Abstract
Background
Imprinting an effector or regulatory phenotype on naïve T cells requires education at induction sites by dendritic cells (DC). In the current studies we analyzed the effect of inflammation on the frequency of mononuclear phagocytes (MP) and the effect of altering their frequency by administration of Flt3-L in chronic ileitis.
Design
Using a TNF-driven model of ileitis (i.e. TNFΔARE) that recapitulates many features of Crohn’s disease (CD), we assessed dynamic changes in the frequency and functional state of MP within the inflamed ileum by flow cytometry, immunofluorescence and real-time reverse-transcription polymerase chain reaction and by generating CX3CR1 GFP-reporter TNFΔARE mice. Finally, we assessed the effect of Flt3-L supplementation on the severity of ileitis, the frequency of CD103+ DC and of FoxP3+ Tregs in TNFΔARE mice.
Results
CD11cHi/MHCII+ MP accumulated in inflamed ilea, predominantly mediated by expansion of the CX3CR1+ MP subpopulation. This coincided with a decreased pro-regulatory CD103+ DC. The phenotype of these MP was that of activated cells, as they expressed increased CD80 and CD86 on their surface. Flt3-ligand administration resulted in a preferential expansion of CD103+ DC that attenuated the severity of ileitis in 20-week-old TNFΔARE mice, mediated by increased CD4+/CD25+/FoxP3+ Tregs.
Conclusions
Our findings support a role for Flt3-L as a potential therapeutic in Crohn’s-like ileitis.
Keywords: Inflammatory bowel disease, mucosal immunology, dendritic cells, small intestine
INTRODUCTION
The gastrointestinal tract is constantly exposed to a vast array of food, bacterial, protozoan and viral-antigens. In this microenvironment the immune system must maintain tolerance to self-antigens, food and commensal microflora while remaining poised to mount robust responses to enteral pathogens. Through constant antigen sampling, antigen-presenting cells play a critical role in intestinal immune surveillance and in limiting over-reactive inflammatory responses. Failure of the pro-regulatory arm of the immune response has been implicated in the development of chronic inflammatory conditions such as Crohn’s disease (CD). Crohn’s is a chronic inflammatory bowel disease (IBD), typified by transmural intestinal inflammation involving the ileum in 60% of patients. Anti-TNF strategies are initially effective in up to 70% of patients[1] yet sustained remission drops significantly at one year[2] leaving an unmet need for newer therapeutics in IBD. Strategies that target other pathways of the chronic inflammatory cascade must therefore be identified[3].
Mononuclear phagocytes (MP) in the intestine can be broadly divided into several functionally distinct subsets, some of which express chemokine receptors[4](e.g. CX3CR1) or specific cell adhesion molecules (i.e. CD103, integrin αE). This CD103+ subset may or may not express CD11b and are classified as dendritic cells (DC)[5]. CX3CR1 is a chemokine receptor, which binds its cognate ligand CX3CL1 or fractalkine[6], a chemokine ligand that exists as a membrane-bound potent arrest chemokine or acts as a soluble chemoattractant upon proteolytic cleavage[7]. CX3CR1+ cells have been shown to drive pro-inflammatory Th17 responses and play a vital role in bacterial clearance in the intestine[8]. They have a significantly higher capacity to sample antigens compared with CD103+ DC[4, 9] due to their ability to extend dendrites into the lumen. CX3CR1+ cells exhibit significantly lower turnover rates, reduced homing to the mesenteric lymph nodes (MLN) and impaired induction of T cell proliferation compared with CD103+ DC[4].
Central to the maintenance of a controlled inflammatory response is the induction of regulatory T cells (Treg). A subset of Tregs is defined by their expression of the transcription factor forkhead box P3 (FoxP3) and release of IL-10[10, 11]. Treg expansion has been reported in human IBD and in murine models[12-15]. Induction of small intestinal homing (CCR9+/α4β7+) on T cells is mediated by the release of retinoic acid (RA), predominantly from CD103+ DC, which in the presence of TGF-β[16, 17] promote induction of a regulatory phenotype in naïve T cells[18]. Compared with CD103+ DC, CX3CR1+ cells express significantly lower levels of aldh1a2 mRNA, which encodes retinaldehyde dehydrogenase 2 (RALDH2), an enzyme critical for the conversion of retinal to RA[16].
Generation of CD103+ DC is partly mediated by the polyonymous FMS-like tyrosine kinase 3 (Flt3) through interaction with its cognate ligand (Flt3-L), a type-1 transmembrane or soluble protein similar in size and structure to other hematopoietic growth factors like CSF-1 and KIT ligand[19]. Originally identified in mice[20], Flt3 was soon found in humans[21]. Flt3 mRNA was detected in pre-B cell, myeloid and monocytic lineages whereas Flt3-L mRNA was detected in most cell lines in humans and mice[22].
Both Flt3 and Flt3-L are highly conserved between mouse and humans. Human Flt3 ligand can bind and activate mouse Flt3 receptor[23] resulting in bone marrow hyperplasia and stimulation of hematopoetic stem and progenitor cell proliferation. While Flt3-L has been shown to synergize with multiple cytokines and growth factors, cytokines such as TNF and TGFβ abrogate its ability to stimulate growth of murine hematopoietic progenitors[24]. The use of Flt3-L to preferentially expand MP in mice has become commonplace for the study of MP function since it was first discovered[25]; however, its preferential proliferative effect on CD103+ DC was identified recently[5].
The role that MP play during induction and perpetuation of CD is not known. To begin to understand their potential contribution to CD we analyzed the relative abundance of MP subsets in a relevant chronic model of Crohn’s-like ileitis generated by deletion of 69 bp within the AU-rich element (ARE) of the TNF gene in mice (i.e. TNFΔARE). The ARE deletion stabilizes TNF mRNA, resulting in systemic TNF overproduction and development of chronic inflammation localized to the terminal ileum, reminiscent of human CD in its histological features and the pivotal role played by TNF in its pathogenesis[26-28]. Here we examined the potential role of intestinal MP subsets in the regulation of chronic ileitis in the TNFΔARE model: one of only two mouse models that recapitulate the histopathologic features of CD. First we investigated whether the inflammatory process affected the overall frequency of CD11c+/MHC+ MP and their activation state. We next assessed if particular MP subsets (CX3CR1+, CD103+) were expanded under conditions of chronic inflammation. We then investigated the capacity of Flt3-L to expand particular MP subsets under conditions of chronic inflammation, and its Treg progeny to modulate disease severity.
MATERIALS & METHODS
Mice
The B6.129S-Tnftm2Gkl/Jarn strain was previously described[29] and kept under specific-pathogen-free conditions. Experimental animals were heterozygous for the ΔARE mutation or homozygous wild-type (WT), which served as controls. CX3CR1GFP/GFP mice and CD103−/− mice on the C57BL6/J background were obtained from Jackson Laboratories (Bar Harbor, ME) and crossed with TNFΔARE or WT mice to generate WT/CX3CR1GFP/+ and TNFΔARE/CX3CR1GFP/+ mice. Fecal samples were negative for Helicobacter, protozoa and helminthes. All animals were handled according to procedures approved by the institutional committee for animal use.
Lymphocyte isolation
Splenocytes, MLN, and LP mononuclear cells were isolated as previously described[30]. Briefly spleens and MLN were passed through a 70μm filter, red cells lysed and single cell suspensions counted. Intraepithelial lymphocytes and epithelial cells were removed from whole ileal tissue using 1mM EDTA and vigorous shaking, tissues were then digested in collagenase VIII (Sigma Aldrich, St Louis, MO), filtered and counted prior to staining.
Flow cytometry
Cells from indicated compartments were incubated with fluorescent rat anti-mouse antibodies including against: mouse CD11c (N418), MHCII (M5/114.15.2), F4/80 (BM8), CD103 (2E7), CD80 (16-10A1), CD86 (GL-1), CD3 (17A2), CD4 (RM4-5), CD25 (PC61.5), E-cadherin (36), and FoxP3 (FJK16S) or their respective isotype controls prior to fixation with 2% paraformaldehyde. Additional controls included cells isolated from CD103-deficient mice. FoxP3 staining was performed according to manufacturer’s instructions (eBiosciences, San Diego, CA). Intracellular cytokine staining was performed by stimulating unfractionated cells with 20ng/ml PMA, 1μg/ml ionomycin and 15μM monensin for 5 h (1×106 cells/well) prior to fixation and permeabilisation using the FoxP3 staining kit as above. Cells were analyzed using the FACS® Canto system (Beckton-Dickinson Immunocytometry Systems, San José, CA). Post-analyses were performed using FLOWJo software (Tree Star Inc, Ashland, OR). Unless otherwise stated CD11cHi/MHCII+ cells were previously gated on F4/80Neg.
Primary cell culture experiments
Freshly isolated cells from the LP of 20-week-old WT and TNFΔARE mice were cultured in complete medium (RPMI supplemented with 10% FBS, 2mM L-glutamine, 100 IU penicillin, 100μg/ml streptomycin; Invitrogen; Carlsbad, CA) in the presence or absence of 5μg/ml LPS for 24h.
RNA isolation, cDNA synthesis and Real time PCR
Ileal tissue was stored in RNAlater (Invitrogen) prior to mRNA isolation (mRNA isolation kit, Qiagen, Valencia, CA). Freshly isolated MP from TNFΔARE mice were enriched by positive selection (CD11c+ N418, Miltenyi Biotec) and FACS sorted based on CD11cHi/MHCII+ and CD103 expression. Transcript quantification was performed using PowerSybr Green and the AB7900 real-time PCR system (Applied Biosystems, Foster City, CA). GAPDH served as an endogenous control. RALDH2 and CX3CR1 were assayed with QuantiTect primers (QT00120477, QT00203434, Qiagen).
Flt3 ligand treatment studies
20-week-old TNFΔARE mice received daily intraperitoneal Flt3-L injections (10μg/injection) or PBS for 9 days. Tissues were collected 24h after the last injection. In some experiments mice were also administered anti-CD25 antibody 200μg/injection on days −3, 0, 3 and 6 (eBioscience, PC65.1). Recombinant human Flt3-L was provided by Dr. Robert Mittler at the Emory Vaccine Center (Atlanta, GA).
Statistics
Statistical analyses were performed using Student t test with Graphpad Prism Data Analysis software (GraphPad Software, La Jolla, CA). Data were expressed as mean ± standard error of the mean (SEM). Statistical significance was set at p<0.05.
RESULTS
Mononuclear phagocytes increased in TNFΔARE mice compared with WT controls
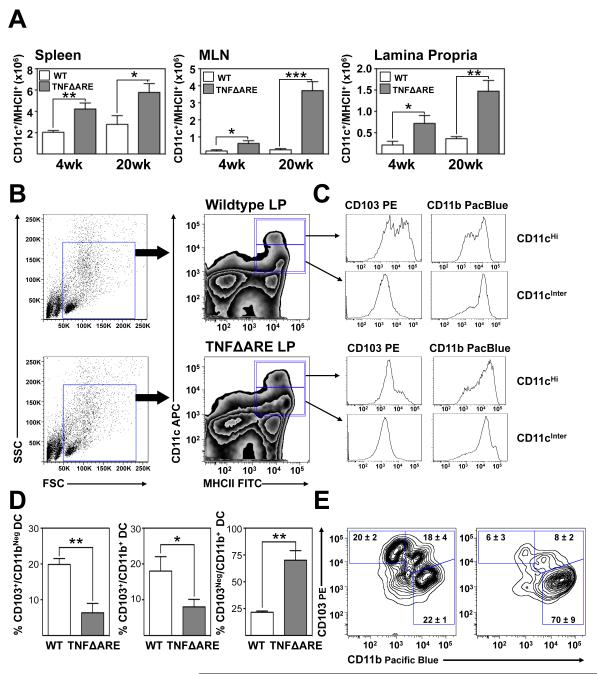
We evaluated the frequency of CD11c+/MHCII+ MP in spleen, MLN and terminal ileal lamina propria (LP) of TNFΔARE mice at 4- and at 20-weeks-of-age compared with WT (figure 1A) using the gating strategy outlined in figure 1B. The total number of MP in the spleen was significantly increased in TNFΔARE mice compared with WT at 4-(p<0.01) and 20-weeks-of-age (p<0.05; figure 1A). This coincided with a significant increase in the MLN at 4- (p<0.05) and at 20-weeks-of-age (p<0.001). Similarly in the LP, there was a significant increase in MP in TNFΔARE mice at 4- (p<0.05) and at 20-weeks-of-age (p<0.01). Evaluation of CD11c+/MHCII+ cells by flow cytometry demonstrated the presence of two distinct subsets based on relative expression of CD11c, one CD11cHi and the other CD11cIntermediate. Assessment of these two subsets, which were both expanded, demonstrated that the CD11cHi subset contained CD103+ DC, whereas the CD11cIntermediate cells were positive for CD11b (figure 1C). Based on the finding that the majority of CD103+ DC were found within the CD11cHi compartment, we focused on this population in subsequent studies. Representative scatter and zebra plots illustrate the effect of inflammation on MP subsets at 20-weeks-of-age. This increase in MP coincided with increased disease severity in TNFΔARE mice (Supplemental figure 1). Thus, MP accumulate within the inflamed terminal ileal LP of TNFΔARE mice and the CD103+ subset express high levels of CD11c.
Figure 1. Increased mononuclear phagocytes in TNFΔARE mice.
(A) Total cell counts from indicated organs of TNFΔARE mice aged 4- and 20-weeks-of-age compared with WT littermate controls (Mean ± SEM from 3 individual experiments, n=3 mice/experiment with cells pooled per genotype and time-point. *p<0.05, **p<0.01, ***p<0.001). (B) Representative scatter plots illustrating the gating strategy utilized, which included F4/80+ cells. (C) Representative zebra plots and histograms illustrating the expression of CD103 and CD11b within CD11cHi/MHCII+ and CD11cIntermediate/MHCII+ MP in the LP of 20-week-old WT and TNFΔARE mice. (D) The percentage of indicated MP subsets were assessed by flow cytometry from the LP of TNFΔARE and WT mice at 20-weeks-of-age (Mean ± SEM, n=4, *p<0.05, **p<0.01). (E) Representative zebra and contour plots from LP MP of WT and TNFΔARE mice.
Both CD103+/CD11b+ and CD103+/CD11bNeg DC subsets decreased in the chronically inflamed ilea of TNFΔARE mice
Recently, Varol et al. demonstrated two distinct pro-regulatory DC subsets present in the small intestinal LP and GALT, which were CD103+/CD11bNeg or CD103+/CD11b+ [5]. Thus, we extended our analyses to include examination of these subsets (figure 1D). The frequency of CD103+/CD11bNeg DC was significantly decreased in 20-week-old TNFΔARE LP compared with WT controls (p<0.01) and similarly the frequency of CD103+/CD11b+ DC were decreased in inflamed ilea compared with WT controls (p<0.05). In contrast the CD103Neg/CD11b+ subset was expanded in inflamed ilea compared with WT mice (p<0.01). Expression of RALDH2 mRNA transcripts, the key enzyme for the production of RA by pro-regulatory DC was examined in CD103+ and CD103Neg DC sorted from the MLN and LP of 20-week-old TNFΔARE mice. Real time RT-PCR revealed significantly higher expression of RALDH2 in CD103+ DC compared with CD103Neg MP from the MLN (p<0.01) and LP (p<0.01) of 20-week-old TNFΔARE mice, consistent with previous findings in WT mice (Supplemental figure 6)[31]. Thus, there is a deficiency of RA-producing tolerogenic CD103+ DC during chronic ileitis.
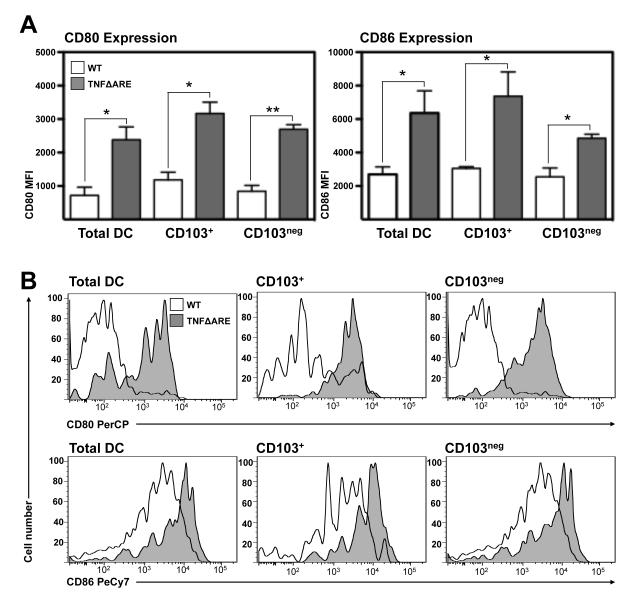
Mononuclear phagocytes from the LP of TNFΔARE mice displayed an activated phenotype
To determine whether chronic inflammation influenced the activation state of MP within the LP, we examined the expression of the co-stimulatory molecules CD80 and CD86 in freshly isolated unfractionated (total) MP, or those gated based on the expression of CD103. MP isolated from 20-week-old TNFΔARE LP expressed higher mean fluorescence intensities (MFI) for both CD80 and CD86 compared with age-matched WT controls (figure 2A). Mean MFI for CD80 in unfractionated MP was 719 ± 243 in WT compared with 2383 ± 381 in TNFΔARE mice (p<0.01) while in CD103+ DC it was 1185 ± 224 in WT compared with 3169 ± 338, in TNFΔARE mice (p<0.05). Similarly MFI on CD103Neg MP was 846 ± 172 in WT compared with 2695 ± 138 in TNFΔARE mice (p<0.01). When the expression of CD86 was assessed on total DC, the MFI in WT mice was 2696 ± 450 compared with 6367 ± 1320 in TNFΔARE mice (p<0.05). Those DC positive for CD103 had an MFI of 3051 ± 107 in WT compared with 8729 ± 89 in TNFΔARE mice (p<0.01) and lastly within the CD103Neg MP subset the MFI was 2546 ± 531 in WT compared with 6170 ± 1324 in TNFΔARE mice (p<0.05; figure 2A). Representative histograms of the above data are presented as figure 2B. LPS treatment negated the differences present between MP from inflamed and non-inflamed mice as CD80 and CD86 MFI increased in WT MP to levels comparable with TNFΔARE MP levels for both markers (data not shown). Therefore, a higher fraction of MP isolated from the terminal ileal LP of TNFΔARE mice displayed an activated phenotype.
Figure 2. Mononuclear phagocytes from TNFΔARE ileal LP display an activated phenotype.
(A) Expression of CD80 and CD86 was analyzed by flow cytometry in the indicated cell subsets and expressed as mean fluorescence intensity (MFI) (Mean ± SEM from 4 independent experiments, n=3 mice/experiment). (B) Representative histograms of the expression of indicated markers on CD11c+/MHCII+ MP freshly isolated from ileal LP of 20-week-old WT (white) and TNFΔARE mice (grey).
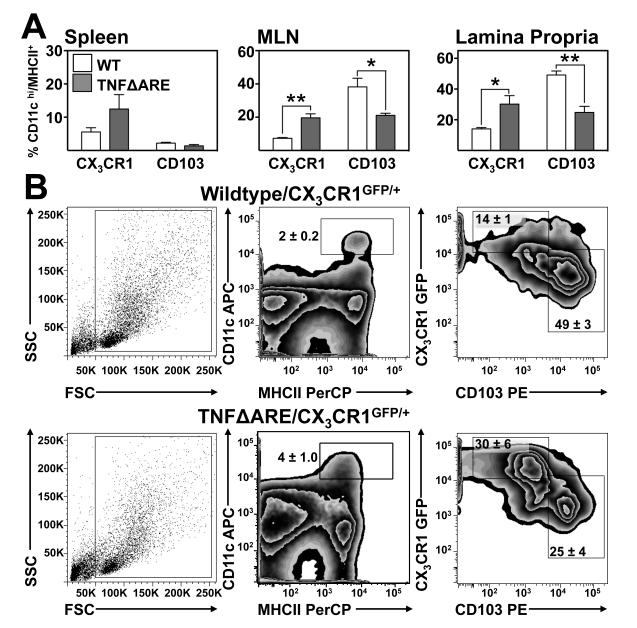
CX3CR1+ mononuclear phagocytes increased while CD103+ DC decreased in chronic ileitis
To evaluate the relative frequency of CX3CR1+ and CD103+ MP in TNF-mediated ileitis we generated a CX3CR1 reporter TNFΔARE substrain (TNFΔARE/CX3CR1GFP/+). Blinded histological examination of the ilea of the resultant progeny revealed no significant differences in the severity of ileitis between TNFΔARE/CX3CR1GFP/+ and TNFΔARE/CX3CR1+/+ mice (Supplemental figure 2). Flow cytometric analysis of 20-week-old TNFΔARE/CX3CR1GFP/+ MP (CD11cHi/MHC+/F4/80Neg) showed no significant differences in the CX3CR1+:CD103+ subset ratio in the spleen; however, in the MLN there was an expansion of the CX3CR1+ MP subset (p<0.01; figure 3A, B) and a decrease in CD103+ DC (p<0.05). Similarly, in the ileal LP the proportion of CX3CR1+ increased (p<0.05) while CD103+ DC decreased (p<0.01; figure 3A). Increased CX3CR1+ MP coincided with up-regulation of CX3CR1 mRNA transcripts within the terminal ilea of TNFΔARE compared with WT mice (Supplemental figure 3). Representative scatter and zebra plots illustrate the inflammation-driven changes on the indicated subsets present in ilea at 20-weeks-of-age (figure 3B). Increased frequency of CX3CR1+ and decreased CD103+ MP was similarly observed when cells expressing the macrophage marker F4/80 were included in the analyses, as F4/80 is expressed predominantly by cells that lack CD103 (Supplemental figure 4). When the frequency of CX3CR1+ cells was analyzed within MHC+/CD11b+ cells, the majority (around 90%) expressed CX3CR1 (Supplemental figure 5). Hence, CX3CR1+ MP increase, whereas CD103+ DC decrease in the MLN and terminal ilea of chronically inflamed TNFΔARE mice.
Figure 3. Increased CX3CR1+: CD103+ MP ratio in the ileal LP of TNFΔARE mice.
(A) CX3CR1+ and CD103+ MP frequency in the spleen, MLN and LP of 20-week-old WT/CX3CR1GFP/+ and TNFΔARE/CX3CR1GFP/+ mice. (Mean ± SEM from 4 independent experiments, n=3 mice/experiment; *p<0.05, **p<0.01). (B) Representative scatter and zebra plots of the expression of CX3CR1+ and CD103+ MP gated on CD11cHi/MHCII+ cells from the LP of 20-week-old mice.
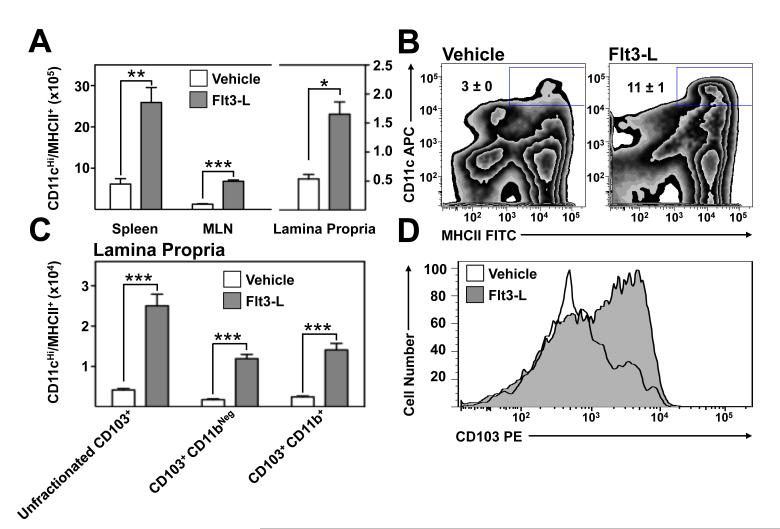
Flt3-L preferentially expands CD103+ pro-regulatory MP subsets in TNFΔARE mice
To begin to understand whether Flt3-L might alter the frequency of specific MP during chronic ileitis, we assessed its effect on the frequency of MP in 20-week-old vehicle- and Flt3-L-treated TNFΔARE mice. Flt3-L supplementation significantly increased the absolute number of CD11cHi/MHCII+ DC in the spleen (p<0.01), MLN (p<0.001) and LP (p<0.05) of TNFΔARE mice compared with vehicle-treated age-matched controls (figure 4A). Representative zebra plots illustrate these findings (figure 4B). Subset analysis of the expanded MP population in the inflamed LP revealed preferential expansion of the absolute numbers of unfractionated CD103+ DC (p<0.001), the CD103+/CD11bNeg subset (p<0.001) and the CD103+/CD11b+ subset (p<0.001; figure 4C). The effect of Flt3L on CD103Neg MP subset was not statistically significant. Representative histograms demonstrate a shift in the expression of CD103 (figure 4D).
Figure 4. Preferential expansion of pro-regulatory CD103+ DC by Flt3-L administration.
(A) Effect of Flt3-L on the number of CD11cHi/MHCII+ cells in the spleen, MLN and ileal LP of 20-week-old TNFΔARE mice. (Mean ± SEM for 3 experiments, n=4 mice/group, ***p<0.001). (B) Representative zebra plots of vehicle and Flt3L-treated mice. (C) Subset analysis of the expanded DC population showing percentages of CD103+/CD11bNeg and CD103+/CD11b+ DC subsets. (Mean ± SEM for n=4 mice/group. **p<0.01, ***p<0.001). Representative histograms of the expansion of CD103+ DC in the terminal ileal lamina propria after Flt3-L treatment.
Real time RT-PCR analysis of ilea from vehicle and Flt3-L-treated demonstrated an up-regulation of RALDH2, the enzyme expressed predominantly by CD103+ MP, critical for the generation of retinoic acid (p<0.05) and a decreased expression of CX3CR1 mRNA transcript (p<0.05; Supplemental figure 7). Furthermore, the frequency of the E-cadherin+ subset, which was increased in the LP of 20-week-old TNFΔARE mice relative to WT littermates (p<0.05), significantly decreased after Flt3-L administration (p<0.001; Supplemental figure 8).
Regulatory T cells increased after Flt3-L-treatment in TNFΔARE mice
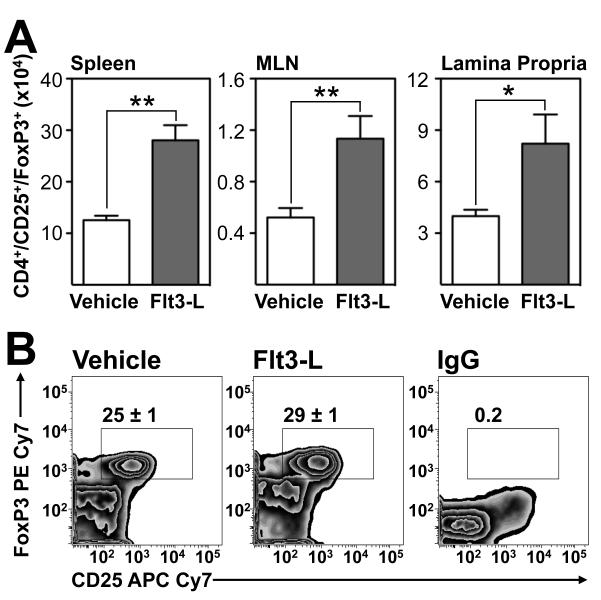
Having demonstrated an expansion of CD103+ DC in the LP of TNFΔARE mice treated with Flt3-L, we examined whether increased CD103+ DC resulted in the induction of regulatory CD4+/CD25+/FoxP3+ Tregs. The absolute number of Tregs significantly increased in 20-week-old Flt3-L-treated TNFΔARE mice spleen (p<0.01), MLN (p<0.01) and LP (p<0.05; figure 5A) compared with vehicle-treated controls, as well as an increase in the thymus of Flt3-L treated TNFΔARE mice (p<0.05). Representative zebra plots demonstrate that the significant increase in total numbers of Tregs seen in the Flt3-treated ileal LP coincides with an increased Treg frequency from 25% to 29% (p<0.05; figure 5B). Hence, stimulation of Flt3 drives induction of CD4+/CD25+/FoxP3+ Tregs.
Figure 5. Increased CD4+/CD25+/FoxP3+ Tregs in Flt3-L-treated mice.
(A) Effect of Flt3-L administration on the absolute counts of CD4+/CD25+/FoxP3+ Tregs in indicated organs isolated from vehicle- or Flt3-L-treated 20-week-old TNFΔARE mice (Mean ± SEM, n=5 mice/group. *p<0.05, **p<0.01). (B) Representative zebra plots display percentages of FoxP3 and CD25 on CD4+ T cells from the LP of vehicle- and Flt3-L-treated mice using PE Cy7 IgG as a control for FoxP3 intracellular staining.
Flt3 ligand administration attenuated chronic ileitis
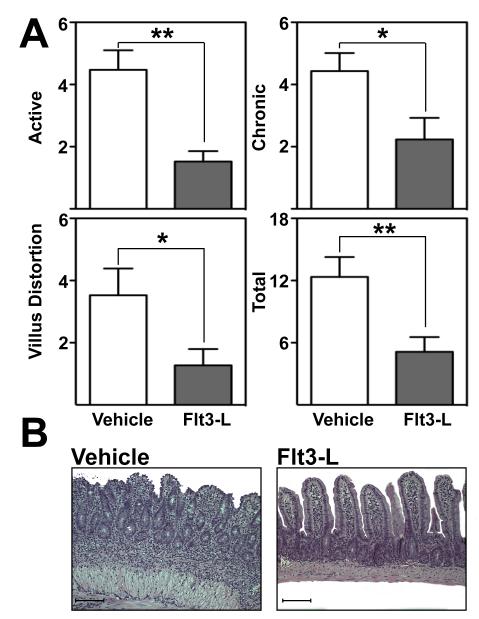
Flt3-L preferentially expanded the CD103+ DC subset and its Treg progeny[5]. To determine whether Flt3-L administration may affect the severity of ileitis during late disease, inflammation was assessed in vehicle- and Flt3-L-treated mice by a pathologist in a blinded fashion. Flt3-L supplementation significantly decreased active (4.5±0.6% vs. 1.5±0.3%; p<0.01), chronic (4±0.6% vs. 2±0.7%; p<0.05), villus distortion (3.5±1% vs. 1±0.5%; p<0.05) and total inflammatory indices (12±2% vs. 5±1.4%; p<0.01) compared with vehicle-treated controls (figure 6A). In addition, histological hallmarks of ileitis such as villus distortion, leukocyte infiltration, goblet cell hyperplasia and muscularis hypertrophy were noticeably decreased (figure 6B). The anti-inflammatory effect was dependent on regulatory T cells as antibody-mediated depletion of functional Tregs with an anti-CD25 antibody abrogated the protective effect of Flt3-L (Supplemental figure 9). Thus, Flt3-L administration exerts a potent anti-inflammatory effect on chronic ileitis mediated by Tregs.
Figure 6. Flt3-L administration attenuated chronic ileitis.
(A) Inflammatory indices from ilea of 20-week-old TNFΔARE mice treated with vehicle or Flt3-L were assessed as described[55] (Mean ± SEM, *p<0.05, n=5/treatment group). (B) Representative H&E micrographs of vehicle- and Flt3-L-treated ilea of 20-week-old TNFΔARE mice (10× magnification, bars=100μm).
DISCUSSION
Mononuclear phagocytes, which include cells that express CX3CR1, CD103 (both CD11b+ and CD11bNeg) and macrophages (CD11b+/F4/80+) play a critical role in intestinal immune surveillance and regulating inflammatory responses. Considerable progress has been made recently in understanding the basic biology of MP in the maintenance of normal gut homeostasis. MP subsets have been identified that appear to be pro- or anti-inflammatory; however, it is not known whether they play a role in the induction and/or maintenance of chronic inflammatory conditions, such as IBD. As such, this study aimed to investigate the effect of chronic inflammation on the frequency of MP subsets in a murine model of chronic ileitis and whether altering this frequency might be of therapeutic value. We noticed expansion of MP in the inflamed LP of TNFΔARE mice. These MP have an activated surface phenotype, consistent with exposure to bacterial antigens, contained a higher proportion of CX3CR1+ cells and show a reduced frequency of pro-regulatory CD103+ MP. We additionally show that Flt3-L attenuated established chronic ileitis in 20-week-old TNFΔARE mice, via an expansion of CD103+ DC and its regulatory CD4+/CD25+/FoxP3+ T cell progeny.
T cell receptor engagement and additional co-stimulatory signals supplied by CD80 and CD86 are required to elicit T cell responses. CD80 and CD86 are up-regulated on the surface of activated MP and serve as surrogates for activation. MP infiltrated the ileum of TNFΔARE mice demonstrated increased activation, in the absence of stimulation. Enhanced activation is concordant with studies in other murine models of IBD [32-34] and in human IBD[35]. MP activation can be reproduced by stimulation with LPS[36-38] and given the disruption of epithelial barrier associated with chronic ileitis[39], it is likely that increased bacterial translocation contributes to the heightened activation of MP in the LP of TNFΔARE mice.
CX3CR1+ MP, also called antigen sampling cells[4, 5] were found to promote ATP-dependent Th17 differentiation. By contrast, CD103+ DC produce RA and indoleamine 2,3-dioxygenase and are critical for the induction of FoxP3+ Tregs in mice and humans [16, 17, 40-43]. Thus we hypothesized that an imbalance of these populations may play a role in the pathogenesis of chronic ileitis and examined their relative frequency in WT and TNFΔARE mice. Indeed we observed a subset imbalance with increased CX3CR1+ and decreased pro-regulatory CD103+ MP in TNFΔARE mice compared with WT controls. The functional implications of the expansion of the CX3CR1+ MP in the TNFΔARE model is unclear at this time and the focus of ongoing studies. Expansion of the CX3CR1+ subset has been demonstrated in human chronic inflammatory conditions such as atopic dermatitis[44]. In separate studies, reconstitution of the CX3CR1 subset exacerbated DSS colitis and impaired tissue repair in a TNF-dependent manner[5] whereas CX3CR1-deficient mice exhibited attenuated colitis[45].
CD103+ DC preferentially drive the induction of a gut-homing regulatory phenotype on naïve T cells in mice[16, 17, 41-43] and humans[42] in a RA/TGFβ-dependent manner. We hypothesized that CD103+ DC might be critical for the regulation of chronic ileitis. While CD103 deficiency is in itself insufficient to inhibit antigen presentation by MP[42], antibody depletion of CD103+ DC exacerbated murine colitis, supporting a protective function for this population[5, 41].
Previous studies have demonstrated that Flt3-L is required for CD103+ DC proliferation[46, 47] and injection of Flt3-L has been shown to increase the number of CD103+ DC and Tregs in the mouse intestine[48]. Thus, we next sought to determine whether administration of Flt3-L might have a therapeutic effect in ileitis. While Flt3-L has been shown previously to block induction of inflammation in a transfer-colitis model[48], this study is the first to demonstrate attenuation of inflammation in a chronic model. In our studies Flt3-L preferentially expanded CD103+/CD11b+ and CD103+/CD11bNeg DC within the LP, driving induction of CD4+/CD25+/FoxP3+ Tregs as has been shown in mice[16, 17, 41-43] and humans[42]. Thus, Flt3-L administration increased the frequency of CD103+ DC in the LP of TNFΔARE mice, expanded the Treg population and attenuated TNF-mediated Crohn’s-like ileitis.
While this manuscript has focused primarily on the pro-regulatory capacity of CD103+ DC in chronic murine ileitis, it must be noted that these are not the only DC population with a proven capacity to expand in response to Flt3-L stimulation and also induce Tregs. Previous studies have demonstrated the capacity of Flt3-L to induce expansion of plasmacytoid DC [49] and migration of this population from peripheral circulation to the inflamed intestine has been proposed to correlate with disease severity in ulcerative colitis and Crohn’s disease [50]. Furthermore, an increase in pDC in the intestinal lamina propria has been demonstrated previously in a murine colitis model [51]. Although numerous studies have demonstrated that CD103+ DC do not express B220 [52], mature plasmacytoid DC may induce Tregs with suppressive function. While the possible contribution of plasmacytoid DC to the anti-inflammatory effect of Flt3-L warrants further evaluation in this model, the capacity of CD103+ DC to induce both gut-homing and regulatory phenotypes place these cells firmly in the forefront of candidates for driving expansion of Tregs seen in the inflamed intestine leading to attenuation of ileitis in vivo.
In summary, our studies examined the frequency of distinct MP subsets in a mouse model that recapitulates many features of CD and demonstrates the therapeutic effect Flt3-L supplementation in a chronic model of IBD. As promoting T cell regulation remains an attractive strategy for induction and maintenance of remission in IBD, the preferential expansion of pro-regulatory DC with Flt3-L maybe further explored as a novel biological therapy. The safety of Flt3-L administration has been demonstrated in healthy human subjects[53] and efforts are currently underway to optimize the large-scale preparation of bioactive Progenipoietin-1, a fusion protein with dual-agonistic properties for both G-CSFR and Flt3, which in vitro has been shown to have synergistic effects on the expansion of the CD103+ subset and its regulatory T cell progeny[54].
Supplementary Material
Supplemental Figure 1 Progression of ileitis in TNFΔARE mice from 4- to 20-weeks-of-age. (A) Inflammatory indices from ilea of 4-, 8- and 20-week-old TNFΔARE+/− mice (Mean ± SEM n=12-20). (B) Representative micrographs of ilea at indicated ages; (H&E, 10× magnification, bars=100μm).
Supplemental Figure 2 Partial deficiency of CX3CR1 did not alter the severity of ileitis. (A) Inflammatory indices from ilea of 20-week-old TNFΔARE/CX3CR1+/+ and TNFΔARE/CX3CR1GFP/+ mice (Mean ± SEM n=9, 6). (B) Representative micrographs; (H&E, 10× magnification, bars=100μm).
Supplemental Figure 3 Increased CX3CR1 mRNA transcripts in ilea of TNFΔARE mice. Analysis of CX3CR1 mRNA transcripts from 4- and 20-week-old WT and TNFΔARE ileal whole tissues Mean ± SEM, for n=6, *p<0.05).
Supplemental Figure 4 Inclusion of F4/80+ leukocytes did not alter the ratio of CX3CR1+ and CD103+ mononuclear phagocytes in chronic ileitis.
The proportion of CD103+ and CX3CR1+ cells, without exclusion of F4/80+ cells, were assessed by flow cytometry from the LP of TNFΔARE/CX3CR1GFP/+ and WT/CX3CR1GFP/+ mice at 20-weeks-of-age compared with WT age-matched control mice. (Mean ± SEM, n=4, *p<0.05, **p<0.01).
Supplemental Figure 5 Most CD11c+/CD11b+ mononuclear phagocytes express CX3CR1 in WT and TNFΔARE/CX3CR1GFP/+ mice. The fraction of CX3CR1+ cells was analyzed by flow cytometry within the CD11c+/CD11b+ MP subset. Representative zebra plots and histograms.
Supplemental Figure 6 CD103+ DC express higher levels of RA synthetic enzymes. Analysis of RALDH2 mRNA transcripts from isolated CD11c+/MHCII+ CD103+ and CD103Neg MP from the MLN and LP of 20-week-old mice using GAPDH as an endogenous control (Mean ± SEM, n=6, *p<0.05).
Supplemental Figure 7 Effect of Flt3-L administration on RALDH2 and CX3CR1 mRNA transcripts in terminal ilea of TNFΔARE mice. Analysis of RALDH2 and CX3CR1 mRNA transcripts from whole ileal LP of 20-week-old TNFΔARE mice treated with vehicle or Flt3-L using GAPDH as an endogenous control (Mean ± SEM, n=6, *p<0.05).
Supplemental Figure 8 Effect of Flt3-L administration on the frequency of E-cadherin+ DC isolated from terminal ilea of TNFΔARE mice. (A) Quantification of CD11cHi/MHCII+/E-cadherin+ DC from ileal LP of 20-week-old WT and TNFΔARE mice treated with vehicle or Flt3-L (Mean ± SEM, n=4, *p<0.05, ***p<0.001). (B) Representative overlaid histograms of the expression of E-cadherin on CD11cHi/MHCII+ cells from the ileal lamina propria of 20-week-old mice. White histogram indicates WT cells, grey histogram represents vehicle-treated TNFΔARE mice and hatched histogram reflects expression in TNFΔARE mice after treatment with Flt3-L.
Supplemental Figure 9 Effect of Anti-CD25 antibody administration on Flt3-L mediated attenuation of ileitis.
Inflammatory indices from ilea of 20-week-old TNFΔARE mice treated with vehicle, Flt3-L or anti-CD25 antibody + Flt3-L were assessed as described[55] (Mean ± SEM, **p<0.01, *p<0.05, n=5/treatment group).
SUMMARY BOX.
What is already known about this subject?
CD103+ dendritic cells preferentially induce regulatory gut homing T cells
Regulatory T cells (Tregs) prevent and treat established disease in mouse models of inflammatory bowel disease (IBD).
Flt3-ligand preferentially expands CD103+ DC in mice and humans.
What are the new findings?
There is expansion of mononuclear phagocytes in chronic ileitis
There is an altered ratio of CX3CR1+ mononuclear phagocytes and CD103+ DC during chronic ileitis
Flt3-ligand administration preferential expands CD103+ DC, FoxP3+ Tregs in vivo and attenuates chronic murine ileitis.
How might it impact on clinical practice in the foreseeable future?
Flt3-ligand has been shown to be safe and effective in human clinical trials previously, these findings therefore have potential translational value for the treatment of patients with IBD.
Acknowledgments
Funding: This project was funded by grants from the NIH/NIDDK USPHS DK080212-01, the Crohn’s and Colitis Foundation of America (CCFA) senior research award 2826 and US Veteran’s administration BLR&D Merit Review Award 1I01BX001051 to J. R-N. and the CCFA 2652 to CBC.
Abbreviations used in this paper
- MP
mononuclear phagocyte
- DC
dendritic cell
- CD
Crohn’s disease
- IBD
inflammatory bowel disease
- Treg
regulatory T cell
- FoxP3
forkhead box P3
- RA
retinoic acid
- RALDH
retinaldehyde dehydrogenase
- Flt3-L
FMS-like tyrosine kinase 3 ligand
- ΔARE
ΔAU-rich element
- LP
lamina propria
- MLN
mesenteric lymph node
- MFI
mean fluorescence intensity
Footnotes
Competing Interests: The authors have no competing financial interests.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article to be published in GUT and any other BMJPGL products and to exploit all subsidiary rights.
REFERENCES
- 1.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 3.van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8:291–300. doi: 10.1097/00054725-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Schulz O, Jaensson E, Persson E, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–12. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 7.Fong AM, Robinson LA, Steeber DA, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–9. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida Y, Hayashi T, Goto T, et al. Essential involvement of CX3CR1-mediated signals in the bactericidal host defense during septic peritonitis. J Immunol. 2008;181:4208–18. doi: 10.4049/jimmunol.181.6.4208. [DOI] [PubMed] [Google Scholar]
- 9.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Requena P, Daddaoua A, Martínez-Plata E, et al. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregulation of interleukin 17. Br J Pharmacol. 2008;154:825–32. doi: 10.1038/bjp.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamias G, Okazawa A, Rivera-Nieves J, et al. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–18. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- 15.Heimesaat MM, Fischer A, Siegmund B, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS ONE. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coombes J, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Lyman SD, James L, Zappone J, et al. Characterization of the protein encoded by the flt3 (flk2) receptor-like tyrosine kinase gene. Oncogene. 1993;8:815–22. [PubMed] [Google Scholar]
- 20.Rosnet O, Marchetto S, deLapeyriere O, et al. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991;6:1641–50. [PubMed] [Google Scholar]
- 21.Rosnet O, Schiff C, Pebusque MJ, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–9. [PubMed] [Google Scholar]
- 22.Brasel K, Escobar S, Anderberg R, et al. Expression of the flt3 receptor and its ligand on hematopoietic cells. Leukemia. 1995;9:1212–8. [PubMed] [Google Scholar]
- 23.Lyman SD, James L, Johnson L, et al. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–801. [PubMed] [Google Scholar]
- 24.Jacobsen SE, Veiby OP, Myklebust J, et al. Ability of flt3 ligand to stimulate the in vitro growth of primitive murine hematopoietic progenitors is potently and directly inhibited by transforming growth factor-beta and tumor necrosis factor-alpha. Blood. 1996;87:5016–26. [PubMed] [Google Scholar]
- 25.Maraskovsky E, Pulendran B, Brasel K, et al. Dramatic numerical increase of functionally mature dendritic cells in FLT3 ligand-treated mice. Adv Exp Med Biol. 1997;417:33–40. doi: 10.1007/978-1-4757-9966-8_6. [DOI] [PubMed] [Google Scholar]
- 26.Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera-Nieves J, Bamias G, Vidrich A, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–82. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 29.Ho J, Kurtz CC, Naganuma M, et al. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 31.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krajina T, Leithäuser F, Möller P, et al. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073–83. doi: 10.1002/eji.200323518. [DOI] [PubMed] [Google Scholar]
- 33.Cruickshank SM, English NR, Felsburg PJ, et al. Characterization of colonic dendritic cells in normal and colitic mice. World J Gastroenterol. 2005;11:6338–47. doi: 10.3748/wjg.v11.i40.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungaro R, Fukata M, Hsu D, et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1167–79. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda Y, Akbar F, Matsui H, et al. Characterization of antigen-presenting dendritic cells in the peripheral blood and colonic mucosa of patients with ulcerative colitis. European journal of gastroenterology & hepatology. 2001;13:841–50. doi: 10.1097/00042737-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Chirdo FG, Millington OR, Beacock-Sharp H, et al. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–40. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 37.Koscielny A, Boerner T, Wehner S, et al. The role of dendritic cells in the gastrointestinal field effect. Transplant Proc. 2006;38:1815–7. doi: 10.1016/j.transproceed.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 38.Kamda JD, Singer SM. Phosphoinositide 3-kinase-dependent inhibition of dendritic cell interleukin-12 production by Giardia lamblia. Infect Immun. 2009;77:685–93. doi: 10.1128/IAI.00718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson TS, Reuter BK, Scott KG, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–52. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 41.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fraticelli P, Sironi M, Bianchi G, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–81. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kostadinova FI, Baba T, Ishida Y, et al. Crucial involvement of the CX3CR1-CX3CL1 axis in dextran sulfate sodium-mediated acute colitis in mice. Journal of Leukocyte Biology. 2010 doi: 10.1189/jlb.1109768. [DOI] [PubMed] [Google Scholar]
- 46.Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darrasse-Jeze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–62. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onai N, Obata-Onai A, Schmid MA, et al. Flt3 in regulation of type I interferon-producing cell and dendritic cell development. Ann N Y Acad Sci. 2007;1106:253–61. doi: 10.1196/annals.1392.015. [DOI] [PubMed] [Google Scholar]
- 50.Baumgart DC, Metzke D, Schmitz J, et al. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–36. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wurbel MA, McIntire MG, Dwyer P, et al. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS ONE. 2011;6:e16442. doi: 10.1371/journal.pone.0016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, et al. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268–81. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 53.Maraskovsky E, Daro E, Roux E, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–84. [PubMed] [Google Scholar]
- 54.O’Keeffe M, Hochrein H, Vremec D, et al. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–30. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 55.Burns RC, Rivera-Nieves J, Moskaluk CA, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Progression of ileitis in TNFΔARE mice from 4- to 20-weeks-of-age. (A) Inflammatory indices from ilea of 4-, 8- and 20-week-old TNFΔARE+/− mice (Mean ± SEM n=12-20). (B) Representative micrographs of ilea at indicated ages; (H&E, 10× magnification, bars=100μm).
Supplemental Figure 2 Partial deficiency of CX3CR1 did not alter the severity of ileitis. (A) Inflammatory indices from ilea of 20-week-old TNFΔARE/CX3CR1+/+ and TNFΔARE/CX3CR1GFP/+ mice (Mean ± SEM n=9, 6). (B) Representative micrographs; (H&E, 10× magnification, bars=100μm).
Supplemental Figure 3 Increased CX3CR1 mRNA transcripts in ilea of TNFΔARE mice. Analysis of CX3CR1 mRNA transcripts from 4- and 20-week-old WT and TNFΔARE ileal whole tissues Mean ± SEM, for n=6, *p<0.05).
Supplemental Figure 4 Inclusion of F4/80+ leukocytes did not alter the ratio of CX3CR1+ and CD103+ mononuclear phagocytes in chronic ileitis.
The proportion of CD103+ and CX3CR1+ cells, without exclusion of F4/80+ cells, were assessed by flow cytometry from the LP of TNFΔARE/CX3CR1GFP/+ and WT/CX3CR1GFP/+ mice at 20-weeks-of-age compared with WT age-matched control mice. (Mean ± SEM, n=4, *p<0.05, **p<0.01).
Supplemental Figure 5 Most CD11c+/CD11b+ mononuclear phagocytes express CX3CR1 in WT and TNFΔARE/CX3CR1GFP/+ mice. The fraction of CX3CR1+ cells was analyzed by flow cytometry within the CD11c+/CD11b+ MP subset. Representative zebra plots and histograms.
Supplemental Figure 6 CD103+ DC express higher levels of RA synthetic enzymes. Analysis of RALDH2 mRNA transcripts from isolated CD11c+/MHCII+ CD103+ and CD103Neg MP from the MLN and LP of 20-week-old mice using GAPDH as an endogenous control (Mean ± SEM, n=6, *p<0.05).
Supplemental Figure 7 Effect of Flt3-L administration on RALDH2 and CX3CR1 mRNA transcripts in terminal ilea of TNFΔARE mice. Analysis of RALDH2 and CX3CR1 mRNA transcripts from whole ileal LP of 20-week-old TNFΔARE mice treated with vehicle or Flt3-L using GAPDH as an endogenous control (Mean ± SEM, n=6, *p<0.05).
Supplemental Figure 8 Effect of Flt3-L administration on the frequency of E-cadherin+ DC isolated from terminal ilea of TNFΔARE mice. (A) Quantification of CD11cHi/MHCII+/E-cadherin+ DC from ileal LP of 20-week-old WT and TNFΔARE mice treated with vehicle or Flt3-L (Mean ± SEM, n=4, *p<0.05, ***p<0.001). (B) Representative overlaid histograms of the expression of E-cadherin on CD11cHi/MHCII+ cells from the ileal lamina propria of 20-week-old mice. White histogram indicates WT cells, grey histogram represents vehicle-treated TNFΔARE mice and hatched histogram reflects expression in TNFΔARE mice after treatment with Flt3-L.
Supplemental Figure 9 Effect of Anti-CD25 antibody administration on Flt3-L mediated attenuation of ileitis.
Inflammatory indices from ilea of 20-week-old TNFΔARE mice treated with vehicle, Flt3-L or anti-CD25 antibody + Flt3-L were assessed as described[55] (Mean ± SEM, **p<0.01, *p<0.05, n=5/treatment group).