Introduction
The mortality in acute liver failure (ALF) remains high despite superior contemporary management. Although orthotopic liver transplantation (OLT) is best for rescue in ALF, donor organ short-ages and contraindications or irreversible neurological complications may restrict OLT. Therefore, alternative therapeutic approaches for ALF are necessary. These are typically aimed at managing complications, ameliorating inflammation, providing hepatic support, and inducing liver repair/regeneration. Whether cell therapy would offer such benefits is of interest for many reasons: cells from one donor could be used for several people; cryopreserved and banked cells could be administered rapidly; cells could be transplanted multiple times; provision of hepatic support from transplanted cells could “bridge” to OLT; reseeding of the liver with transplanted cells could promote tissue repair; secretion of appropriate factors from transplanted cells could aid liver regeneration, which might avoid OLT altogether; cell therapy is technically simple and is also “reversible” because the native liver need not be removed. Allogeneic hepatocytes are normally rejected over 7–10 days but immunosuppression might not be required for short-term cell therapies in ALF.
Recent insights into the potential of various cell types in liver regeneration, mechanisms of liver repopulation and tissue engineering support the possibility of cell therapy for ALF. New sources of cells for transplantation, including candidate stem cells, will expand the supply of transplantable cells.
A major question in ALF is whether one must reseed the native liver with cells, which is difficult, or whether creating a mass of transplanted cells elsewhere in simpler ways will suffice.
Here, we highlight issues in intrahepatic versus extrahepatic transplantation of cells for ALF. Findings from animal studies are included in this discussion.
Initial clinical experience of cell therapy in ALF
The worldwide experience up to 2006 of hepatocyte transplantation included 37 children and adults (Table 1) [1]. We are not aware subsequently of hepatocyte transplantation in more people with ALF. To date, all studies have been uncontrolled. Most cases received allogeneic adult human hepatocytes, often repeatedly. The routes of cell administration varied – into portal vein, spleen or peritoneal cavity. The sample sizes were small for intergroup comparisons. After hepatocyte transplantation, 7 of 37 cases (19%) recovered without OLT and 8 (22%) cases recovered after OLT. Thus, hepatocyte transplantation was ‘successful” in 15 out of 37 (41%) cases.
Table 1.
Clinical experience to 2006 of hepatocyte transplantation in ALF.*
| Etiology of ALF | Total treated by cell therapy | Pediatric | Adult | Beneficial outcomes ↓ Recovery | |
|---|---|---|---|---|---|
| without OLT | with OLT | ||||
| Drugs | 20 | 5 | 15 | 3 | 3 |
| Viral | 9 | 1 | 8 | 1 | 2 |
| Idiopathic | 6 | 4 | 2 | 2 | 3 |
| Mushroom | 1 | 0 | 1 | 1 | |
| Post-surgical | 1 | 0 | 1 | ||
| Total | 37 | 10 | 27 | 7** | 8*** |
From Fisher and Strom [1].
Cells transplanted via portal vein (3 cases), spleen (1 case), and peritoneal cavity (3 cases).
Cells transplanted via portal vein (4 cases), and spleen (4 cases).
However, critical questions were unanswered: did transplanted hepatocytes … engraft? … proliferate? … produce liver regeneration? If so, how, and what was the fate of transplanted cells in liver, spleen or peritoneal cavity? Understanding the mechanistic basis of recovery after cell therapy in ALF is necessary for therapeutic development. For instance, the nature of interventions would change if liver must be reseeded with cells compared with transplantation of cells in peritoneal cavity, since the latter is simpler.
Integrating evidences from animal studies in ALF
Reseeding of the liver with transplanted cells in ALF carries limitations. After hepatocyte transplantation via portal vein or spleen, only 1–5% of the hepatocyte mass was replaced in the liver [2]. Transplanted hepatocytes occluded portal vessels and hepatic sinusoids, albeit temporarily, but with significant hepatic injury and inflammation [3]. Days were required for engraftment and weeks for proliferation (to small extent) of transplanted hepatocytes in acute liver injury [4].
By contrast, the greater space in peritoneal cavity accommodated larger numbers of transplanted cells than the liver. In the presence of scaffolds, transplanted hepatocytes engrafted in peritoneal cavity [5]. Transplanted cells remained in peritoneal cavity without migrating elsewhere, e.g. to the liver. After cell transplantation in peritoneal cavity in ALF, liver injury subsided, liver regenerated, and animals rapidly recovered. Besides hepatic support, engrafted cells provided paracrine factors, e.g., VEGF, FGFs, G-CSF, etc., for hepatic protection and promotion of liver regeneration [5,6]. Liver recovered due to proliferation of native cells since transplanted cells in the liver did not proliferate [5]. Use of absorbable scaffolds for cell engraftment in peritoneal cavity has long been without complications in animals and this should not interfere with further surgery if needed. The role of paracrine factors in rescue of ALF was supported by studies with other cell types, e.g. transplantation of mouse endothelial progenitor cells secreting another hepatoprotective cytokine, cardiotrophin- 1 [7]. This is significant since endothelial cells have increasingly been recognized to contribute in liver regeneration.
Recently, pigs with ALF were rescued by intraportal transplantation of human bone marrow-derived mesenchymal cells, perhaps with hepatic transdifferentiation [8], although more studies are needed to confirm this possibility. Interestingly, comparison of adult, fetal, and embryonic stem cell-derived mouse hepatocytes indicated that adult hepatocytes most effectively rescued mice in ALF [9].
Future perspectives
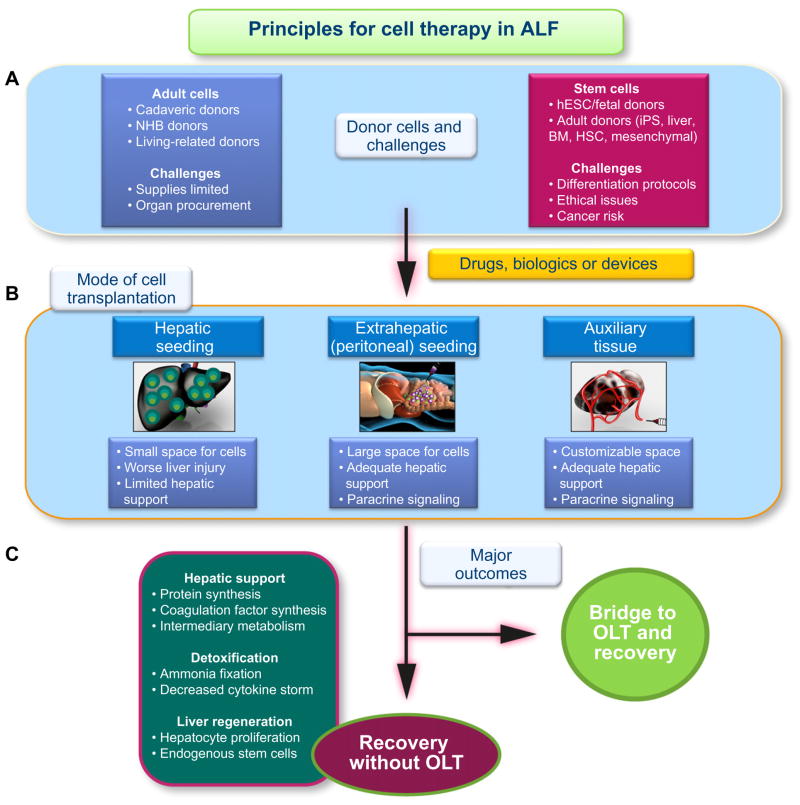
Remarkably, studies of non-heart-beating (NHB) donor animals as new sources of hepatocytes, showed the liver remained intact long after death [10]. Despite molecular lesions, hepatocytes from NHB donors did engraft and proliferate in animals. Another donor source could be stem cells with highly efficient protocols for hepatic differentiation. Tissue engineering with decellularized–recellularized substrates incorporating relevant cell types should advance further directions for cell therapy (Fig. 1). Transplantation of hepatocytes along with other cell types, e.g. endothelial cells, may be helpful.
Fig. 1. Schematic representation of principles for cell therapy in ALF.
(A) Major categories of donor cells are listed. Adult cells include hepatocytes and liver sinusoidal endothelial cells. Cells could be isolated from cadaveric, non-heart beating (NHB) or living-related donors. The challenges include limited supply of cadaveric donors. Successful use of NHB donors will require organ procurement and cell isolation programs. Superior cryopreservation protocols are essential. Candidate stem cells include human embryonic (hESC) or fetal cells. Adult donors types include induced pluripotent stem (iPS) cells, stem cells from discarded human livers, or stem cells from bone marrow, blood, mesenchymal cells, adipose tissue, etc. Challenges with stem cells concern efficient differentiation protocols, extent of cell differentiation, expansion of differentiated cells in vitro, restrictions in engraftment and proliferation of cells in vivo, and mitigation of cancer risks. (B) indicates potential anatomic sites for cell transplantation. Transplanting cells in the liver itself seems appropriate, although hepatic space is small, hepatocyte transplantation worsens liver injury, and transplanted cells in small numbers should provide little hepatic support. By contrast, transplantation of cells into extrahepatic sites, such as peritoneal cavity, would accommodate far larger numbers of cells. This will permit adequate hepatic support along with paracrine signaling from transplanted cells to accelerate liver regeneration. The space in an auxiliary liver, e.g. with decellularized–recellularized matrix, should be customizable for appropriate hepatic support and paracrine signaling. (C) Desirable outcomes are listed. “Bridge” to OLT or recovery without OLT will constitute good outcomes. This should concern restoration of proteins, coagulation factors and intermediary metabolism, ammonia fixation and decrease in cytokine storm, and liver regeneration, including recruitment of endogenous stem cells. Incorporating suitable drugs, biological agents and devices should be appropriate and helpful.
Acknowledgments
Financial support
Supported in part by NIH Grants R01 DK071111, R01 DK088561, P30 DK41296 and P30 CA13330.
Footnotes
Conflict of interest
The underlying research reported in the study was funded by the NIH Institutes of Healthnice.
References
- 1.Fisher R, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 2.Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. Studies of liver repopulation using the dipeptidyl peptidase IV deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology. 1996;23:482–496. doi: 10.1002/hep.510230313. [DOI] [PubMed] [Google Scholar]
- 3.Krohn N, Kapoor S, Enami Y, Follenzi A, Bandi S, Joseph B, et al. Hepatocyte transplantation-induced liver inflammation is driven by cytokines–chemokines associated with neutrophils and Kupffer cells. Gastroenterology. 2009;136:1806–1817. doi: 10.1053/j.gastro.2009.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Rajvanshi P, Irani AN, Palestro CJ, Bhargava KK. Integration and proliferation of transplanted cells in hepatic parenchyma following D-galactosamine- induced acute injury in F344 rats. J Pathol. 2000;190:203–210. doi: 10.1002/(SICI)1096-9896(200002)190:2<203::AID-PATH521>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Bandi S, Joseph B, Berishvili E, Singhania R, Wu YM, Cheng K, et al. Perturbations in Atm signaling pathways following drug-induced acute liver failure and their reversal during rescue of animals by cell therapy. Am J Pathol. 2011;178:161–174. doi: 10.1016/j.ajpath.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandi S, Cheng K, Joseph B, Gupta S. Spontaneous origin from human embryonic stem cells of early developmental stage liver cells displaying conjoint meso-endodermal phenotype with hepatic functions. J Cell Sci. 2012;125:1274–1283. doi: 10.1242/jcs.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Ruiz V, Kawa M, Berasain C, Iñiguez M, Schmitz V, Martinez-Ansó E, et al. Treatment of murine fulminant hepatitis with genetically engineered endothelial progenitor cells. J Hepatol. 2011;55:828–837. doi: 10.1016/j.jhep.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, et al. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 2012 Mar 16; doi: 10.1002/hep.25722. http://dx.doi.org/10.1002/hep.25722. [Epub ahead of print] [DOI] [PubMed]
- 9.Kamimura R, Ishii T, Sasaki N, Kajiwara M, Machimoto T, Saito M, et al. Comparative study of transplantation of hepatocytes at various differentiation stages into mice with lethal liver damage. Cell Transplant. 2012 Apr 2; doi: 10.3727/096368912X636957. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Enami Y, Joseph B, Bandi S, Lin J, Gupta S. Molecular perturbations restrict potential for liver repopulation of hepatocytes isolated from nonheart-beating donor rats. Hepatology. 2012;55:1182–1192. doi: 10.1002/hep.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]