Abstract
Objective
The value of metabolic syndrome (MetS) in childhood and adolescence and its stability into young adulthood have been questioned. This study compared the MetS in late childhood (mean age 13) versus a cluster score of the MetS components as predictors of young adult (mean age 22) cardiovascular risk.
Methods
Anthropometrics, blood pressure, lipid profile, and insulin resistance (insulin clamp) were obtained in 265 individuals at mean ages 13 and 22. MetS was defined dichotomously by current pediatric and adult criteria. The MetS cluster score used the average of deviates of the MetS components standardized to their means and standard deviations at mean age 13.
Results
The MetS was rarely present at mean age 13 and did not predict MetS at mean age 22 but identified individuals who continued to have adverse levels of risk factors at mean age 22. In contrast to the standard MetS definition, the MetS cluster score tracked strongly and at mean age 22 was significantly higher in the individuals with MetS at mean age 13 (0.78 ± 0.71) than those without MetS at mean age 13 (0.09 ± 0.70, p<0.0001).
Conclusions
Although MetS at mean age 13, using the conventional definition, is not a reliable method for predicting MetS at mean age 22, it does predict adverse levels of cardiovascular risk factors. A cluster score, using the MetS components as continuous variables, is more reliable in predicting young adult risk from late childhood.
Key Terms: Metabolic Syndrome, Risk Factors, Obesity, Insulin Resistance, Children
Introduction
The increasing prevalence of childhood obesity has led to an intensive effort to identify children at risk for development of adult cardiovascular disease and type 2 diabetes. Prominent among the approaches proposed is the classification of children according to the metabolic syndrome (MetS), with modification of the adult components (waist circumference, triglycerides, high density lipoprotein [HDL] cholesterol, fasting glucose, blood pressure) to comply with childhood norms (1;2). There is wide recognition that the adult MetS, as defined by the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program (3;4), is associated with obesity in general, and in particular, with visceral adiposity (5), and at any given level of fatness, MetS increases the long term risk of cardiovascular disease and type 2 diabetes (6). Thus, obesity and MetS in childhood would be expected to be predictors of MetS and cardiovascular risk factors in adulthood.
The prevalence of pediatric MetS in 12–19 year olds increased from 4.2% in NHANES III (1) to 6.4% in NHANES 1999–2000 (7). The prevalence of MetS in children is lower than in adults, with an overall childhood prevalence between 2% and 9.4% and even higher rates (12.4 – 44.2%) in obese children and adolescents (8). Despite the significant prevalence of the MetS as currently defined, the appropriateness of combining the five cardiovascular risk components into a syndrome and the relation of MetS to insulin resistance and obesity has recently been questioned in adults (9), and similar questions have been raised in childhood, as noted in a scientific statement from the American Heart Association (10) highlighting the challenges of defining the MetS in children. Relevant questions have been raised about dichotomizing risk factors that are known to represent continuous levels of risk, failure to develop a unified pediatric definition for MetS, and the instability of the childhood classification of risk that cast doubt on the use of MetS to predict adult risk (11–13), Although alternative methods to stratify cardiovascular risk in children have been proposed (14), no consensus has been reached.
The present study was based on the hypotheses that: 1) risk factor clustering exists in childhood, 2) the tracking of risk factors from childhood into young adulthood is strong, and 3) methods other than the categorical/dichotomous definitions used in the MetS, such as a cluster score method (15–23), would be more powerful estimates for predicting levels of adult cardiovascular risk factors. The study was conducted in a cohort participating in a longitudinal evaluation between late childhood and early young adulthood of the influence of obesity and insulin resistance on the development of cardiovascular risk factors.
Methods
Participants
Participants in this study were randomly recruited after blood pressure, height and weight screening of 12,043 5th–8th grade Minneapolis Public School students (3,819 black, 4,216 white, 4,008 other; 6,035 male, 6,008 female), representing 93% of all students in those grades (24). An initial clinic examination, euglycemic hyperinsulinemic clamp and other laboratory studies were conducted at mean age 13 (range 11–15 years) in 357 Black and non-Hispanic white children and repeated at mean age 22 (range 19–24 years). For this analysis we had complete data available at childhood and young adulthood for 265 participants. This study was approved by the Human Subjects Committee of the University of Minnesota. Consent was obtained from all children and their parents/guardians.
Anthropometry and Blood Pressure
The children underwent a complete physical examination including Tanner staging and anthropometric measurements. Height was measured by a wall-mounted stadiometer. Weight was measured by a balance scale. Body fat percentage and lean body mass (LBM), or fat-free mass, was calculated by skinfold analysis (25) at mean age 13 and using dual energy x-ray absorptiometry (DXA) (Prodigy, 3M, Madison, WI, USA) at mean age 22. Since DXA was not available at the mean age 13 examination, the body fat percentage and LBM values at mean age 13 were calibrated to DXA values according to equations derived from studies in siblings of the present cohort, and within the same age range (26). Blood pressure was measured twice on the right arm using a random-zero sphygmomanometer with participants seated; the averages of the two measurements (systolic blood pressure [SBP] and 5th phase Korotkoff diastolic blood pressure [DBP]) were used in the analyses.
Measurement of Insulin Sensitivity
Euglycemic hyperinsulinemic clamp studies were conducted in the University of Minnesota Clinical Research Center as previously described (24). Blood samples for fasting serum insulin and lipoproteins were obtained at baseline (before starting the insulin infusion). Plasma glucose was measured at baseline and every five minutes during the age 13 clamp and every 10 minutes during the mean age 22 clamp. The insulin infusion of 1 mU/kg/min was started at time 0 and continued for 3 hours. An infusion of 20% glucose was started at time 0 and adjusted, based on plasma glucose levels, to maintain plasma glucose at 5.6 mmol/l (100 mg/dl). Insulin sensitivity, M, was determined from the amount of glucose administered over the final 40 minutes of the euglycemic clamp and was expressed as Mlbm (i.e., glucose utilization/ kg lean body mass/ minute). Blood samples were analyzed for glucose immediately at the bedside with a Beckman Glucose Analyzer II (Beckman Instruments Inc., Fullerton, CA). Insulin levels (radioimmunoassay) and serum lipids were determined in the laboratory of the Fairview- University Medical Center.
Definitions of Metabolic Syndrome
Categorical designation of MetS at mean age 22 was based on the previously published adult ATP III criteria (3;4). The definition of MetS at mean age 13 was based on modification of ATP III criteria from two sources: 1) a study of NHANES data in children (1); and 2) adult criteria modified for children by the International Diabetes Federation (2).
Calculation of the Metabolic Syndrome Cluster Score
A MetS cluster score was developed, consisting of the average of standardized deviates of the primary components of the MetS (i.e., the sum of the z scores of waist circumference, SBP, triglycerides, inverse HDL-cholesterol, and fasting glucose divided by 5) at mean age 13. As base values, we used means and standard deviations for the five MetS components at mean age 13, namely the values observed in our initial examination of the cohort (Table 1). Thus, the score at any age can be computed as: 1/5*((waist circumference-77.7)/11.4 −(HDL-cholesterol-44.6)/10.2 + (triglycerides-90.5)/52.9 + (SBP-107.6)/9.2 + (glucose-88.2)/7.4). The concept allows the score to grow with age and changes in the MetS components by inserting the observed values of waist circumference, HDL-cholesterol, triglycerides, SBP, and fasting glucose for that age into the formula. A higher z-score indicates that the components tend to cluster in the higher sections of the distributions, i.e., represent overall higher risk. While it is theoretically possible that a high cluster score could arise from an extreme value of a single component, we verified that high scores are almost always the result of high values in two or more components.
Table 1.
Clinical Characteristics of the Cohort (N = 265) at Mean Ages 13 and 22 and Correlations between Mean Ages 13 and 22
| Variable | Mean age 13 | Mean age 22 | Correlation |
|---|---|---|---|
| Age (years) | 13 ± 1.2 | 21.6 ± 1.6 | |
| Gender (number) | |||
| Males | 151 (57%) | ||
| Females | 114 (43%) | ||
| Race (number) | |||
| White | 218 (82%) | ||
| Black | 47 (18%) | ||
| Body Mass Index (kg/m2) | 22 ± 4.7 | 26.2 ± 6.5 | 0.80 |
| Waist Circumference (cm) | 77.7 ± 11.4 | 87.1 ± 16.2 | 0.73 |
| Body Fat (%) | 29.7 ± 11.3 | 28.5 ± 12.5 | 0.76 |
| Total Cholesterol (mg/dL) | 151.2 ± 29.3 | 161.0 ± 31.4 | 0.50 |
| LDL Cholesterol (mg/dL) | 88.6 ± 25.4 | 95.8 ± 27.6 | 0.57 |
| HDL Cholesterol (mg/dL) | 44.6 ± 10.2 | 46.1 ± 11.4 | 0.54 |
| Triglycerides (mg/dL) | 90.5 ± 52.9 | 96.6 ± 54.1 | 0.29 |
| Systolic Blood Pressure (mmHg) | 107.6 ± 9.2 | 110.2 ± 10 | 0.42 |
| Diastolic Blood Pressure (mmHg) | 56.0 ± 13.1 | 65.1 ± 10.2 | 0.31 |
| Fasting Glucose (mg/dL) | 88.2 ± 7.4 | 85.6 ± 9.4 | 0.21 |
| Fasting Insulin (mU/L) | 11.4 ± 8.9 | 8.8 ± 12.4 | 0.13 |
| Mlbm (mg/kg/min) | 12.8 ± 4.4 | 11.7 ± 4.1 | 0.15 |
| MetS Cluster Score | −0.01 ± 0.58 | 0.14 ± 0.72 | 0.51 |
Data are presented as mean ± standard deviation. All tracking correlations are statistically significant.
Statistical Analysis
Data from all participants were combined considering participant age but ignoring Tanner stage, based on the fact that there was a similar relation of insulin sensitivity to body mass index (BMI) across Tanner stages as previously reported in this cohort (27). Data were expressed as mean ± standard deviation unless otherwise stated. Variables that were not normally distributed (i.e., fasting insulin, triglycerides) were log-transformed; however, results were not qualitatively different from those using non-transformed data and non-transformed data were presented for simplicity and were used for the calculation of the MetS cluster score.
Prediction of development of young adult cardiovascular risk from late childhood factors was assessed by three methods of analysis, as follows: 1) partial Pearson correlation analysis for tracking of the risk factors between mean ages 13 and 22; 2) using the modified ATP III classification of MetS at mean age 13 to predict the ATP III MetS at mean age 22; and 3) using the MetS cluster score, referenced to the mean age 13 risk factor values, to predict the mean age 22 ATP III MetS and risk factors. In the second set of analyses we used logistic regression analysis with dependent variable MetS at mean age 22 (adult ATP III criteria) and adjustments made for sex, race, and age. In the third set of analyses we performed parallel linear regression with dependent variable MetS cluster score at mean age 22.
Results
Baseline factors (anthropometric measures, blood pressure, fasting insulin, fasting glucose, and fasting lipids) were not significantly different between the participants included in the present cohort and those examined at mean age 13 but not included at mean age 22. Table 1 shows the clinical characteristics of the cohort at mean ages 13 and 22 along with tracking correlations for each of the variables. The cohort consisted of 265 youth (age 13 ± 1 years old; 151 (57%) males; 218 (82%) white). All correlations of the individual risk factors were statistically significant, and the strongest correlations were related to body fatness (BMI, waist circumference, percent body fat). Correlations of the other risk factors (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, SBP, DBP, fasting glucose, Mlbm, fasting insulin) were somewhat weaker, suggesting considerable within person variation during this period of maturation.
At mean age 13, pediatric MetS (1) was diagnosed in 18 (7%) participants. Of these, only 3 continued to have MetS at mean age 22, and of the 12 participants with pediatric MetS according to criteria proposed by the International Diabetes Federation (2), only one continued to have MetS at mean age 22. In a stepwise multiple regression analysis of the five MetS components, only waist circumference was a significant predictor of young adult MetS: OR = 1.05 (95% CI = 1.01–1.09, p = 0.008). For every 1 cm increase in waist circumference at mean age 13, the odds for developing MetS at mean age 22 increased by 5%. For every one standard deviation (11.5 cm) increase in waist circumference at mean age 13, the odds for developing MetS at mean age 22 increased by 78% (OR = 1.78; 95% CI = 1.16–2.72, p = 0.008). Similar to waist circumference, for every 1 unit (kg/m2) increase in BMI at mean age 13, the odds for developing MetS at mean age 22 increased by 13% (OR = 1.13; 95% CI = 1.04–1.23, p = 0.003). For every one standard deviation (4.7 BMI units) increase in BMI at mean age 13, the odds for developing MetS at mean age 22 increased by 80% (OR = 1.80; 95% CI = 1.22–2.67, p = 0.003).
Despite the failure of MetS at mean age 13 to show a tracking effect, the individuals with MetS had significantly adverse levels of many risk factors at mean age 22 when compared to the individuals without mean age 13 MS (Table 2). The mean age 22 risk factor levels were significantly higher for BMI, waist circumference, percent body fat, and triglycerides, and significantly lower for HDL cholesterol and Mlbm. Total cholesterol, LDL cholesterol, SBP, DBP, fasting glucose, and fasting insulin were not significantly different between the two groups. In contrast, the difference in the cluster score at mean age 22, based on MetS at mean age 13, was striking (Figure 1.)
Table 2.
Clinical Characteristics of the Cohort (N = 265) at Mean Age 22 According to Metabolic Syndrome Status at Mean Age 13
| Variable | No MetS at Age 13 (N = 247) | MetS at Age 13 (N = 18) | P-Value |
|---|---|---|---|
| Body Mass Index (kg/m2) | 25.7 ± 6.0 | 32.6 ± 9.5 | <0.0001 |
| Waist Circumference (cm) | 85.7 ± 14.9 | 106.4 ± 20.7 | <0.0001 |
| Body Fat (%) | 27.8 ± 12.5 | 39.4 ± 8.1 | 0.0007 |
| Total Cholesterol (mg/dL) | 160.1 ± 31.0 | 173.6 ± 35.4 | 0.104 |
| LDL Cholesterol (mg/dL) | 94.9 ± 27.2 | 107.8 ± 31.1 | 0.071 |
| HDL Cholesterol (mg/dL) | 46.5 ± 11.3 | 40.0 ± 10.7 | 0.021 |
| Triglycerides (mg/dL) | 94.2 ± 52.2 | 128.9 ± 69.7 | 0.013 |
| Systolic Blood Pressure (mmHg) | 110.1 ± 10.2 | 111.2 ± 7.1 | 0.538 |
| Diastolic Blood Pressure (mmHg) | 65.2 ± 10.4 | 64.2 ± 8.6 | 0.743 |
| Fasting Glucose (mg/dL) | 85.4 ± 9.6 | 87.2 ± 6.9 | 0.294 |
| Fasting Insulin (mU/L) | 8.6 ± 12.7 | 10.9 ± 6.9 | 0.436 |
| Mlbm (mg/kg LBM/min) | 11.9 ± 3.9 | 9.6 ± 5.3 | 0.037 |
Data are presented as mean ± standard deviation. All analyses were adjusted for sex, race, and age.
Figure 1.
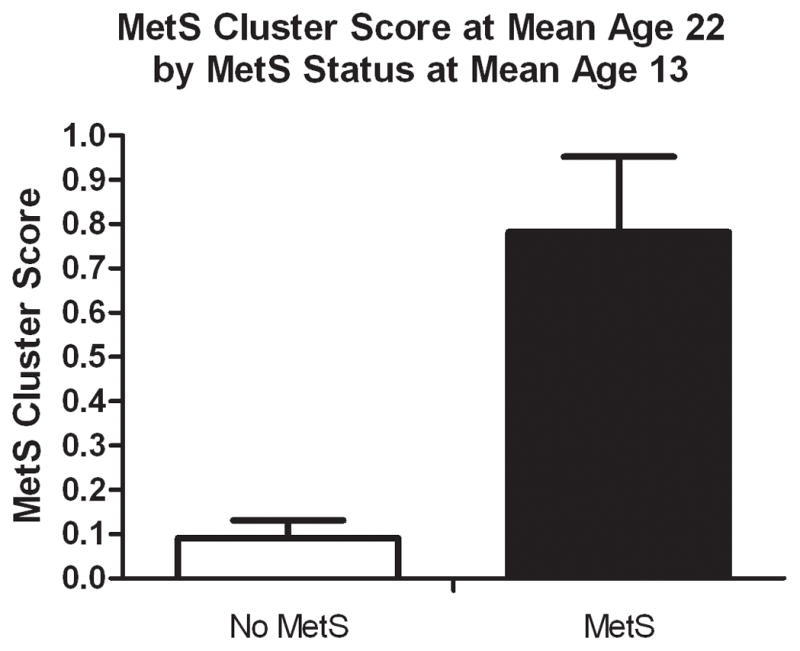
Metabolic Syndrome Cluster Score at Mean Age 22 According to Metabolic Syndrome Status at Mean Age 13
We also looked retrospectively at tracking of MetS by comparing the clinical characteristics at mean age 13 between individuals with (N=16) and without MetS at mean age 22. Although all but three with MetS at mean age 22 did not have pediatric MetS, the group had significantly greater mean values at mean age 13 for BMI, waist circumference, body fat percent, total cholesterol, LDL cholesterol, and the MetS cluster score than the group without mean age 22 MetS. The mean levels of SBP, triglycerides, glucose and fasting insulin were higher and the mean levels for Mlbm and HDL were lower, but these differences were not statistically significant.
The tracking correlation for the MetS cluster score was substantial (r = 0.51, p<0.0001), indicating that relative rank of the MetS cluster score at mean age 13 predicted its rank at mean age 22 (Table 1.). Tracking by quartile of cluster score was also significant; 82% of those in quartile 1 (lowest cluster scores) at mean age 13 were in either quartile 1 or quartile 2 at mean age 22 and 76% of those in quartile 4 at mean age 13 were in either quartile 4 or quartile 3 at mean age 22. Table 3 shows risk factors at mean age 22 by mean age 13 cluster score quartile. All risk factors, including insulin resistance (Mlbm), worsened across increasing quartiles of MetS cluster score, except DBP and fasting insulin.
Table 3.
Clinical Characteristics of the Cohort (N = 265) at Mean Age 22 According to Quartile of Metabolic Syndrome Cluster Score at Mean Age 13
| Variable | Quartile 1 (N = 59) (range −1.440 to −0.411) | Quartile 2 (N = 67) (range −0.400 to −0.073) | Quartile 3 (N = 73) (range −0.072 to 0.317) | Quartile 4 (N = 66) (range 0.318 to 2.044) | P-Trend |
|---|---|---|---|---|---|
| Body Mass Index (kg/m2) | 23.2 ± 4.9 | 25.4 ± 5.5 | 26.9 ± 6.5 | 29.0 ± 7.6 | <0.0001 |
| Waist Circumference (cm) | 78.9 ± 11.6 | 84.6 ± 14.9 | 88.6 ± 14.5 | 95.3 ± 18.7 | <0.0001 |
| Body Fat (%) | 24.7 ± 12.2 | 25.9 ± 11.9 | 29.7 ± 12.8 | 33.1 ± 11.8 | <0.0001 |
| Total Cholesterol (mg/dL) | 149.6 ± 27.7 | 160.7 ± 36.9 | 162.6 ± 28.0 | 169.8 ± 29.6 | 0.0004 |
| LDL Cholesterol (mg/dL) | 82.8 ± 23.4 | 95.6 ± 31.7 | 97.5 ± 23.7 | 105.7 ± 26.7 | <0.0001 |
| HDL Cholesterol (mg/dL) | 53.1 ± 13.2 | 46.5 ± 10.3 | 44.2 ± 10.0 | 41.5 ± 9.1 | <0.0001 |
| Triglycerides (mg/dL) | 71.7 ± 35.9 | 93.1 ± 51.9 | 104.8 ± 59.1 | 113.3 ± 56.7 | <0.0001 |
| Systolic Blood Pressure (mmHg) | 106.5 ± 9.2 | 109.7 ± 10.6 | 111.3 ± 8.8 | 112.8 ± 10.4 | 0.0004 |
| Diastolic Blood Pressure (mmHg) | 64.5 ± 9.2 | 66.3 ± 9.7 | 63.6 ± 9.6 | 66.2 ± 12.1 | 0.65 |
| Fasting Glucose (mg/dL) | 83.1 ± 10.9 | 85.5 ± 8.7 | 85.0 ± 9.2 | 88.5 ± 8.4 | 0.002 |
| Fasting Insulin (mU/L) | 7.6 ± 10.0 | 8.0 ± 5.0 | 8.4 ± 5.1 | 11.2 ± 21.6 | 0.28 |
| Mlbm (mg/kg LBM/min) | 13.0 ± 4.4 | 11.4 ± 3.5 | 11.6 ± 4.2 | 11.0 ± 3.8 | 0.02 |
| MetS Cluster Score | −0.38 ± 0.60 | 0.06 ± 0.64 | 0.24 ± 0.66 | 0.57 ± 0.64 | <0.0001 |
Data are presented as mean ± standard deviation. All analyses were adjusted for sex, race, and age.
Conclusions
The main findings of this study are as follows 1) MetS at mean age 13 (using two separate categorical definitions) does not predict MetS in young adulthood but, nevertheless, identifies individuals who continue to have significantly adverse levels of cardiovascular risk factors in young adulthood, thus, tracking of MetS across adolescence into young adulthood is masked by the arbitrary dichotomization of the risk factors; 2) combining the MetS risk factors into a cluster score that considers each risk factor as a continuous variable, as opposed to the dichotomization used in the MetS, not only resulted in a substantial tracking correlation between mean age 13 and mean age 22, but also showed that the mean age 13 cluster models discriminated mean age 22 risk factors better than any single component of the cluster. Thus, these findings suggest that MetS, as a dichotomous designation, is not a reliable predictor of adults MetS and that alternative classifications, such as a cluster score (15–23), may be preferred methods of identifying youth at risk of developing early cardiovascular disease and type 2 diabetes.
The MetS was originally defined in adults as the presence of abnormal levels for three out of five cardiovascular risk factors (waist circumference, triglycerides, HDL cholesterol, blood pressure, and fasting glucose) (3) and there is general agreement that these MetS risk factors tend to cluster more frequently than would otherwise be expected. However, there continues to be debate regarding the use of the MetS diagnosis to assign levels of risk of cardiovascular disease and type 2 diabetes, as opposed to giving greater consideration to individual levels of each of the MetS constituent parts (28). Nevertheless, the MetS concept is conceptually grounded in mutually occurring and reinforcing adverse metabolic processes and has both theoretical and practical utility (9;29). In an attempt to adapt the MetS to children, arbitrary definitions, analogous to the adult definition, were constructed by modifying the same five risk factors to conform to pediatric standards (1;2). However, the questions raised about the validity of an adult MetS (28) are also relevant for children (10), based on an incomplete understanding of the underlying pathophysiology of MetS, lack of consensus on appropriate pediatric cut-points for specific components, the effect of puberty on some cardiovascular risk factors and lack of hard clinical endpoints and outcomes data. The present study supports the current concerns about the MetS in childhood, as suggested by the instability of the risk factors reported in several studies (11–13) and underscores the need to examine alternative methods of risk stratification (15–23). Ongoing research in this area has provided initial evidence supporting the validation of a continuous MetS score in children (21).
The current study, because of the longitudinal evaluation from late childhood to early young adulthood, expands on those prior findings and helps clarify the relation of risk factors and insulin resistance in childhood to establishment of adult cardiovascular risk. While showing that use of the current dichotomous definitions of childhood MetS is a poor identifier of at-risk children, the broader concepts (i.e., that metabolic clustering is already present in childhood and tracks into adulthood) remain sound. By deriving a cluster score consisting of the five commonly used MetS components considered as continuous variables, it was possible to show the significant relation between childhood MetS and adult cardiovascular risk that otherwise was lost. We suggest that prediction from this risk score will grow stronger as the subjects are followed into later adulthood and levels of the risk factors and potential morbid events are magnified.
The tracking correlation for insulin resistance (Mlbm) was low ( r = 0.15), but significant, in contrast to the higher correlation previously reported from this cohort (r = 0.42) between ages 13 and 19 (30). This suggests that the overall tracking effect for Mlbm in a randomly selected cohort may decrease with time. However, the relevance of insulin resistance to the MetS was established since almost all individuals with MetS were below the median for insulin sensitivity (i.e., relative insulin resistance) at mean age 13 (95%) and mean age 22 (88%), consistent with previous studies reporting a modest relation between insulin resistance and MetS in children (5;31). Moreover, the MetS cluster score at mean age 13 was directly related to insulin resistance at mean age 22. Thus, despite the low degree of tracking between mean ages 13 and 22, when MetS is present, it is accompanied by higher levels of insulin resistance. Previous studies from the present cohort using the insulin clamp have shown an interaction between insulin resistance and body fatness that is related cross-sectionally to an adverse cardiovascular risk factor profile in adolescence (32), and insulin resistance, independent of BMI, predicts future individual cardiovascular risk factors (30). Analysis of relations between insulin resistance and cardiovascular risk during childhood and adolescence may be influenced by the significant changes in insulin resistance that occur during pubertal development (27) and the additional gender-specific changes in insulin resistance that are independent of puberty and occur during the second decade of life (30).
As has been well recognized in prior studies, measures of body fatness (i.e., waist circumference and BMI) in this study tracked strongly from adolescence into young adulthood. Waist at mean age 13 was the only significant predictor of young adult MetS among the five MetS components. Longitudinal reports from the Bogalusa Heart Study (33) and Cardiovascular Risk in Young Finns Study (34) reported that obesity is a primary factor involved in risk factor clustering in childhood and strongly predicts cardiovascular risk factors and vascular outcomes in adulthood. A recent report from the Muscatine Study Longitudinal Adult Cohort highlighted BMI as the strongest childhood predictor of adult MetS (35). Therefore, data from the current study, along with these previous reports, suggest that adiposity in childhood is the single strongest predictor of future cardiovascular risk in adulthood.
This study has some limitations that should be noted. The sample size of the cohort was modest and the age-range of the participants was relatively narrow. We had complete MetS risk factor data at baseline and follow-up for only 265 (sample size reported in this study) of the 357 participants in the cohort. However, baseline factors were similar between those without full data at mean age 22 (excluded from the analysis) and those included in the current analysis. The generalizability of the results may be limited since the cohort was mainly white and the study was conducted in only one region.
In conclusion, the findings of this study show that the childhood MetS, using the five MetS factors as dichotomous variables, is not a reliable predictor of MetS in young adulthood and underestimates the magnitude of risk factor tracking. Nevertheless, it may continue to have a role in childhood healthcare because it identifies children and adolescents who will continue to have significantly adverse levels of risk factors in young adulthood. The MetS cluster score improves upon the dichotomized definition by better predicting levels of young adulthood cardiovascular risk factors, suggesting it may be a useful method of predicting the risk factor trajectory and clustering of children and adolescents into adulthood. Thus, these data highlight the limitations of a dichotomous definition of the MetS in children and adolescents and underscore the importance of exploring alternative methods, such as risk factor cluster scoring, for identifying youth at risk of developing early cardiovascular disease and type 2 diabetes.
Acknowledgments
Grant Support: This project was supported by National Institutes of Health grants HL-52851 and M01-RR-00400 (General Clinical Research Center).
Footnotes
Disclosures: The authors have no relevant conflicts of interest.
Reference List
- 1.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003 Aug;157(8):821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007 Jun 23;369(9579):2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004 Jan 27;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr. 2008 Feb;152(2):177–84. doi: 10.1016/j.jpeds.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006 Aug;91(8):2906–12. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 7.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among u.s. Adolescents, 1999–2000. Diabetes Care. 2004 Oct;27(10):2438–43. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 8.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008 Feb;152(2):165–70. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005 Jun;51(6):931–8. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 10.Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009 Feb 3;119(4):628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 11.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007 May 1;115(17):2316–22. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Ford ES, Huang TT, Sun SS, Goodman E. Patterns of change in cardiometabolic risk factors associated with the metabolic syndrome among children and adolescents: the Fels Longitudinal Study. J Pediatr. 2009 Sep;155(3):S5–16. doi: 10.1016/j.jpeds.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson JK, Yanoff LB, Easter BD, et al. The Stability of Metabolic Syndrome in Children and Adolescents. J Clin Endocrinol Metab. 2009 Oct 16; doi: 10.1210/jc.2008-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brambilla P, Lissau I, Flodmark CE, et al. Metabolic risk-factor clustering estimation in children: to draw a line across pediatric metabolic syndrome. Int J Obes (Lond) 2007 Apr;31(4):591–600. doi: 10.1038/sj.ijo.0803581. [DOI] [PubMed] [Google Scholar]
- 15.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006 Jul 22;368(9532):299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 16.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994 Aug 22;154(16):1842–7. [PubMed] [Google Scholar]
- 17.Batey LS, Goff DC, Jr, Tortolero SR, et al. Summary measures of the insulin resistance syndrome are adverse among Mexican-American versus non-Hispanic white children: the Corpus Christi Child Heart Study. Circulation. 1997 Dec 16;96(12):4319–25. doi: 10.1161/01.cir.96.12.4319. [DOI] [PubMed] [Google Scholar]
- 18.Brage S, Wedderkopp N, Ekelund U, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS) Diabetes Care. 2004 Sep;27(9):2141–8. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 19.DuBose KD, Eisenmann JC, Donnelly JE. Aerobic fitness attenuates the metabolic syndrome score in normal-weight, at-risk-for-overweight, and overweight children. Pediatrics. 2007 Nov;120(5):e1262–e1268. doi: 10.1542/peds.2007-0443. [DOI] [PubMed] [Google Scholar]
- 20.Eisenmann JC, Katzmarzyk PT, Perusse L, Tremblay A, Despres JP, Bouchard C. Aerobic fitness, body mass index, and CVD risk factors among adolescents: the Quebec family study. Int J Obes (Lond) 2005 Sep;29(9):1077–83. doi: 10.1038/sj.ijo.0802995. [DOI] [PubMed] [Google Scholar]
- 21.Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetol Metab Syndr. 2010;2:8. doi: 10.1186/1758-5996-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzmarzyk PT, Perusse L, Malina RM, Bergeron J, Despres JP, Bouchard C. Stability of indicators of the metabolic syndrome from childhood and adolescence to young adulthood: the Quebec Family Study. J Clin Epidemiol. 2001 Feb;54(2):190–5. doi: 10.1016/s0895-4356(00)00315-2. [DOI] [PubMed] [Google Scholar]
- 23.Raitakari OT, Porkka KV, Rasanen L, Ronnemaa T, Viikari JS. Clustering and six year cluster-tracking of serum total cholesterol, HDL-cholesterol and diastolic blood pressure in children and young adults. The Cardiovascular Risk in Young Finns Study. J Clin Epidemiol. 1994 Oct;47(10):1085–93. doi: 10.1016/0895-4356(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 24.Sinaiko AR, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001 Nov;139(5):700–7. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 25.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988 Oct;60(5):709–23. [PubMed] [Google Scholar]
- 26.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 2005 Nov;29(11):1346–52. doi: 10.1038/sj.ijo.0803026. [DOI] [PubMed] [Google Scholar]
- 27.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999 Oct;48(10):2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 28.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005 Sep;28(9):2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 29.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 30.Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008 May 6;117(18):2361–8. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care. 2007 Aug;30(8):2091–7. doi: 10.2337/dc07-0203. [DOI] [PubMed] [Google Scholar]
- 32.Sinaiko AR, Steinberger J, Moran A, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005 Apr 19;111(15):1985–91. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 33.Freedman DS, Patel DA, Srinivasan SR, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008 May;32(5):749–56. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 34.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003 Nov 5;290(17):2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 35.Burns TL, Letuchy EM, Paulos R, Witt J. Childhood predictors of the metabolic syndrome in middle-aged adults: the Muscatine study. J Pediatr. 2009 Sep;155(3):S5–26. doi: 10.1016/j.jpeds.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]


