Abstract
Objectives
Guidelines for the management of patients with metastatic bone disease (MBD) have been available to the orthopaedic community for more than a decade, with little improvement in service provision to this increasingly large patient group. Improvements in adjuvant and neo-adjuvant treatments have increased both the number and overall survival of patients living with MBD. As a consequence the incidence of complications of MBD presenting to surgeons has increased and is set to increase further. The British Orthopaedic Oncology Society (BOOS) are to publish more revised detailed guidelines on what represents ‘best practice’ in managing patients with MBD. This article is designed to coincide with and publicise new BOOS guidelines and once again champion the cause of patients with MBD.
Methods
A series of short cases highlight common errors frequently being made in managing patients with MBD despite the availability of guidelines.
Results
Despite guidelines for the management of patients with MBD being available for more than a decade basic errors in management continue to be made, affecting patient survival and quality of life.
Conclusions
It is hoped that by publicising the new BOOS guidelines the management of patients with MBD will improve over the next decade, significantly more than it has over the last decade.
Keywords: Metastatic bone disease, Guidelines, Harrington, Endoprosthesis, BOOS, Guidelines
Article focus
Highlighting the continued errors being made in managing patients with metastatic bone disease (MBD) despite guidelines being available
Publicising new guidelines with the hope that this enlarging patient group will be managed better in the next decade
Key messages
Patients with MBD are living longer and will present more frequently to the orthopaedic surgeon. More ‘aggressive’ intervention reduces overall dependence on medical services
Improvements in reconstruction techniques facilitate better quality of life than those previously offered with many clinicians being unaware of current management options
Definitive referral pathways should exist with MBD being managed by appropriately trained surgeons in appropriate institutions supported by a multidisciplinary team
Strengths and limitations
This is a level-IV evidence study describing common errors being made in the management of patients with MBD
The authors are from a ‘world-renowned’ institution frequently involved in the ‘salvage’ of patients with MBD previously managed at other institutions
Introduction
Metastatic bone disease (MBD) results from the spread of a primary cancer to bone and represents the most common form of bone cancer. It is not age-specific and affects young adults and the elderly populations alike with potentially devastating effects.
In recent years advances in adjuvant and neo-adjuvant treatments for patients with cancer has had two main effects. First, the number of patients living with MBD has increased significantly, and secondly the overall survival of patients with MBD has increased. These have resulted in a greatly increased incidence of complications of MBD presenting to orthopaedic surgeons. Despite this, the vast majority of orthopaedic surgeons have little experience in managing such patients resulting in potentially sub-optimal treatment and outcomes.
In 1999 the British Society of Surgical Oncology published guidelines on the management of patients with MBD in the United Kingdom.1 In 2001, these guidelines were formally adopted by the British Orthopaedic Association (BOA) to provide the general orthopaedic community guidance in terms of ‘best practice’ in the management of patients with MBD.2 In addition and more recently, the National Institute for Health and Clinical Excellence has published guidelines on the management and treatment of patients with metastatic spinal cord compression3 and malignant metastatic disease of unknown primary origin.4
The NHS is currently evolving from a service provider with volume-based priorities to one striving for ‘Equity and Excellence’ where quality of patient care, improved patient outcome and overall patient experience are paramount. Lord Darzi5 initiated this process with the development of Best Practice Tariffs in cohorts of patients with common problems in which huge discrepancies in treatment and outcome were identified. Patients with MBD represent one such patient group.
This annotation comes at a time when a working party from the British Orthopaedic Oncology Society is about to publish more detailed guidelines on the management of patients with MBD. A decade of guidelines has to date still not achieved the objective of providing optimal care to this growing patient group. Errors that ultimately affect patient quality of life and survival remain unfortunately common events, reflected by this series of short cases treated in a single unit in the last 12 months. The purpose of this article is to champion the cause of this group of patients and to advise that guidance on best practice is available.
Case 1
A 68-year-old man with a previous history of nephrectomy for renal cell carcinoma presented to a Major Trauma Centre fracture clinic complaining of pain in the left hip. There was no history of metastatic spread and imaging showed a solitary metastasis in the proximal femur. Without biopsy the patient underwent uncomplicated prophylactic intramedullary nailing with post-operative radiotherapy.
The patient’s pain persisted and he presented 18 months later to a specialist Orthopaedic Oncology unit. Radiographs showed significant disease progression with risk of impending implant fracture (Fig. 1a). MRI showed a large proximal tumour mass with distal spread of disease. No further metastatic sites were identified. The patient underwent selective embolisation of the proximal metastatic deposit (Fig. 1b) and total femoral replacement (Fig. 1c). The patient is alive and mobilising fully weight-bearing at six months post-operatively.
Figs. 1a - 1c.
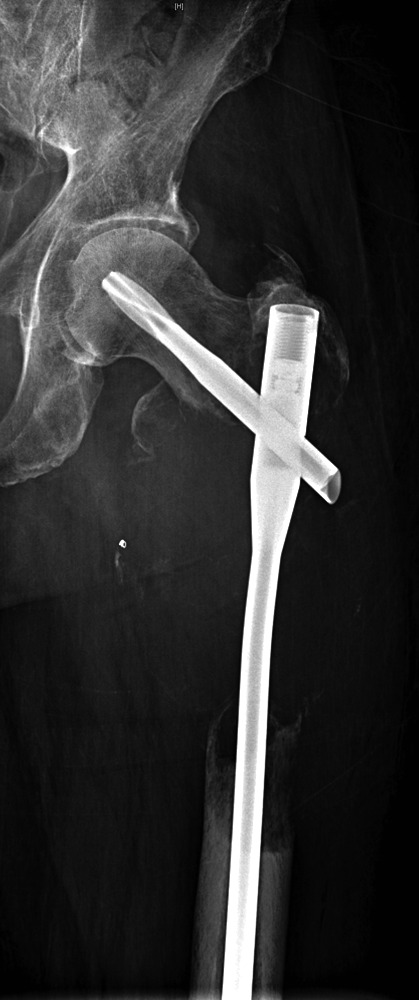
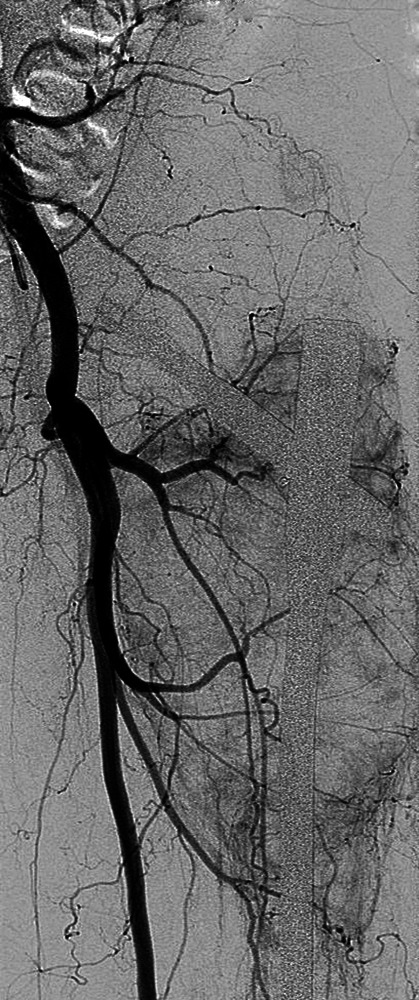
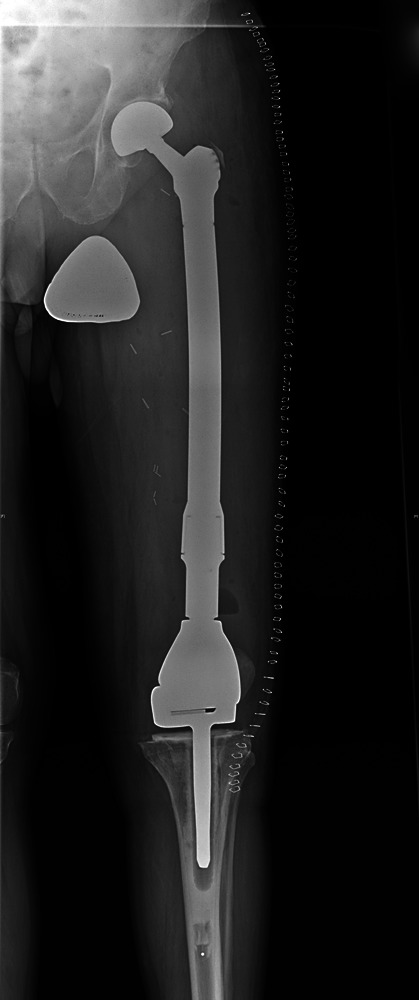
Case 1. Figure 1a – anteroposterior (AP) radiograph upon presentation to the specialist orthopaedic oncology unit, showing distal progression of the disease and impending fracture of the implant. Figure 1b – angiogram showing selective embolisation of the proximal metastatic deposit. Figure 1c – post-operative AP radiograph taken three days after total femoral replacement.
A solitary bony lesion with or without a known previous malignancy must always be biopsied in order to make a histological diagnosis. In renal cell carcinoma, resection of a solitary metastasis is potentially curative. Intramedullary nailing does not excise the tumour, stop local disease progression or successfully eliminate pain. It can spread disease to a distal site and compromise survival.
Case 2
A 65-year-old male with a known history of nephrectomy for renal cell carcinoma (and no known metastatic disease) was referred to our unit unable to bear weight with a pathological fracture of the right acetabulum and a lytic lesion of the ipsilateral proximal femur (Fig. 2a). Biopsy performed at our institution confirmed the diagnosis as metastatic renal cell carcinoma.
Figs. 2a - 2b.
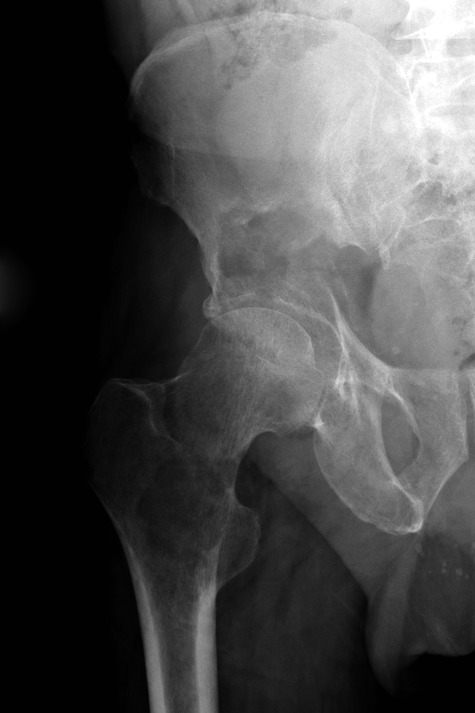
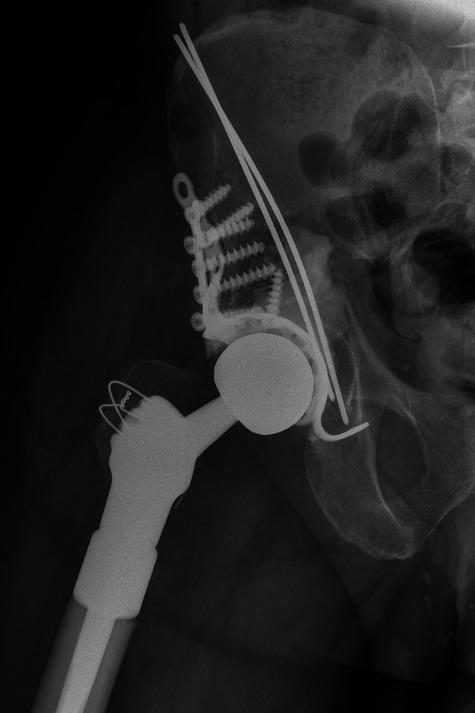
Case 2. Radiographs a) on presentation, showing a pathological fracture of the right acetabulum and a lytic lesion of the ipsilateral proximal femur, and b) at three days after proximal femoral replacement and acetabular reconstruction using a Graft Augmentation Prosthesis and the Harrington pin technique.
No other evidence of metastatic disease was identified and after selective embolisation the patient underwent proximal femoral replacement and acetabular reconstruction using a Graft Augmentation Prosthesis (GAP cage; Stryker UK, Newbury, United Kingdom) and Harrington Pin technique to reconstitute the medial wall (Fig. 2b). At three months post-operatively the patient remained independently ambulant and pain-free.
In renal cell carcinoma resection of a solitary metastasis is potentially curative. Up to 50% of pathological fractures fail to unite6 and the analgesic efficacy of radiotherapy is greatly overestimated.7 The principles of management involve biopsy and a single operation with complete resection of the solitary metastasis, and reconstruction using an implant that allows immediate mobilisation. This should be undertaken by a specialist orthopaedic oncologist with multidisciplinary team support.
Case 3
A 49-year-old man with known lung adenocarcinoma presented non-weight-bearing in a wheelchair with bilateral hip pain. He had known multiple metastases to bone and brain. Imaging showed large lytic lesions in the left proximal femoral metaphysis and right acetabular dome. No CT scanning was performed and the patient was treated with a fully cemented left total hip replacement and staged right total hip replacement with curettage and cementation of the acetabular tumour deposit. He was immediately fully able to bear weight. Six weeks later the patient represented with pain and imaging showed medial migration of the cementoma and was referred to a specialist orthopaedic oncology unit (Fig. 3a). CT scans showed a Harrington type III defect (loss of medial wall and posterior column with large dome defect). The patient underwent revision reconstruction using a GAP cage, mesh and Harrington pin technique to reconstitute the posterior wall (Fig. 3b). The patient was independently mobile until his death six months later.
Figs. 3a - 3b.
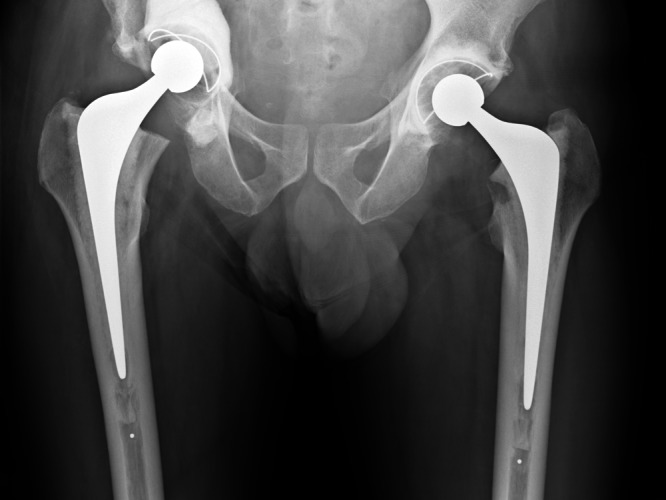
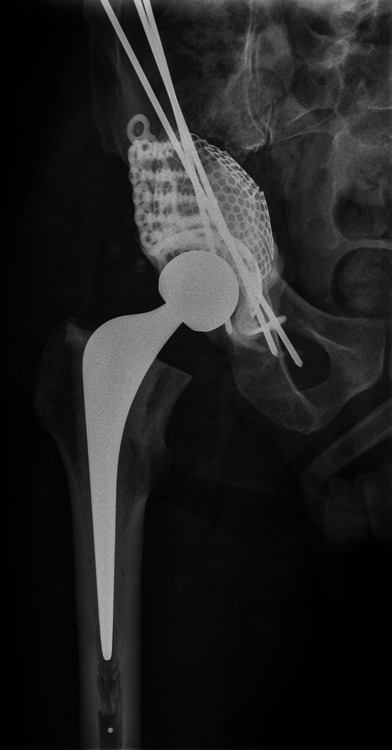
Case 3. Radiographs a) on presentation to the specialist orthopaedic oncology unit, showing medial migration of the cementoma, and b) at two days after acetabular reconstruction using a Graft Augmentation Prosthesis, mesh and the Harrington pin technique.
Metastatic disease around the acetabulum must be investigated appropriately and managed by surgeons trained in the appropriate reconstruction technique, such as cage reconstruction and Harrington pin technique.8
Case 4
A 93-year-old man with a past history of Dukes’ B adenocarcinoma of the bowel was referred to our unit for prophylactic stabilisation of large pain-free ‘metastatic’ deposits of both distal tibiae identified on bone scan (with smaller proximal deposits). MRI performed by the referring surgeon confirmed bilateral lytic deposits with cortical loss of nearly 50% (Fig. 4). No biopsy was performed before referral.
Fig. 4.
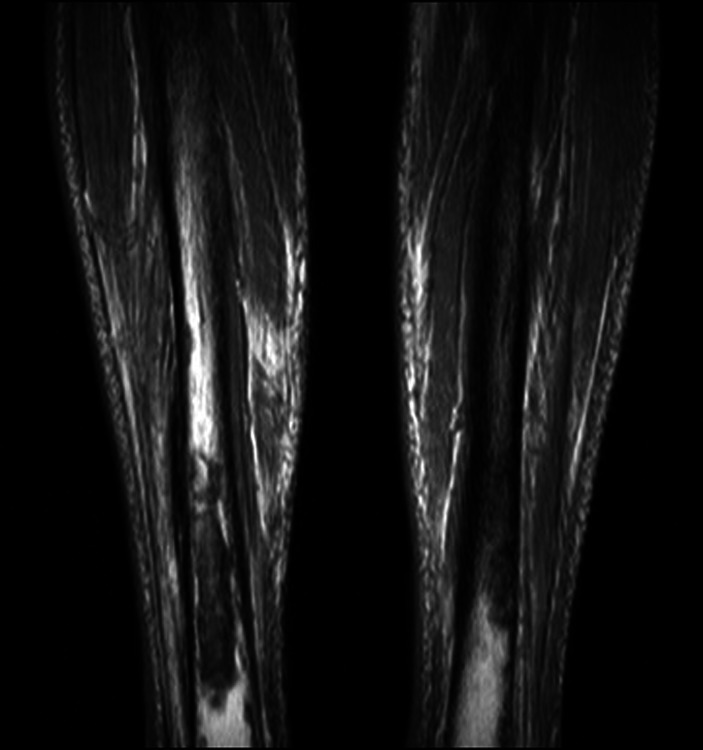
Case 4. T1-weighted MRI showing bilateral tibial lytic lesions with reported circumferential cortical loss of nearly 50% in the diaphyseal axial plane.
Clinically the patient was mobilising fully weight-bearing with non-tender tibiae but evidence of chronic cellulitis. Biopsy performed in our unit confirmed the underlying pathology as chronic osteomyelitis and the patient was referred and managed appropriately.
Biopsy must always be undertaken before surgical intervention when metastatic disease first presents or is suspected. Failure to do say may result in inappropriate management that may compromise patient survival. The only exception to this is where a patient is already known to have multiple metastatic deposits, although intra-operative tissue samples should always be sent for histopathological analysis to confirm the diagnosis.
Case 5
A 56-year-old man with a previous history of nephrectomy for renal cell carcinoma was referred to our unit after presenting with a pathological fracture of his left femoral diaphysis. There was no history of metastatic disease. Before fracture the patient presented three times to his GP complaining of thigh pain and was referred non-urgently to local orthopaedic services without imaging being undertaken. He fractured before review.
Biopsy performed before transfer via a lateral approach confirmed the underlying diagnosis as metastatic renal cell carcinoma. This represented a first solitary metastasis and the patient underwent segmental intercalary resection, excising the fracture site en bloc with a tissue cuff (intra-operative frozen section showing clear margins) and reconstruction with a proximally and distally locked segmental prosthesis (WG Healthcare, Letchworth, United Kingdom) (Fig. 5). He was treated with adjuvant radiotherapy. Six months post-reconstruction he was mobilising fully weight-bearing with no local or distal metastatic recurrence.
Fig. 5.
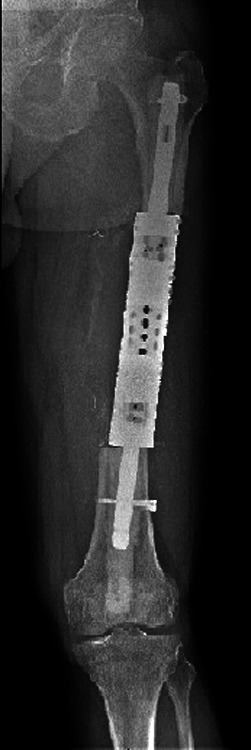
Case 5. Anteroposterior radiograph showing reconstruction with a proximally and distally locked segmental prosthesis after segmental intercalary resection.
Ideally biopsy should be performed at the unit where definitive surgery is to be undertaken. Otherwise it should be done after consultation with the operating surgeon and the biopsy tract excised at surgery. Inappropriate biopsy sites can compromise surgical approaches and increase the risk of local recurrence. Any patient with bone pain in the presence of a known primary malignancy must be investigated immediately for the presence of metastatic disease. Fracture contaminates the surrounding tissue bed with tumour, and resection will necessitate wider tissue margins. Both result in increased risk of local recurrence and may impair function post-operatively. Ultimately survival may be compromised.
Case 6
A 68-year-old woman a previous history of nephrectomy for renal cell carcinoma presented in an Acute Fracture Clinic with acute pain around her right knee/distal femur and an inability to fully bear weight. There was no known history of metastatic disease. Radiographs showed a large lytic lesion of the distal femoral metaphysis (Mirel’s score of 11/12). No biopsy was undertaken and stabilisation was initially planned using either a locking plate or retrograde intramedullary nail.
The patient was eventually referred to our institution. Imaging and biopsy were performed, confirming the presence of a first isolated metastatic renal cell deposit. She underwent distal femoral resection with clear tissue margins and adjuvant radiotherapy (Fig. 6). She was immediately fully weight-bearing and well 12 months later with no local or distant metastatic disease.
Fig. 6.
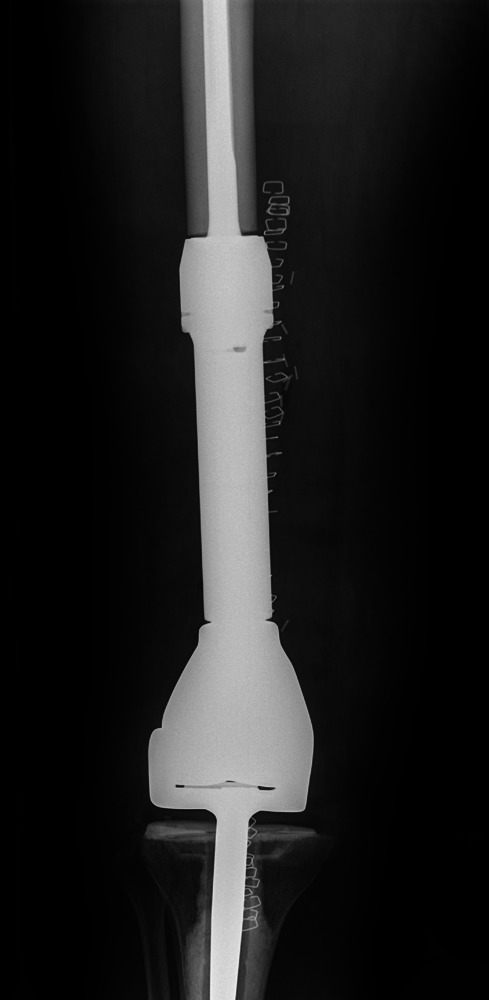
Case 6. Anteroposterior radiograph showing distal femoral resection and replacement using a Stanmore distal femoral endoprosthesis (Stanmore Implants, Stanmore, United Kingdom).
The efficacy of adjuvant therapies and survival of patients with metastatic disease is widely underestimated. Plates and intramedullary nails used in prophylactic stabilisation have disadvantages, in that they may fail through fatigue caused by cyclical loading in ununited fractures, they do not stop local disease progression and they may not control pain. This can result in increased morbidity, further surgery and loss of independence. Surgery should be definitive, allowing immediate full weight-bearing and should be carried out by an appropriately trained orthopaedic oncology surgeon, using the appropriate technique in an appropriate location with the appropriate multidisciplinary team support. Anything less compromises patient care.
Discussion
Patients with MBD have simple priorities, in that they want to remain ambulant, free of pain, independent and out of hospital. Traditionally many patients have simply been treated with prophylactic fixation/open reduction and internal fixation of fractures with or without radiotherapy. Up to 50% of pathological fractures fail to unite, and radiotherapy has variable efficacy in controlling pain. There is now sufficient evidence to support a more ‘interventional’ approach in managing this patient group with superior outcomes, improved survival and fewer complications being achieved.9-18
However, surgeons must be competent and trained in techniques that are often only applied to this specific patient group. Cement augmentation of intramedullary nails, Harrington and modified Harrington pin techniques and the use of tumour endoprosthetic implants are techniques essential in the repertoire of surgeons undertaking such cases. A degree of improvisation may be needed, with long-term ‘construct-survival’ not being the prime objective. It is hoped therefore that surgery for MBD be recognised as a subspecialty in its own right.
In order to provide an optimum service to patients with MBD, surgeons must have multidisciplinary team support from Oncologists, Specialist Nurses, Pathologists, Radiologists and other surgical disciplines. We do not advocate that all orthopaedic surgeons undertake this work, although it is hoped that through educational courses (such as the Oxford Metastatic Course) and the more detailed provision of guidelines, colleagues will become increasingly aware of improved reconstruction options as well as when and where to refer such cases. All hospitals should have an Orthopaedic Metastatic Lead who can provide this information to colleagues. Professor Keith Willett (National Clinical Director for Trauma Care) is currently revolutionising the provision of Trauma services in the United Kingdom with the development of Major Trauma Networks using a ‘hub and spoke’ model. It would be envisaged that a similar model could be developed for MBD.
Furthermore, the short-term costs in managing patients with MBD can be high.19 Commissioners therefore need to see the broader perspective that ‘aggressive’ early intervention maintains patient independence, reduces complications and overall reduces the burden that these patients place on scarce NHS resources. Implants costs can also be reduced through the centralisation of services and subsequent economies of scale.
Conclusions
Patients with MBD represent a significant minority but increasing population of patients. To date, no orthopaedic subspecialty has sought to take ownership of this patient group, resulting in poor patient care and poor outcomes. Guidelines for the management of such patients have been in place for more than a decade with little improvement in service provision.
With improved awareness, education and detailed guidelines it is once again hoped that as the number of patients with MBD increases the next decade sees a significant improvement in patient care.
Funding Statement
None declared
Footnotes
Author contributions:P. Harvie: Original idea, Writing of manuscript
D. Whitwell: Expert opinion, Editing of manuscript
ICMJE Conflict of Interest:None declared
References
- 1.No authors listed British Association of Surgical Oncology Guidelines: the management of metastatic bone disease in the United Kingdom: the Breast Specialty Group of the British Association of Surgical Oncology Eur J Surg Oncol 1999;25:3–23 [PubMed] [Google Scholar]
- 2.British Orthopaedic Association. Metastatic bone disease: a guide to good practice, December 2001. http://www.boa.ac.uk/Publications/Documents/ (date last accessed 25 January 2013).
- 3.National Institute for Health and Clinical Excellence. NICE Clinical Guidelines CG75 – Metastatic spinal cord compression: diagnosis and management of adults at risk of and with metastatic spinal cord compression, November 2008. http://guidance.nice.org.uk/CG75 (date last accessed 25 January 2013).
- 4.National Institute for Health and Clinical Excellence. NICE Clinical Guidelines CG104 – Metastatic malignant disease of unknown primary origin, July 2010. http://www.nice.org.uk/cg104 (date last accessed 25 January 2013). [PubMed]
- 5.Darzi A. High quality care for all: NHS Next Stage Review final report. London: Department of Health, 2008. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_085825 (date last accessed 25 January 2013).
- 6.Helmstedter CS, Goebel M, Zlotecki R, Scarborough MT. Pathologic fractures after surgery and radiation for soft tissue tumors. Clin Orthop Relat Res 2001;389:165–172 [DOI] [PubMed] [Google Scholar]
- 7.Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy: a systematic review of the randomised trials. Cochrane Database Syst Rev 2004;2:CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington KD. Orthopaedic management of extremity and pelvic lesions. Clin Orthop Relat Res 1995;312:136–147 [PubMed] [Google Scholar]
- 9.Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer: indications, complications and survival. Acta Orthop 2012;83:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker MJ, Khan AZ, Rowlands TK. Survival after pathological fractures of the proximal femur. Hip Int 2011;21:526–530 [DOI] [PubMed] [Google Scholar]
- 11.Steensma M, Boland PJ, Morris CD, Athanasian E, Healey JH. Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin Orthop Relat Res 2012;470:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey N, Ahlmann ER, Allison DC, Wang L, Menendez LR. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin Orthop Relat Res 2012;470:684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified Harrington procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg [Br] 2008;90-B:84–87 [DOI] [PubMed] [Google Scholar]
- 14.Ji T, Guo W, Yang RL, Tang S, Sun X. Clinical outcome and quality of life after surgery for peri-acetabular metastases. J Bone Joint Surg [Br] 2011;93-B:1104–1110 [DOI] [PubMed] [Google Scholar]
- 15.Kotwal SY, Finn HA. A 17-year follow-up of modified “Harrington” reconstruction after acetabular resection. J Arthroplasty 2011;26:1570–1521 [DOI] [PubMed] [Google Scholar]
- 16.Tillman RM. The role of the orthopaedic surgeon in metastatic disease of the appendicular skeleton: Working Party on Metastatic Bone Disease in Breast Cancer in the UK. J Bone Joint Surg [Br] 1999;81-B:1–2 [DOI] [PubMed] [Google Scholar]
- 17.Marco RA, Sheth DS, Boland PJ, et al. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg [Am] 2000;82-A:642–651 [DOI] [PubMed] [Google Scholar]
- 18.Yasko AW, Rutledge J, Lewis VO, Lin PP. Disease- and recurrence-free survival after surgical resection of solitary bone metastases of the pelvis. Clin Orthop Relat Res 2007;459:128–132 [DOI] [PubMed] [Google Scholar]
- 19.Ashford RU, Hanna SA, Park DH, et al. Proximal femoral replacements for metastatic bone disease: financial implications for sarcoma units. Int Orthop 2010;34:709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]


