635–40. doi: 10.3748/wjg.v9.i4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathurin P, Raynard B, Dharancy S, et al. Meta-analysis: Evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17:1247–61. doi: 10.1046/j.1365-2036.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 13.Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/ adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: A systematic review. Hepatobiliary Pancreat Dis Int. 2009;8:124–33. [PubMed] [Google Scholar]
- 14.Samuel M, Chow PK, Chan Shih-Yen E, et al. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009:CD001199. doi: 10.1002/14651858.CD001199.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono T, Yamanoi A, Nazmy El Assal O, et al. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: Metaanalysis of three randomized controlled trials. Cancer. 2001;91:2378–85. [PubMed] [Google Scholar]
- 16.Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2010;40:943–53. doi: 10.1111/j.1872-034X.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 17.Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975–81. doi: 10.1002/bjs.6731. [DOI] [PubMed] [Google Scholar]
- 18.Zhang CH, Xu GL, Jia WD, et al. Effects of interferon alpha treatment on recurrence and survival after complete resection or ablation of hepatocellular carcinoma: A meta-analysis of randomized controlled trials. Int J Cancer. 2009;124:2982–8. doi: 10.1002/ijc.24311. [DOI] [PubMed] [Google Scholar]
- 19.Miao RY, Zhao HT, Yang HY, et al. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: A meta-analysis. World J Gastroenterol. 2010;16:2931–42. doi: 10.3748/wjg.v16.i23.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake Y, Takaki A, Iwasaki Y, et al. Meta-analysis: Interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287–92. doi: 10.1111/j.1365-2893.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 21.Shen YC, Hsu C, Chen LT, et al. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): A meta-regression approach. J Hepatol. 2010;52:889–94. doi: 10.1016/j.jhep.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Singal AK, Freeman DH, Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851–8. doi: 10.1111/j.1365-2036.2010.04414.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: The efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–12. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 24.Chu KJ, Lai EC, Yao XP, et al. Vitamin analogues in chemoprevention of hepatocellular carcinoma after resection or ablation – a systematic review and meta-analysis. Asian J Surg. 2010;33:120–6. doi: 10.1016/S1015-9584(10)60021-8. [DOI] [PubMed] [Google Scholar]
- 25.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–51. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 28.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7. [PubMed] [Google Scholar]
- 29.Poon RT, Fan ST, Lo CM, et al. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094–101. doi: 10.1200/JCO.2000.18.5.1094. [DOI] [PubMed] [Google Scholar]
- 30.Shimada M, Yamashita Y, Hamatsu T, et al. Surgical indications for advanced hepatocellular carcinoma. Hepatogastroenterology. 2000;47:1095–9. [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. < www.cochrane-handbook.org> (Accessed January 2012). [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto M, Arii S, Sugahara K, et al. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83:336–40. doi: 10.1002/bjs.1800830313. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa K, Takayama T, Ijichi M, et al. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: A randomized trial. Hepatology. 2006;44:891–5. doi: 10.1002/hep.21341. [DOI] [PubMed] [Google Scholar]
- 35.Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: A randomized controlled trial. Ann Surg Oncol. 2010;17:3137–44. doi: 10.1245/s10434-010-1148-3. [DOI] [PubMed] [Google Scholar]
- 36.Izumi R, Shimizu K, Iyobe T, et al. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295–301. [PubMed] [Google Scholar]
- 37.Ueno S, Tanabe G, Yoshida A, et al. Postoperative prediction of and strategy for metastatic recurrent hepatocellular carcinoma according to histologic activity of hepatitis. Cancer. 1999;86:248–54. [PubMed] [Google Scholar]
- 38.Tanaka S, Shimada M, Shirabe K, et al. A novel intrahepatic arterial chemotherapy after radical resection for advanced hepatocellular carcinoma. Hepatogastroenterology. 2005;52:862–5. [PubMed] [Google Scholar]
- 39.Lai EC, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: A randomized controlled trial. Arch Surg. 1998;133:183–8. doi: 10.1001/archsurg.133.2.183. [DOI] [PubMed] [Google Scholar]
- 40.Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: A prospective randomized study. Semin Oncol. 1997;24:S6-18–S6-25. [PubMed] [Google Scholar]
- 41.Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228–32. doi: 10.1053/jhep.2000.9409. [DOI] [PubMed] [Google Scholar]
- 42.Kubo S, Nishiguchi S, Hirohashi K, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963–7. doi: 10.7326/0003-4819-134-10-200105150-00010. [DOI] [PubMed] [Google Scholar]
- 43.Kubo S, Nishiguchi S, Hirohashi K, et al. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418–22. doi: 10.1046/j.0007-1323.2001.02054.x. [DOI] [PubMed] [Google Scholar]
- 44.Nishiguchi S, Tamori A, Kubo S. Effect of long-term postoperative interferon therapy on intrahepatic recurrence and survival rate after resection of hepatitis C virus-related hepatocellular carcinoma. Intervirology. 2005;48:71–5. doi: 10.1159/000082098. [DOI] [PubMed] [Google Scholar]
- 45.Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299–306. doi: 10.7326/0003-4819-138-4-200302180-00008. [DOI] [PubMed] [Google Scholar]
- 46.Lin SM, Lin CJ, Hsu CW, et al. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376–82. doi: 10.1002/cncr.20004. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaferro V, Romito R, Schiavo M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–54. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 48.Sun HC, Tang ZY, Wang L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: A randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458–65. doi: 10.1007/s00432-006-0091-y. [DOI] [PubMed] [Google Scholar]
- 49.Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–42. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–7. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 51.Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med. 1999;340:1046–7. doi: 10.1056/NEJM199904013401315. [DOI] [PubMed] [Google Scholar]
- 52.Mizuta T, Ozaki I, Eguchi Y, et al. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: A pilot study. Cancer. 2006;106:867–72. doi: 10.1002/cncr.21667. [DOI] [PubMed] [Google Scholar]
- 53.Hotta N, Ayada M, Sato K, et al. Effect of vitamin K2 on the recurrence in patients with hepatocellular carcinoma. Hepatogastroenterology. 2007;54:2073–7. [PubMed] [Google Scholar]
- 54.Kakizaki S, Sohara N, Sato K, et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol. 2007;22:518–22. doi: 10.1111/j.1440-1746.2007.04844.x. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida H, Shiratori Y, Kudo M, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532–40. doi: 10.1002/hep.24430. [DOI] [PubMed] [Google Scholar]
- 56.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet. 2000;356:802–7. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 57.Weng DS, Zhou J, Zhou QM, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63–71. doi: 10.1097/CJI.0b013e31815a121b. [DOI] [PubMed] [Google Scholar]
- 58.Hui D, Qiang L, Jian W, et al. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Kuang M, Peng BG, Lu MD, et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574–9. doi: 10.1158/1078-0432.ccr-03-0071. [DOI] [PubMed] [Google Scholar]
- 60.Lau WY, Leung TW, Ho SK, et al. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: A prospective randomised trial. Lancet. 1999;353:797–801. doi: 10.1016/s0140-6736(98)06475-7. [DOI] [PubMed] [Google Scholar]
- 61.Lau WY, Lai EC, Leung TW, et al. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: A prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43–8. doi: 10.1097/SLA.0b013e3181571047. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Shen ZY, Chen XG, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45:269–76. doi: 10.1002/hep.21465. [DOI] [PubMed] [Google Scholar]
- 63.Liu CJ, Lee PH, Lin DY, et al. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: A randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50:958–68. doi: 10.1016/j.jhep.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 64.Pan CC, Huang ZL, Li W, et al. Serum alpha-fetoprotein measurement in predicting clinical outcome related to autologous cytokine-induced killer cells in patients with hepatocellular carcinoma undergone minimally invasive therapy. Chin J Cancer. 2010;29:596–602. doi: 10.5732/cjc.009.10580. [DOI] [PubMed] [Google Scholar]
- 65.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avila MA, Berasain C, Sangro B, et al. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866–84. doi: 10.1038/sj.onc.1209550. [DOI] [PubMed] [Google Scholar]
- 67.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 68.Ikeda K, Saitoh S, Suzuki Y, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: A pilot study. Cancer. 1998;82:827–35. doi: 10.1002/(sici)1097-0142(19980301)82:5<827::aid-cncr5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 69.Kumada T, Nakano S, Takeda I, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1053/jhep.1997.v25.pm0008985270. [DOI] [PubMed] [Google Scholar]
- 70.Chen YJ, Yeh SH, Chen JT, et al. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119:431–40. doi: 10.1053/gast.2000.9373. [DOI] [PubMed] [Google Scholar]
- 71.Ng IO, Guan XY, Poon RT, et al. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol. 2003;199:345–53. doi: 10.1002/path.1287. [DOI] [PubMed] [Google Scholar]
- 72.Cheung ST, Chen X, Guan XY, et al. Identify metastasis-associated genes in hepatocellular carcinoma through clonality delineation for multinodular tumor. Cancer Res. 2002;62:4711–21. [PubMed] [Google Scholar]
- 73.Sargent D. General and statistical hierarchy of appropriate biologic endpoints. Oncology (Williston Park) 2006;20:5–9. [PubMed] [Google Scholar]
- 74.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 75.Michiels S, Piedbois P, Burdett S, et al. Meta-analysis when only the median survival times are known: A comparison with individual patient data results. Int J Technol Assess Health Care. 2005;21:119–25. doi: 10.1017/s0266462305050154. [DOI] [PubMed] [Google Scholar]


