Abstract
This review will highlight the unique features of immune reconstitution following unrelated cord blood transplantation (UCBT) that lead to heightened risk of infection related mortality (IRM) in the early post UCBT period. There is no evidence that the innate immunity is uniquely compromised after UCBT, however, the development of antigen-specific cellular immunity is affected by numerical and qualitative deficits in the early post-UCBT period, primarily within the first 100 days. Nevertherless, beyond the first few months after UCBT there is no evidence for reduced graft-versus-leukemia (GVL) or anti-viral immunity compared to other hematopoietic cell therapy (HCT) modalities. Immune reconstitution is addressed in both myeloablative and the non-myeloablative settings. Novel cellular therapies that are about to enter the clinical setting in the form of natural killer (NK) cell and T cell therapies in the form of donor lymphocyte infusion (DLI) are also discussed.
INTRODUCTION
Near ablation of the host immune system is an unavoidable consequence of most myeloablative preparative regimens. Interestingly, it was found that regardless of the graft source, most essential cellular members of the innate immune system, namely phagocytes and natural killer lymphocytes, appear to recover rapidly, within weeks after hematopoietic cell transplantation (HCT). This can be observed even after transplantation of highly pure CD34+-selected grafts, used in the haploidentical setting.1,2 The early cellular recovery is in stark contrast with the recovery of functional B and T lymphocytes (adaptive immunity) that may take several months to years.3-5 The first wave of T cells emerging in the lymphopenic host are peripherally expanding T lymphocytes representing the thymic-independent pathway via peripheral expansion.6 However, the antigen-driven expansion leads to a limited and skewed T cell receptor (TCR) repertoire primarily shaped by the allogeneic environment and pathogens present in the host.6 Several weeks or months after HCT a second wave of T cells may emerge derived from so called common lymphocyte progenitors (CLP) as the result of de novo thymopoiesis. In the absence of significant graft-versus-host disease (GvHD) this thymic-dependent pathway3,4 is solely responsible for a fully diverse T cell repertoire displayed by ‘Recent Thymic Emigrants’ (RTE), the most ‘primitive’ subset of the naïve CD45RA+ T cell pool. These T cells express CCR7 and L-selectin (CD62L) along with CD45RA.
UNIQUE FEATURES OF IMMUNE RECOVERY AFTER MYELOABLATIVE CONDITIONING AND UCBT
The limited TCR diversity that characterizes thymic-independent T cell regeneration is in sharp contrast with the infused cord blood T cells that represent a particularly broad repertoire that appears fully constituted at it’s full diversity at birth.7 Cord blood T cells are almost exclusively naïve and antigen inexperienced and display the characteristic CD45RA+/CD45RO-/CD62L+ surface phenotype of RTE. However, the long term persistence of RTE in the circulation depends on the absence of any significant GvHD that may compromise thymic recovery beyond damage inflicted by pretransplant chemo or radiation. Infused RTE lose their characteristic phenotype within a few weeks as peripheral expansion and apoptosis takes its toll in the lymphopenic environment and immunosuppressive drugs. Very young children with abundant thymic tissue and no prior therapy before transplant, e.g. infants with inborn errors of metabolism, may recover T cells and specifically RTE via the central pathway the fastest. Ultimately, it is this thymic-dependent pathway that is capable of generating a long –lasting fully diverse T cell repertoire essential to control any infectious challenge for the life of the patient.4,8,9 Interestingly, TCR diversity was higher in CB recipients than in recipients of BMT 2 years after HCT as measured by TCR rearrangement excision circles (TRECs)10 indicating the existence of an efficient thymic regeneration pathway from CB lymphoid progenitors despite the low number of cells infused. Although mitogenic responses may already reach normal range in children 6-9 months after UCBT, T cell reconstitution is gradual and typically does not reach age appropriate numbers before 9 months contrasting with adults where it typically extends even beyond the first year, presumably as a result from age-dependent decline in pre-UCBT thymic function.11 A detailed analysis in a cohort of adults who underwent UCBT for hematological malignancies demonstrated extremely severe T cell lymphopenia that extended all throughout the first year.12
Immune recovery was significantly worse in these UCBT recipients compared to adults receiving bone marrow (BM) grafts. However, the observed delay may reflect not only thymopoietic failure triggered by chemo and radiation therapy and/or numerical or functional deficits of CB-derived CLP, but may also reflect the prolonged detrimental effects of thymoglobulin, in this single center cohort.12
In contrast to T cells, natural killer (NK) cell recovery is prompt in both adults and children by the first 2 months both in numbers and function similar to recipients of BM.12-14 Significant B cell recovery starts approximately 3-4 months after transplant that may reach normal numbers by 6 months.12,15
While the incidence of life threatening viral infections (opportunistic infections [OI]) is high in the first 6 months after UCBT suggesting deficits in T cell numbers or function, the speed of T cell recovery seems to be at least comparable16 to or even better than that seen after unrelated bone marrow transplantation (BMT), when monitored beyond 9 months post-transplant.8,9,14 Most importantly, the cumulative incidence of serious infections was not significant between pediatric recipients of unrelated CB versus BM, regardless whether the BM was T cell depleted or not.17 In fact, it appears that while UCB recipients are at increased risk for OI in the first 6 months they have fewer infections beyond day 180 corresponding with the time when thymic contribution accelerates.
Investigators from the Cord Blood Transplantation Study (COBLT) analyzed antigen-specific proliferation after UCBT.18 Children with malignancies were longitudinally tested over the first 3 years post transplant for herpes virus specific responses (HSV, VZV, CMV). Approximately 43% of the patients studied eventually developed a positive T-lymphocyte proliferative response to at least one herpes virus at some point over the 3 year observational period. In a few, proliferative responses developed as early as within the first 30-50 days, indicating that naive T lymphocytes transferred in the graft can give rise to antigen-specific T-lymphocyte immunity before thymic recovery.18 Significantly, patients with a proliferative response at any time in the first 3 years to any of the herpes viruses had a lower probability of leukemia relapse and a higher overall survival (OS).19 One may speculate that the superior proliferative T cell response represents a powerful surrogate marker for functional immune reconstitution leading to more effective graft-versus-leukemia (GVL) activity. However, the development and kinetics of protective antigen-specific function was not evaluable.19
IMMUNE RECONSTITUTION FOLLOWING NON-MYELOABLATIVE CONDITIONING AND UNRELATED UCBT
We have previously characterized the immunophenotypic immune subsets in over 8,000 umbilical CB units prior to cryopreservation that were collected, processed and cryopreserved during the COBLT supported by the National Heart, Lung and Blood Institute (NHLBI) (Table 1).20,21 As mentioned above, despite these immune populations present in cryopreserved umbilical CB units, there is a significant delay in immune reconstruction following myeloablative conditioning therapy and UCBT. We recently analyzed the results of immune reconstitution in our original cohort of 21 children and adolescents, as well as an additional 25 patients for a total of 46 children and adolescents following non-myeloablative conditioning and UCBT (Table 2).22,23 The absolute number of CD3 (T cell), CD19+ (B cell) and CD56+ (NK cell) cells at 6 months were 379 ± 108, 778 ± 223, and 171 ± 23, respectively.22,23 The 12 month CD3 (T cell), CD19 (B cell) and CD56+ (NK cell) counts increased significantly to 1598 ± 271, 1244 ± 153 and 255 ± 38, respectively (Table 2).22,23 Serum immunoglobulin levels (IgG, IgA, IgM) were close to normal values by six months and were within the normal range by one year. The six month IgG, IgA, and IgM levels were 629 ± 74, 62 ± 14, and 55 ± 15 mg/dL, respectively (Table 2).22,23 Furthermore, there was no significant difference in the CD3+, CD19+, or CD56+ immune subsets or immunoglobulin levels at 6 and 12 months between those children receiving a myeloablative vs. a non-myeloablative conditioning regimen and UCBT.22,23
Table 1.
Immunophenotypic Composition of Banked Umbilical Cord Blood Units (COBLT)
| Subtype | CD3+ | CD3+/CD4+ | CD3+/CD8+ | CD19+ | CD16+/CD56+ |
|---|---|---|---|---|---|
| Mean ± SD | 17.8 ± 9.5 × 107 | 12.8 ± 7.0 × 107 | 5.0 ± 3.0 × 107 | 4 ± 3.0 × 107 | 6.3 ± 4.1 × 107 |
| 5%-95% ± SD | 7.7 ± 33.4 × 107 | 5.2 ± 24.4 × 107 | 1.9 ± 10.2 × 107 | 1.4 ± 9.7 × 107 | 2.0 ± 13.5 × 107 |
Adapted from Cairo MS, Wagner EL, Fraser J, et al. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856-866. Wiley Interscience.
Table 2.
Immune Reconstitution Following Reduced Intensity Conditioning and Umbilical Cord Blood Transplant in Pediatric Recipients
| CD3+ | CD19+ | CD56+ | IgG | IgA | IgM | |
|---|---|---|---|---|---|---|
| Mean ± SEM/mm3 | Mean ± SEM/mm3 | Mean ± SEM/mm3 | Mean ± SEM/dl | Mean ± SEM/dl | Mean ± SEM/dl | |
| Day 180 | 379 ± 108 | 778 ± 223 | 171 ± 23 | 629 ± 74 | 62 ± 14 | 55 ± 15 |
| 1 year | 1598 ± 27 | 1294 ± 153 | 255 ± 38 | 772 ± 73 | 74 ± 14 | 94 ± 21 |
INFECTIONS ARE THE MAJOR CAUSE OF DEATH AFTER UCBT, MOST OCCURRING IN THE FIRST 100 DAYS
Despite the considerable progress over the past decade in supportive care after HCT, infections remain a major cause of morbidity and mortality. Infection-related mortality (IRM) is the primary or secondary cause of death (with or without another major cause such as GvHD) in ≥50% of deaths after UCBT with the majority of them occurring in the first 100 days.24-27 The impact of early infections is highlighted by additional studies. Investigators from the International Bone Marrow Transplant Registry (IBMTR) reported on the outcome after transplantation from either CB (150 patients) or from BM that was from HLA-matched (367 patients) or mismatched for one human leukocyte antigen (HLA) (83 patients).28 The proportion of deaths that were related to infections within 100 days after transplantation was significantly higher among recipients of mismatched cord blood than among recipients of either HLA-matched marrow or mismatched marrow (45%, 21%, and 24%, respectively; P=0.01). However, beyond day 100, the proportions of infection-related deaths were similar in the three groups. In a different study, when the occurrence of severe infections was evaluated in 192 consecutive adult recipients of unrelated donor HCT the proportion of IRMs that occurred during the first 100 days was also higher after UCBT (73%) than in the BMT/peripheral blood stem cell (PBSC) groups (50%; p=0.02).29 Similarly, over the first 6 months after unrelated HCT at the University of Minnesota, the cumulative incidence of serious infections in the UCBT group (n=60) was higher only between day +43 and +100, (58%) than in recipients (n=52) of unmanipulated BM 35%, p=0.04).17 However, subsequently there was a trend towards less serious infection in the UCBT group demonstrating similar findings to the IBMTR report above. 28Overall these findings support the notion that the immune deficit that seems to be so heightened in the immediate post-UCBT period is followed by significant improvements of post-transplant immunity. Improvement in immunity after 100 days post-UCBT, which coincides with the time of thymic recovery, may even exceed that typically seen after bone marrow transplantation. Interestingly, Barker et al analyzed two BMT cohorts separately according to whether or not T cell depletion (TCD) was performed as GvHD prophylaxis.17 Notably, even beyond day +180 after HCT, patients in the TCD group demonstrated significantly more viral (p<0.01), but not bacterial, infections than either UCBT or unmodified bone marrow recipients. The absence of adoptively transferred post-thymic T cells in the TCD group, despite the lower incidence of acute GvHD and resultant lower use of corticosteroids, was associated with a higher incidence of serious viral infections that extended beyond 6 months. These findings together suggest an important protective role for post-thymic T cells infused in the graft whether or not they include antigen experienced (marrow) or solely antigen naïve (cord blood) T cells.
PATIENT AND GRAFT SPECIFIC FACTORS PREDICT THE RISK OF DEATH FROM OPPORTUNISTIC INFECTIONS (OI) IN THE FIRST 6 MONTHS AFTER UCBT
Over the past few years we have studied the reconstitution of immunity in the immediate post-UCBT period (prior to thymic recovery) in >150 pediatric recipients of single unit UCB at Duke University to identify surrogate immune markers for those at risk for OI.
To determine the impact of patient and graft-specific factors on 6-month post-UCBT OI-related mortality we reviewed all consecutive pediatric UCB recipients transplanted at Duke between June 1999 and October 2005 to overlap with a parallel immune monitoring study that evaluated the clinical relevance of Day +50 immune monitoring.30 Three hundred thirty (330) pediatric recipients of single UCB grafts were identified. Those receiving a second transplant for primary graft failure were excluded. Two hundred twenty (220) of the 330 patients (67%) were alive at 6 months (Figure 1). Of those who died by 6 months, 58% were identified with OI (viral, fungal, protozoal infections) implicated as a cause of death (Figure 1). Those who died prior to 6 months and for whom OI was not implicated as a cause of death were omitted from the study dataset, resulting in 284 patients. Of these 284 patients, 220 patients (77%) were alive at 6 months and 64 (23%) died at or before 6 months with cause of death related to OI. Twenty two (22) patients died related to adenovirus infection and twelve (12) due to CMV infection, rendering these two viruses the cause in >50% of all OI related deaths.
Figure 1. Kaplan-Meier curve of survival (months) after UCBT in 330 consecutive patients.
Death related to OI is the major cause of failure, most occurring by 6 months. Immune reconstitution after unrelated cord blood transplantation. Szabolcs P, Niedzwiecki D. Cytotherapy. 2007;9(2):111-122. Reprinted by permission of Taylor & Francis Group, http://www.informaworld.com.
In univariate analyses, gender (p=0.28), race (0.12) and total body irradiation (TBI) (p=0.80) did not predict 6-month death due to OI. Malignancy (p=0.07) was marginally associated with a greater probability of 6-month death due to OI. Malignancy without TBI was also associated with a marginally higher probability of 6-month death due to OI (p=0.04). A significantly greater probability of 6-month OI-related death was associated with cytomegalovirus (CMV) positive serology (p<0.0001), greater HLA mismatch (p=0.006), and older age (p=0.0009). Higher total graft cell dose (p=0.001), CD34+ cell dose (p=0.014) and CD3+ cell dose (0.014) were associated with lower probability of death due to OI at 6 months.
Since treatment with TBI was closely related to age two multivariable models were fit. Model 1 included: CMV (p=0.0004), HLA mismatch (p=0.042) and age (p=0.03). Model 2 included: CMV (p<0.0001), HLA mismatch (p=0.005) and malignancy without TBI (p=0.04). Since total graft cell dose, CD34+ cell dose and CD3+ cell dose were also highly correlated, each of these variables was introduced into models 1 and 2 separately. Total graft cell dose was the strongest predictor when cell dose variables were added to Models 1 (p=0.0097) and 2 (p=0.004). CD34+ cell dose contributed less significantly to both Models (p=0.02 both models). CD3+ cell dose did not significantly contribute to Model 1, however was marginally significant in Model 2 (p=0.05). In Model 1 total graft cell dose and CD34+ cell dose replaced age because cell dose/kg inversely correlates with age.
Thus, in the pediatric cohort 6-month death due to OI can be predicted by the following risk factors: older age, positive CMV serology, >1 HLA mismatch, malignancy without TBI, and lower graft cell dose (total, CD34+ and CD3+).30 In contrast, gender, race and TBI alone do not predict 6-month death due to OI.30
DENDRITIC AND T CELL SUBSETS AT DAY +50 AFTER UCBT SERVE AS SURROGATE MARKERS OF PROTECTION FROM OI
To identify patients who were at increased risk for developing OI in the first 100 days, a prospective cross-sectional study has been conducted at approximately day + 50 post-UCBT,31 extended to 111 patients. Utilizing Trucount™ methodology32-34 4-color surface and intracellular (ic) FACS was employed to accurately enumerate and characterize lymphocyte and dendritic cell (DC) subsets.
All patients received myeloablative conditioning regimes, (TBI/cyclophosphamide [CY], busulfan [Bu]/CY, Bu/melphalan [MEL], TBI/MEL) and equine anti-thymocyte globulin (ATG) at 30mg/kg/day between day-3 to day-1. All received identical GvHD prophylaxis consisting of cyclosporine A and methyl-prednisolone, slowly tapered after day+21 in the absence of active ≥grade II acute GvHD (aGvHD).
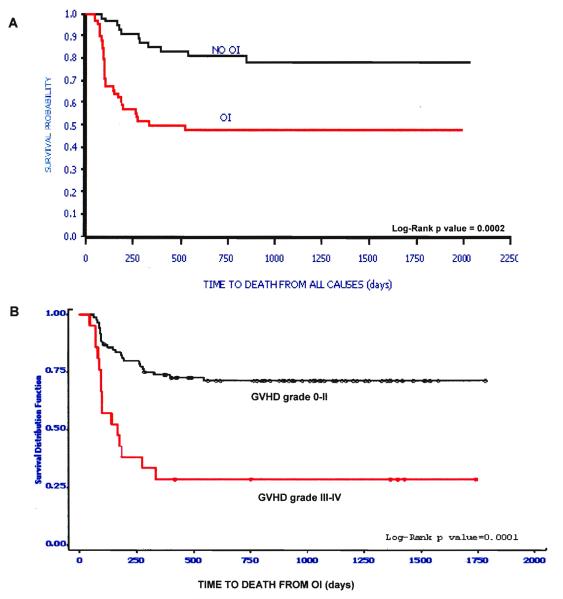
Except for the complete absence of B lymphocytes, there was great variability. Table 3 lists those immune parameters that remain significant predictors for the presence of de novo developed OI. Figure 2 shows that individuals that develop OI by day +100 have a significantly reduced probability of OS (Figure 2A) and that death due to OI is related to grade III/IV GvHD (Figure 2B). Based on these31 and data not shown, we hypothesize that the increased prevalence of CD8+ T cells expressing/secreting HLA-DR, IFNγ, granzymes A, B, perforin represent an effort by the immune system to control the infectious agent. These changes accompany down regulation of CD28 and CD27 expression inCD8+ T cells along with CD57 upregulation. These findings together reflect an evolution towards ‘effector’ phenotype and function. Along with the skewing of the T cell profile away from the infused “naïve” phenotype, significantly fewer CD123+ plasmacytoid/lymphoid DC circulate in those with infection (p=0.007) demonstrating that antigen presenting cell(APC) deficiency occurs in parallel with lymphocyte alterations.
Table 3.
Continuous variables of immunity associated with OI incidence in the first 100 days. Measurements in the “Day +50”= group.
| Variable | Median Value For Patients with OI |
Median Value For Patients without OI |
Logistic Regression (P-value) |
|---|---|---|---|
| Abs # CD4+ T Cells (cell # /μl) | 44 | 137 | 0.02 without age in model |
| % CD8+ T Cells | 44 | 14 | <0.0001 |
| % CD57+/CD28−/CD8+ T cells | 9 | 3 | <0.02 |
| % CD25+/CD3+T Cells | 22 | 40 | <0.016 |
| % TCRγδ T cell subset | 2.3 | 0.97 | <0.017 |
| % ‘activated’ HLA-DR+ T cells | 53 | 38 | <0.009 |
| % ‘NKT’ CD3+/CD56+ T cells | 8 | 4 | <0.01 |
| % IFNγ Secreting T cells | 18 | 4 | <0.006 |
Confounders tested: Race, age, gender, weight, CMV status, HLA mismatch, malignancy, TBI, GvHD, High Dose steroid pulse (yes, no), Anti-CD25/Daclizumab pulse (yes, no), infused total cell dose/kg, CD34+ cell/kg, CD3+ T cell dose/kg.
OI, opportunistic infection.
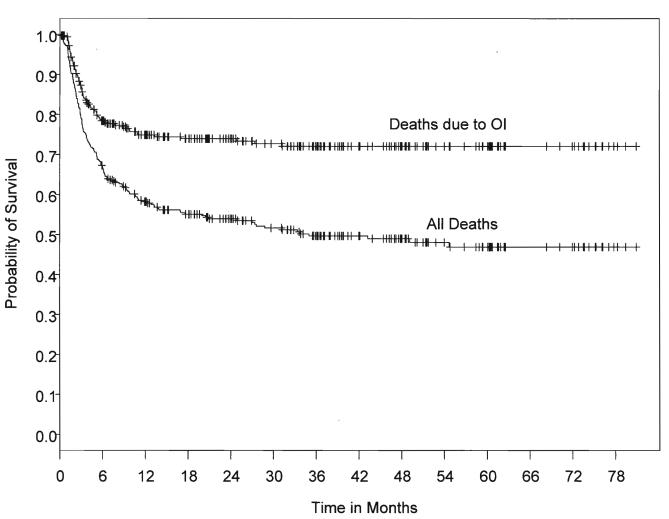
Figure 2And 2B.
(A) Time to death from all causes in the “Day 50” cohort by OI status. (B) Time to death from OI by presence or absence of severe GvHD.
Immune reconstitution after unrelated cord blood transplantation. Szabolcs P, Niedzwiecki D. Cytotherapy. 2007;9(2):111-122. Reprinted by permission of Taylor & Francis Group, http://www.informaworld.com.
HEMATOLOGICAL MALIGNANT RELAPSE FOLLOWING UNRELATED UCBT: IMPACT OF IMMUNE RECONSTITUTION
We and others have demonstrated the success of using unrelated UCB units as an alternative allogeneic stem cell source to successfully reconstitute both children and adults with hematological malignant disease.26-28,35-38 The probability of relapse in children and adolescents with acute leukemia following UCBT varies between 15% and 40% at 2 years post UCBT (Table 4).39-43 The incidence of relapse in children and adolescents with acute leukemia occurs primarily between month 3 and 12 following UCBT.26-28,35-43 It is during this time that there is a significant delay in both quantitative and qualitative T cell immune reconstitution.18 The influence of immune reconstitution on the risk of relapse in children with acute leukemia following UCBT was reported by Parkman et al. in the Pediatric Acute Leukemia COBLT trial.19 The cumulative incidence of leukemia relapse in children with acute leukemia following UCBT appears to be directly related to their ability to generate a T cell proliferative response during a 36 month observation period following UCBT (Figure 3) (P=0.003).19 Parkman et al. specifically analyzed antigen-specific T cell immunity to herpes viruses and correlated these results with the risk of relapse following UCBT in children with acute leukemia treated on the COBLT study.19 Patients who had a positive antigen-specific proliferative response (>3000CPM increase over control) were considered to have a positive immune response. In a multivariate analysis, patients with a negative antigen-specific T cell proliferative response had a hazard ratio (HR) of 3.6, P<0.0002 which was associated with a significant increase in the risk of relapse following UCBT (Figure 3).19 Although immune reconstitution following UCBT in children with acute leukemia is significantly dependent on T cell immune reconstitution, there are other risk factors (stage, HLA, cell dose) that have been demonstrated to be associated with an increased risk of leukemia relapse. Kurtzberg et al. reporting results from the Pediatric Acute Leukemia COBLT study demonstrated that the incidence of leukemia relapse in children with acute leukemia following UCBT was significantly dependent on the stage of disease at the time of UCBT (Figure 4A & 4B).40 Similarly, Eapen et al. reporting for the Center for International Blood and Marrow Transplant Research (CIBMTR) demonstrated that both HLA mismatch and cell dose following UCBT in children with acute leukemia is significantly associated with leukemia-free survival.39
Table 4.
Probability of Relapse at 1-2 yrs in Children and Adults with Acute Leukemia Following UCBT
| Author/Study | Childhood ALL |
Childhood AML |
Combined (Childhood ALL/AML) |
Adult ALL | Adult AML | Combined (Adult ALL/AML) |
|---|---|---|---|---|---|---|
| Kurtzberg/COBLT 40 | N/A | N/A | 19% | --- | --- | --- |
| Eapen/CIBMTR 39 | N/A | N/A | 20-40% | --- | --- | --- |
| Michel/Eurocord41 | N/A | 15-25% | NA | --- | --- | --- |
| Wall/COBLT43 | N/A | N/A | 30% | --- | --- | --- |
| Rocha/EBMT42 | N/A | N/A | 38% | --- | --- | --- |
| Laughlin/multicenter28 | --- | --- | --- | N/A | N/A | 20-30% |
| Rocha/EBMT26 | --- | --- | --- | N/A | N/A | 23% |
| Ooi/University of Tokyo45 |
--- | --- | --- | N/A | 26% | NA |
| Atsuta/JCBBN44 | --- | --- | --- | 27-31% | 20-21% | N/A |
| Takahashi/University of Tokyo46 | --- | --- | --- | N/A | N/A | 16% |
UCBT, umbilical cord blood transplantation; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; COBLT, Cord Blood Transplantation Study; CIBMTR, Center for International Blood and Marrow Transplant Research; EBMT, The European Group for Blood and Marrow Transplantation; JCBBN, Japan Cord Blood Bank Network.
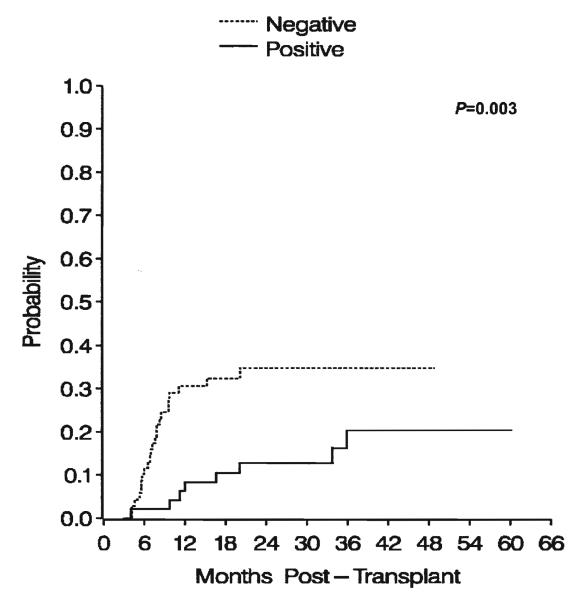
Figure 3.
Cumulative incidence of leukemic relapse by positive (solid line) or negative (dotted line) proliferative response status. P=0.003, log-rank test for difference between curves.
Reprinted from Biol Blood Marrow Transplant, Vol 12, Parkman R, Cohen G, Carter SL, et al., Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation, 919-927, ©2006, with permission from Elsevier.
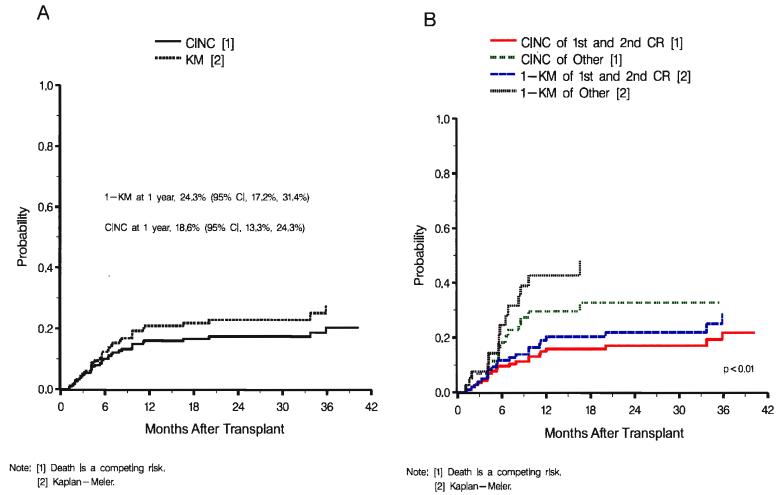
Figure 4A and 4B. Cumulative incidence of relapse.
(A) Overall cumulative incidence and 1-KM probability of relapse. (B) Cumulative incidence and 1-KM probability f relapse by stage of disease (first and second CR vs. other).
This research was originally published in Blood. Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318-4327. © the American Society of Hematology.
The risk of relapse in adults with acute leukemia following UCBT ranges from a low of 16% to as high as 30% (Table 4).26,28,44-46 There appears to be no significant difference in the risk of relapse in adults with acute myeloid leukemia vs. acute lymphoblastic leukemia following UCBT.44,45 Similar to children with acute leukemia, the risk of relapse in adults with acute leukemia occurs primarily between month 3 and 12 following UCBT.26,28,44-46 Several studies in adult recipients of UCBT have demonstrated significant delays of immune reconstitution during the first 12 months post UCBT.11,12 Specific delays in T-cell recovery, function and subsets are quite prevalent in adult UCBT recipients during this time period at highest risk of leukemia relapse. Unfortunately, there are no specific studies that investigated whether delayed immune reconstitution is a significant risk factor for relapse in adults with acute leukemia following UCBT. However, Ooi et al. demonstrated that adults with acute leukemia with either high risk status (HR 2.86, P=0.042) and unfavorable cytogenetics (HR 4.94, P=0.048) were associated with a significant decrease in event-free survival following UCBT.45
Adults with both non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) have also benefited from UCBT.47-50 In adults with both NHL and HL both full intensity and non-myeloablative conditioning have been utilized prior to UCBT.47-50 The incidence of relapse in adults with both NHL and HL ranges between 30% and 50%.47-50 In adults with NHL following non-myeloablative or reduced intensity conditioning and UCBT, the cumulative incidence of relapse is approximately 30% to 45%, which occurs predominantly within the first nine months post UCBT. The lymphoma free survival varies between 30% and 75% depending on the histology of NHL (Figure 5A and 5B).47,49 The only known risk factors associated with a lower risk of relapse or progression include chemosensitive disease,( P=0.05) and the use of double UCBT (P=0.009).49 As with adults with acute leukemia, adults with NHL and HL who have received UCBT, there have been no immune reconstitution studies performed to date therefore, it is unknown whether a low or delay in immune reconstitution is associated with relapse or progression in adults with lymphoma following UCBT.
Figure 5A and 5B.
(A) Cumulative incidence of relapse after nonmyeloablative umbilical cord blood transplantation for patients with follicular lymphoma/chronic lymphocytic leukemia (—), large-cell /mantle-cell lymphoma (---), and HL (- - -).
Reprinted from Biol Blood Marrow Transplant, Vol 15, Brunstein CG, Cantero S, Cao Q, et al., Promising progression-free survival for patients low and intermediate grade lymphoid malignancies after nonmyeloablative umbilical cord blood transplantation, 214-222, ©2009, with permission from Elsevier.
(B) Estimated progression-free survival (PFS) according to histologic subtype. Patients with indolent non-Hodgkin’s lymphoma (NHL); yellow line), mantle-cell lymphoma (blue line), aggressive NHL (grey line) and Hodgkin’s lymphoma (red line).
Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Rodrigues, CA et al: J Clin Oncol 27(2), 2009:256-263.
These UCBT studies in children and adults with hematological malignancies suggest that stage, HLA match, nucleated cell dose/kg and chemosensitivity of disease at the time of UCBT may be important risk factors for malignant relapse or progression. Others have also demonstrated that CD34/kg cell dose following UCBT can be associated with a significant difference in OS.51,52 Clearly, Parkman et al. demonstrated in children following UCBT that delay in T cell functional reconstitution following UCBT is significantly associated with an increased incidence of leukemia relapse. Further studies are required to investigate in more depth the role of immune reconstitution both quantitatively and qualitatively, as well as which immune subsets and/or function are most critical to prevent hematological relapse following UCBT.
UNRELATED CORD BLOOD AS A SOURCE OF ENRICHED T-CELLS FOR DONOR IMMUNE CELL INFUSION
Adoptive transfer of naturally primed and ex vivo restimulated T lymphocytes in the form of donor leukocyte infusions (DLI) has demonstrated efficacy to prevent/treat EBV-associated lymphomas, and post-transplant viral infections.53 Despite advances in the applicability and outcome following UCBT, currently there is no obviously available post-transplant source for DLI from the transplanted unit. Impaired Th1/Tc1 cytokine production54 and cytotoxicity,8,55 collectively lead to impaired cord blood antiviral immunity.56
Recently, we and others have demonstrated the feasibility of ex vivo CB T cell expansion57,58 drawing from the pioneering work by June et al.59 utilizing paramagnetic Dynal beads, coated with anti-CD3 and anti-CD28 stimulatory antibodies. These artificial APCs simultaneously provide agonistic TCR and co-stimulatory signals that trigger sufficient T cell proliferation to generate clinically relevant DLI products from living donors.60-62
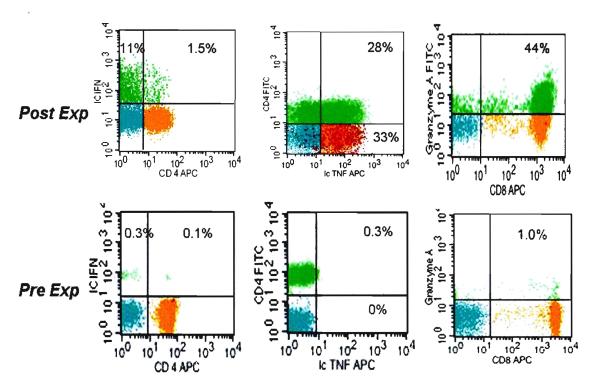
While ≥100 fold expansion may be attainable in 10-14 days few if any “terminally differentiated, effector memory” CD8+ T cells (CD57+, CD27−/CD28−) are generated. The majority of the expanded cells in the culture retain several surface markers of the starting pool of “naïve/resting” T cells with >90% of expanded progeny expressing CD62L, CD27 and CD28, thus favoring homing potential to secondary lymphoid organs which is a desired destination for unprimed DLI infusions. However, significant activation has occurred in these cultures, based on the high expression of HLA-DR, CD25 and the expression of IL-12 receptor. Moreover, TNFα IL-2 and IFNγ secreting cells are more frequent compared to pre-expansion state and the Granzyme A expressing CD8+ T cell pool is remarkably reconstituted, as illustrated in Figure 6. Nevertheless, compared to resting adult peripheral blood, fewer CD8+ T cells store Granzyme B and no CD57+/ Perforin+ cells could be detected likely responsible for the complete absence of cytotoxicity against a highly immunogenic allogeneic lymphoma cell line, IM9. The lack of cytotoxicity has been extended to patient fibroblasts as well.57 Although the clinical implication of these studies is very encouraging, further studies and possible modifications should further improve on these pre-clinical findings to reduce apoptosis. This would result in potentially higher DLI dose levels so adults of all sizes may benefit. Additional functional maturation towards Th1/Tc1 competence is also desirable. It is likely critical to retain low/minimal allogeneic cytotoxicity and sufficiently high expression of homing molecules necessary for entry and retention in secondary lymphoid organs thus avoiding significant alloreactivity and post-infusion GVHD. Once these conditions are satisfied, of ex vivo expanded T cell infusions would have the potential for more effective control and/or reduction of opportunistic viral infections 56 and may enhance GVL activity .
Figure 6. Th1/Tc1 cytokine secretion profile and Granzyme A expression in the expanded T cell progeny.
4-color FACS dotplot profile of viable T lymphocytes contrasting the starting day 0 and post-expansion day 14 progeny. Surface detection of indicated antibodies is presented except for IFNγ, IL-2, TNFα and Granzyme B which were detected following permeabilization and intracellular staining. The relative size of the indicated T cell subsets in a quadrant is expressed as the percentage of total viable T cells. Representative experiment, n=10.
Reprinted from Biol Blood Marrow Transplant, Vol 14, Mazur MA, Davis CC, Szabolcs P, Ex Vivo Expansion and Th1/Tc1 Maturation of Umbilical Cord Blood T Cells by CD3/CD28 Costimulation, 1190-1196, ©2008, with permission from Elsevier.
As opposed to enhancing the immune responsiveness of UCBT recipients, investigators at the University of Minnesota have initiated clinical trials to test the safety and efficacy of ex vivo expanded T reg cells from cord blood to attenuate the incidence and severity of GVHD. As of this date, there are no results available yet to discuss the benefits and/or limitations of this novel approach.63,64
In parallel with the antigen-nonspecific approaches described above, several centers have made significant progress to engineer leukemia-specific or virus-specific T cells 65,66 from antigen-inexperienced T cells. This is a rapidly growing area67 that over the next 2-3 years should yield data on the safety and efficacy of these novel targeted T cell therapies.
UNRELATED CORD BLOOD AS A SOURCE OF ENRICHED NATURAL KILLER CELLS FOR DONOR IMMUNE CELL INFUSION
We and others have demonstrated that critically important immunoregulatory cytokines that control natural killer (NK) cell development and functional activation such as IL-12, IL-15, and IL-18 are significantly decreased at the genetic and protein level in umbilical versus peripheral blood mononuclear cell populations.68-70 Furthermore, in vitro studies with IL-12, IL-15, and IL-18 with UCB mononuclear cells has been demonstrated to significantly increase UCB-NK cytotoxicity and interferon gamma production.68-71 Recently, Dunbar et al. prospectively evaluated NK reconstitution in 209 patients receiving reduced intensity and full ablative allogeneic stem cell transplantation for high-risk hematological malignancies.72 In this multivariate analysis, they demonstrated that low day 60 absolute NK cell counts in patients following reduced intensity conditioning was independently associated (HR 20.2) with an increased risk of hematological relapse.72 These data suggests that enhanced NK reconstitution following allogeneic stem cell transplantation may be associated with a reduced risk of hematological relapse and therefore an increase in overall survival.72
Willemze et al. more recently reported that KIR-ligand incompatibility in the graft-verus-host direction significantly improves the outcome after unrelated UCBT in patients with acute leukemia.73 In this international retrospective analysis, Willemze et al. demonstrated that NK KIR-ligand incompatibility in the graft-versus-host direction was associated with a significant decrease in the relapse rate in patients with acute leukemia following unrelated UCBT (20 ± 5 vs 37 ± 4%, P=0.03).73 These studies and those by Dunbar et al. suggest that adoptive ex vivo enriched NK cell infusions particularly those with KIR-ligand incompatibility may enhance NK cell immune reconstitution and potentially decrease the relapse rate in patients with acute leukemia following unrelated UBCT.72,73
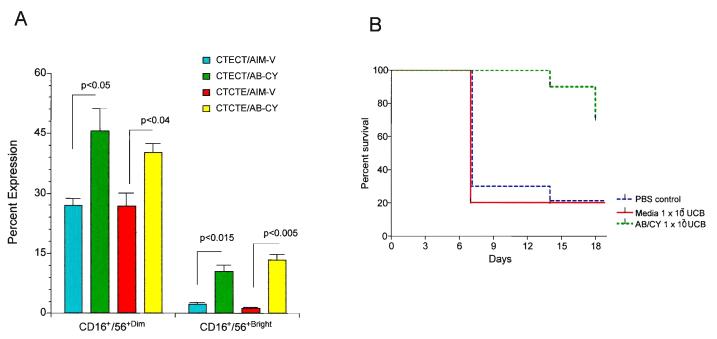
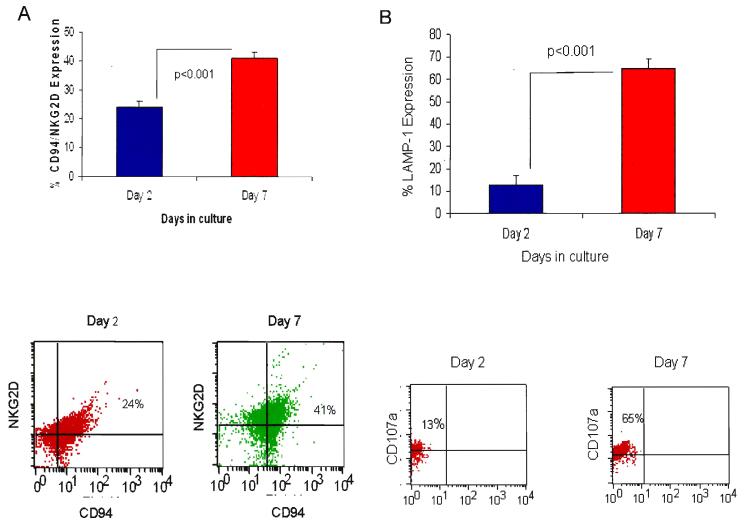
Several studies have demonstrated the safety of ex vivo expanded UCB cells as an adjuvant following unrelated UCBT.74,75 We and others have demonstrated methods of ex vivo expanding human UCB into increased numbers and active CD3−/CD56+ NK cytotoxic cells (Table 5).76-80 We originally demonstrated the ability to ex vivo expand UCB with an antibody/cytokine cocktail consisting of anti-CD3 antibody and IL-2, IL-7, and IL-12 in fresh cord blood to expand both NK and T cell immune subsets.80 Subsequently, we compared the expansion using this antibody/cytokine cocktail to expand NK cells from frozen UCB versus frozen UCB that was re-cryopreserved after thawing and frozen UCB that was ex vivo expanded with this cocktail and then re-cryopreserved.77 In these studies, we demonstrated a significant increase in both CD3−/CD16+/CD56+ dim cells and CD3−/CD60+/CD56bright cells after 48 hours of ex vivo expansion (Figure 7A).77 Utilizing these ex vivo expanded cells after 48 hours of incubation with these antibody/cytokine cocktails, we demonstrated a significant increase in leukemia-free survival in a human/mouse xenograft model utilizing K562 human leukemia cells and NOD/SCID xenograft in mouse (Figure 7B).77 More recently, we increased the duration of the expansion from 48 hours to 7-10 days using the same antibody/cytokine cocktail.76 In this study, we demonstrated a significant increase in in vitro cytotoxic activity against a variety of tumor targets including K562, Daudi, S5YSY and Kasumi cell lines, as well as a significant increase in NK cells expressing the NKG2D C-lectin activating receptor and expression of the CD107a (LAMP-1) NK activation receptor (Figure 8A and 8B).76 Lastly, we have developed a more novel method of using genetically reengineered antigen presenting cells (APC) by transfecting K562 cells with a construct expressing membrane bound IL-15 and 4-1BB ligand (K562-mb IL15-4-1BBL).81 Utilizing these genetically reengineered APCs, we have been able to significantly increase cord blood NK cells from cord blood mononuclear cells following both 7 and 14 day ex vivo expansion resulting in a 2500 to 3500% increase from the original input of cord blood CD3−/CD56+ NK cells.81 We are currently characterizing the in vitro and in vivo functional properties of these expanded cord blood NK cells. In the future these CB NK cells will be further transduced with a construct encoding a chimeric antigen receptor against CD20 with NK signaling molecules including CD3ζ and 4-1BB (MSCD-antiCD20-BB-CD3ζ).81
Table 5. Cytolytic activity of ex-vivo expanded natural killer (NK) cells in AB/CY; day 7 vs. day 2.
NK cytotoxicity was measured by standard europium release assay using as tumor targets: an NK sensitive cell line, K562, a LAK sensitive cell line Daudi, and human cell lines AML, Kasumi-1and neuroblastoma, SYSY5Y at an effector /target ratio of 20:1.
| Tumor target | % Cytotoxicity @ d2 (Mean±SEM)) |
% Cytotoxicity @ d7 (Mean±SEM)) |
p value |
|---|---|---|---|
| K562 | 53.83±3.91 | 71.46±0.81 | p<0.001 |
| Daudi | 31.83±1.8 | 63.95±0.74 | p<0.001 |
| SYSY5 | 57.53±3.41 | 76.77±6.59 | p<0.05 |
| Kasumi | 38.0±1.16 | 56.65±0.47 | p<0.001 |
Reprinted from Exp Hematol, Ayello J, van de Ven C, Cairo E, et al., Characterization of natural killer (NK) and natural killer-like T (NKT) cells derived from ex-vivo expanded and activated cord blood mononuclear cells: Implications for adoptive cellular immunotherapy (ACI), ©2009, with permission from Elsevier.
Figure 7A and 7B.
(A) CD3−/CD16+/CD56+dim/bright subset expansion as determined by flow cytometry. The CD3− lymphocyte population was gated and used as a reference to determine the percentages of CD3−/CD16+/CD56+dim and CD3−/CD16+/CD56+bright expressions of non-adherent UCB MNCs from CTE versus CTCTE versus CTECT after 48 hours in culture with AB/CY versus SF medium alone. Results represent mean ± SEM (n=3); CD56+dim: P <.05, CTE, CTECT, CTCTE AB/CY versus medium alone; CD56+bright: P <.01, CTE, CTECT, CTCTE AB/CY versus medium alone.
(B) NK Cytotoxicity in NOD/SCID Mouse Xenografted with K562 cells. Effect of UCB ex vivo expanded in medium alone versus AB/CY on survival of NOD/SCID mice that received a xenograft of K562 cells. NOD/SCID mice were injected with 10 × 106 human K562 cells 3 days after tumor cell injection; groups of mice (n=10) received intraperitoneal injections of CTECT UCB cells (1 × 107 cells/animal) stimulated with medium alone or CTECT UCB cells (1 × 107 cells/animal) stimulated with AB/CY. Parallel sham injections of sterile PBS served as a control group. Injections of ex vivo expanded UCB cells or PBS continued every 7 days for 14 days. Tumor survival was monitored daily and animals were killed when they become moribund with disseminated tumor burden.
Reprinted from Biol Blood Marrow Transplant, Vol 12, Ayello J, van de Ven C, Fortino W, et al., Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering, 608-622, ©2006, with permission from Elsevier.
Figure 8A and 8B.
(A) Expression of activating c-lectin receptor CD94/NKG2D after ex-vivo expansion of cryopreserved/thawed/re-cryopreserved/re-thawed (CTCT) cord blood (CB) cells cultured for 2 to 7 days in AB/CY as determined by flow cytometry. CD94/NKG2D expression was significantly increased after incubation at 7 days versus 2 days (p <0.001). Results represent mean ± SEM (n=6). Representative dot plot of CD94/NKG2D expression of CB mononuclear cells (MNCs) after 2-7 days in culture. The lymphocyte population was gated and used as a reference to determine the specific subsets. (B) Expression of NK degranulation marker LAMP-1 (CD107a) of cryopreserved/thawed/re-cryopreserved/re-thawed/ex-vivo expanded (CTCTE) cord blood (CB) cells cultured for 2 to 7 days in AB/CY as determined by flow cytometry. Expression of CD107a in CB CTCTE cells was significantly increased (p <0.001) when comparing day 7 to day 2. Results represent mean ±SEM (n=6). Representative dot plot of CD107a expression of CTCTE CB mononuclear cells (MNCs) after 2-7 days in culture. The lymphocyte population was gated and used as a reference to determine the specific subsets.
Reprinted from Exp Hematol, Ayello J, van de Ven C, Cairo E, et al., Characterization of natural killer (NK) and natural killer-like T (NKT) cells derived from ex-vivo expanded and activated cord blood mononuclear cells: Implications for adoptive cellular immunotherapy (ACI), ©2009, with permission from Elsevier.
These studies suggest that NK cells may play a pivotal role following unrelated UCBT to reduce hematological relapse and possibly the incidence of opportunistic infection. Further studies will be required to determine the optimum method of isolation and expansion of UCB NK cells, optimal method of administration and which NK subsets are critical for both reducing hematological relapse and/or reducing the incidence of opportunistic infections.
SUMMARY
Unrelated cord blood transplantation is an excellent and viable alternative allogeneic stem cell source for both children and adults with both malignant and non malignant disease. Delays in the reconstitution of antigen specific cellular immunity currently predispose UCBT recipients to an increased risk of opportunistic infections (OI) and possible hematological malignant relapse. New strategies to enhance early and more robust recovery of antigen specific cellular immunity by ex-vivo cellular engineering, third party transplants, adoptive cellular immunotherapy and donor vaccination strategies are currently under investigation and on the horizon and will be critical in the future to circumvent this current limitation.
ACKNOWLEDGMENT
The authors would like to thank Erin Morris, RN for her expert and invaluable assistance and advice during the preparation of this manuscript.
M.C. was supported in part by grants from National Heart, Lung and Blood Institute (NIH N01-HB-67136), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21 AR49330; MSC), Pediatric Cancer Research Foundation, BevanMar Foundation, Marisa Fund, Sonia Scaramella Fund, Paul Luisi Foundation, Brittany Barron Fund (MSC) P. Sz. was supported in part by grants from R01-CA132110, The Childrens’ Miracle Network, The National Marrow Donor Program-Marrow Transplant Research Grant and 1PO1-HL-67314-01A1 (PI: N.Chao)
Footnotes
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Schwinger W, Weber-Mzell D, Zois B, Rojacher T, Benesch M, Lackner H, et al. Immune reconstitution after purified autologous and allogeneic blood stem cell transplantation compared with unmanipulated bone marrow transplantation in children. Br J Haematol. 2006;135:76–84. doi: 10.1111/j.1365-2141.2006.06244.x. [DOI] [PubMed] [Google Scholar]
- 2.Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 3.Crooks GM, Weinberg K, Mackall C. Immune reconstitution: from stem cells to lymphocytes. Biol Blood Marrow Transplant. 2006;12:42–46. doi: 10.1016/j.bbmt.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005;35(Suppl 1):S53–57. doi: 10.1038/sj.bmt.1704848. [DOI] [PubMed] [Google Scholar]
- 5.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–78. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 6.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 7.Garderet L, Dulphy N, Douay C, Chalumeau N, Schaeffer V, Zilber MT, et al. The umbilical cord blood alphabeta T-cell repertoire: characteristics of a polyclonal and naive but completely formed repertoire. Blood. 1998;91:340–346. [PubMed] [Google Scholar]
- 8.Broxmeyer HE, editor. Cord Blood: Biology, Immunology, Banking and Clinical Transplantation. AABB Press; Bethesda, MD: 2004. American Association of Blood Banks. [Google Scholar]
- 9.Koh LP, Chao NJ. Umbilical cord blood transplantation in adults using myeloablative and nonmyeloablative preparative regimens. Biol Blood Marrow Transplant. 2004;10:1–22. doi: 10.1016/j.bbmt.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99:1458–1464. doi: 10.1182/blood.v99.4.1458. [DOI] [PubMed] [Google Scholar]
- 11.Klein AK, Patel DD, Gooding ME, Sempowski GD, Chen BJ, Liu C, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7:454–466. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 12.Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmi Z, Hommel-Berrey G, Smith F, Thomson B. NK cells recover early and mediate cytotoxicity via perforin/granzyme and Fas/FasL pathways in umbilical cord blood recipients. Hum Immunol. 2001;62:782–790. doi: 10.1016/s0198-8859(01)00275-0. [DOI] [PubMed] [Google Scholar]
- 14.Moretta A, Maccario R, Fagioli F, Giraldi E, Busca A, Montagna D, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001;29:371–379. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- 15.Niehues T, Rocha V, Filipovich AH, Chan KW, Porcher R, Michel G, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children -- a Eurocord analysis. Br J Haematol. 2001;114:42–48. doi: 10.1046/j.1365-2141.2001.02900.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomson BG, Robertson KA, Gowan D, Heilman D, Broxmeyer HE, Emanuel D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- 17.Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O’Brien MR, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11:362–370. doi: 10.1016/j.bbmt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, Kurtzberg J, et al. Antigen-specific T-lymphocyte function after cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:1335–1342. doi: 10.1016/j.bbmt.2006.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkman R, Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Cairo MS, Wagner EL, Fraser J, Cohen G, van de Ven C, Carter SL, et al. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856–866. doi: 10.1111/j.1537-2995.2005.04429.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL, et al. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–855. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 22.Bradley MB, Satwani P, Baldinger L, Morris E, van de Ven C, Del Toro G, et al. Reduced intensity allogeneic umbilical cord blood transplantation in children and adolescent recipients with malignant and non-malignant diseases. Bone Marrow Transplant. 2007;40:621–631. doi: 10.1038/sj.bmt.1705785. [DOI] [PubMed] [Google Scholar]
- 23.Cairo MS, Jin Z, van de Ven C, Tallamy B, Schonfeld T, Moore V, et al. Reduced intensity conditioning (RIC) is associated with a significant decrease in day +100 non-relapse mortality (NRM), grade II-IV AGVHD and overall survival (OS) in pediatric recipients following cord blood transplantation (CBT) Biol Blood Marrow Transplant. 2009;15:77. (abstract) [Google Scholar]
- 24.Kurtzberg J, Carter SL, Baxter-Lowe L, Feig SA, Guinan EC, Kamani NR, et al. Results of the cord blood transplantation study (COBLT): Clinical outcomes of 193 unrelated donor umbilical cord blood transplantation in pediatric patients with malignant conditions. Biol Blood Marrow Transplant. 2005;2:6. (abstract) [Google Scholar]
- 25.Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12:34–41. doi: 10.1016/j.bbmt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 28.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 29.Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Szabolcs P, Niedzwiecki D, Chao N, Kurtzberg J. Multivariate Analysis of Patient and Graft Specific Factors among 330 Recipients of Unrelated Cord Blood Transplant (UCBT) To Predict Risk of Death from Opportunistic Infections in the First 6 Months after UCBT. Blood. 2006;108:2860a. (abstract) [Google Scholar]
- 31.Szabolcs P, Park KD, Marti L, Reese M, Lee M, Deoliveria D, et al. The impact of immune reconstitution in the early post grafting period on the development of opportunistic infections after unrelated cord blood transplantation: a multivariate analysis of host, graft, and day +50 immune profile. Biol Blood Marrow Transplant. 2004;10:24. (abstract) [Google Scholar]
- 32.Szabolcs P, Park KD, Marti L, Deoliveria D, Lee YA, Colvin MO, et al. Superior depletion of alloreactive T cells from peripheral blood stem cell and umbilical cord blood grafts by the combined use of trimetrexate and interleukin-2 immunotoxin. Biol Blood Marrow Transplant. 2004;10:772–783. doi: 10.1016/j.bbmt.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31:708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 34.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem Cells. 2003;21:296–303. doi: 10.1634/stemcells.21-3-296. [DOI] [PubMed] [Google Scholar]
- 35.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–4678. [PubMed] [Google Scholar]
- 36.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 37.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 38.Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 39.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 40.Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michel G, Rocha V, Chevret S, Arcese W, Chan KW, Filipovich A, et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: a Eurocord Group analysis. Blood. 2003;102:4290–4297. doi: 10.1182/blood-2003-04-1288. [DOI] [PubMed] [Google Scholar]
- 42.Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 43.Wall DA, Carter SL, Kernan NA, Kapoor N, Kamani NR, Brochstein JA, et al. Busulfan/melphalan/antithymocyte globulin followed by unrelated donor cord blood transplantation for treatment of infant leukemia and leukemia in young children: the Cord Blood Transplantation study (COBLT) experience. Biol Blood Marrow Transplant. 2005;11:637–646. doi: 10.1016/j.bbmt.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Atsuta Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, Kai S, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 45.Ooi J, Takahashi S, Tomonari A, Tsukada N, Konuma T, Kato S, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2008;14:1341–1347. doi: 10.1016/j.bbmt.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi S, Iseki T, Ooi J, Tomonari A, Takasugi K, Shimohakamada Y, et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004;104:3813–3820. doi: 10.1182/blood-2004-03-1001. [DOI] [PubMed] [Google Scholar]
- 47.Brunstein CG, Cantero S, Cao Q, Majhail N, McClune B, Burns LJ, et al. Promising progression-free survival for patients low and intermediate grade lymphoid malignancies after nonmyeloablative umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2009;15:214–222. doi: 10.1016/j.bbmt.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107:3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 50.Yuji K, Miyakoshi S, Kato D, Miura Y, Myojo T, Murashige N, et al. Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant. 2005;11:314–318. doi: 10.1016/j.bbmt.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Styczynski J, Cheung YK, Garvin J, Savage DG, Billote GB, Harrison L, et al. Outcomes of unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant. 2004;34:129–136. doi: 10.1038/sj.bmt.1704537. [DOI] [PubMed] [Google Scholar]
- 52.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 53.Fujita Y, Rooney CM, Heslop HE. Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant. 2008;41:193–198. doi: 10.1038/sj.bmt.1705906. [DOI] [PubMed] [Google Scholar]
- 54.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 55.Risdon G, Gaddy J, Stehman FB, Broxmeyer HE. Proliferative and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cell Immunol. 1994;154:14–24. doi: 10.1006/cimm.1994.1053. [DOI] [PubMed] [Google Scholar]
- 56.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant. 2008;14:1190–1196. doi: 10.1016/j.bbmt.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parmar S, Robinson SN, Komanduri K, St John L, Decker W, Xing D, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8:149–157. doi: 10.1080/14653240600620812. [DOI] [PubMed] [Google Scholar]
- 59.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 60.Laport GG, Levine BL, Stadtmauer EA, Schuster SJ, Luger SM, Grupp S, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 61.Levine BL, Bernstein WB, Aronson NE, Schlienger K, Cotte J, Perfetto S, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 62.Porter DL, Levine BL, Bunin N, Stadtmauer EA, Luger SM, Goldstein S, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 63.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 64.June CH, Blazar BR. Clinical application of expanded CD4+25+ cells. Semin Immunol. 2006;18:78–88. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T-cells that target CMV, adenovirus and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009 doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108:1770–1773. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serrano LM, Pfeiffer T, Olivares S, Numbenjapon T, Bennitt J, Kim D, et al. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. doi: 10.1182/blood-2005-09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SM, Suen Y, Chang L, Bruner V, Qian J, Indes J, et al. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood. 1996;88:945–954. [PubMed] [Google Scholar]
- 69.Qian JX, Lee SM, Suen Y, Knoppel E, van de Ven C, Cairo MS. Decreased interleukin-15 from activated cord versus adult peripheral blood mononuclear cells and the effect of interleukin-15 in upregulating antitumor immune activity and cytokine production in cord blood. Blood. 1997;90:3106–3117. [PubMed] [Google Scholar]
- 70.Satwani P, Ayello J, Ven C, O’Neill AF, Simpson LL, Baxi L, et al. Immaturity of IL-18 gene expression and protein production in cord blood (CB) versus peripheral blood (PB) mononuclear cells and differential effects in natural killer (NK) cell development and function. Br J Haematol. 2005;130:284–292. doi: 10.1111/j.1365-2141.2005.05592.x. [DOI] [PubMed] [Google Scholar]
- 71.Lee SM, Suen Y, Qian J, Knoppel E, Cairo MS. The regulation and biological activity of interleukin 12. Leuk Lymphoma. 1998;29:427–438. doi: 10.3109/10428199809050903. [DOI] [PubMed] [Google Scholar]
- 72.Dunbar EM, Buzzeo MP, Levine JB, Schold JD, Meier-Kriesche HU, Reddy V. The relationship between circulating natural killer cells after reduced intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica. 2008;93:1852–1858. doi: 10.3324/haematol.13033. [DOI] [PubMed] [Google Scholar]
- 73.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socie G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaroscak J, Goltry K, Smith A, Waters-Pick B, Martin PL, Driscoll TA, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–5067. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 75.Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 76.Ayello J, van de Ven C, Cairo E, Hochberg J, Baxi L, Satwani P, et al. Characterization of natural killer (NK) and natural killer-like T (NKT) cells derived from ex-vivo expanded and activated cord blood mononuclear cells: Implications for adoptive cellular immunotherapy (ACI) Exp Hematol. 2009 doi: 10.1016/j.exphem.2009.07.009. (in press) [DOI] [PubMed] [Google Scholar]
- 77.Ayello J, van de Ven C, Fortino W, Wade-Harris C, Satwani P, Baxi L, et al. Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering. Biol Blood Marrow Transplant. 2006;12:608–622. doi: 10.1016/j.bbmt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Condiotti R, Zakai YB, Barak V, Nagler A. Ex vivo expansion of CD56+ cytotoxic cells from human umbilical cord blood. Exp Hematol. 2001;29:104–113. doi: 10.1016/s0301-472x(00)00617-2. [DOI] [PubMed] [Google Scholar]
- 79.Frias AM, Porada CD, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. Generation of functional natural killer and dendritic cells in a human stromal-based serum-free culture system designed for cord blood expansion. Exp Hematol. 2008;36:61–68. doi: 10.1016/j.exphem.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson KL, Ayello J, Hughes R, van de Ven C, Issitt L, Kurtzberg J, et al. Ex vivo expansion, maturation, and activation of umbilical cord blood-derived T lymphocytes with IL-2, IL-12, anti-CD3, and IL-7. Potential for adoptive cellular immunotherapy post-umbilical cord blood transplantation. Exp Hematol. 2002;30:245–251. doi: 10.1016/s0301-472x(01)00781-0. [DOI] [PubMed] [Google Scholar]
- 81.Hochberg J, Mar B, Ayello J, Day N, van de Ven C, Ricci A, et al. Genetic engineering and significant ex-vivo expansion of cord blood natural killer cells: implications for post-transplant adoptive cellular immunotherapy. Blood. 2008;112:209. (abstract) [Google Scholar]