Abstract
Rationale
The Z-line, alternatively termed the Z-band or Z-disc, is a highly ordered structure at the border between two sarcomeres. Enigma subfamily proteins (Enigma, Enigma homolog protein and Cypher) of the PDZ-LIM domain protein family are Z-line proteins. Among the Enigma subfamily, Cypher has been demonstrated to play a pivotal role in the structure and function of striated muscle, whereas the role of Enigma homolog protein (ENH) in muscle remains largely unknown.
Objective
We studied the role of Enigma homolog protein in the heart using global and cardiac-specific ENH knockout mouse models.
Methods and Results
We identified new exons and splice isoforms for ENH in the mouse heart. Impaired cardiac contraction and dilated cardiomyopathy were observed in ENH null mice. Mice with cardiac specific ENH deletion developed a similar dilated cardiomyopathy. Like Cypher, ENH interacted with Calsarcin-1, another Z-line protein. Moreover, biochemical studies showed that ENH, Cypher short isoform and Calsarcin-1 are within the same protein complex at the Z-line. Cypher short isoform and Calsarcin-1 proteins are specifically downregulated in ENH null hearts.
Conclusions
We have identified an ENH-CypherS-Calsarcin protein complex at the Z-line. Ablation of ENH leads to destabilization of this protein complex and dilated cardiomyopathy.
Keywords: Enigma homolog, ENH, dilated cardiomyopathy, Z-line, ENH-Cypher-Calsarcin-1 protein complex
Introduction
The Z-line, also termed the Z-band or Z-disc, is an electron dense structure formed by multiple highly ordered protein complexes which is responsible for transmitting force between sarcomeres during contraction.1, 2 The Z-line also connects myofibrils to the sarcolemmal membrane and ultimately the extracellular matrix.3, 4 The Z-line is not only a basic structural anchor but also an important focus for signaling within striated muscle required for multiple aspects of muscle structure and function.5, 6 Mutations in Z-line proteins have been linked to cardiomyopathy or myofibrillar myopathy both in humans and in transgenic mouse models.7-9
The Enigma subfamily members are Z-line proteins which interact directly with α-actinin-2. Enigma (PDLIM7 or LIM mineralization protein, LMP), Enigma homolog protein (also known as ENH or PDLIM5), and Cypher (ZASP in human) are three Enigma subfamily members, which belong to the PDZ-LIM protein family.10, 11 Cypher has been extensively studied by ourselves and others, and has been found to play a pivotal role in maintaining sarcomeric structure of striated muscle in humans and animals.8, 12-19 Cypher-deficient mice die within five days after birth with multiple striated muscle defects, including dilated cardiomyopathy and disorganized Z-lines. Many mutations in the human Cypher/ZASP gene have been indentified in patients with dilated and/or hypertrophic cardiomyopathy.17, 20-23 Recently, Cypher/ZASP mutations were also found in a portion of patients with myofibrillar myopathies, termed zaspopathy.8, 24
ENH, another member of the Enigma subfamily, is highly homologous to Cypher/ZASP and Enigma.25-27 In man, there are four ENH splice isoforms which have been identified.28 One long isoform (ENH1), containing three LIM domains at its C-terminus, has been found to be expressed ubiquitously in all tissues.26, 27 Three short isoforms (ENH2-4) are expressed predominantly in cardiac and skeletal muscle.27, 28 Splice isoforms of ENH are differentially expressed in heart during development and in heart diseases.29 ENH has been reported to interact with a-actinin,25, 28 protein kinase C,27, 30, 31 protein kinase D,32 L-type calcium channel,32 and a DNA transcription inhibitor, ID2. 33
We hypothesized that ENH, like its homologue Cypher, has an important role in the formation and/or maintenance of the normal Z-line in cardiac and skeletal muscle with an associated role in normal cardiac function. To begin to test this hypothesis, we analyzed ENH splice isoforms in the mouse heart and generated global and cardiac-specific ENH knockout mouse lines in which all ENH isoforms were ablated. We found wider Z-lines and dilated cardiomyopathy in ENH null mouse hearts. In addition, we indentified that ENH forms a protein complex with Cypher short isoform (CypherS) and Calsarcin-1 at the Z-line. CypherS and Calsarcin-1 proteins are significantly downregulated in ENH null mouse hearts resulting in wider and destabilized Z-lines, ultimately giving rise to dilated cardiomyopathy.
Methods
Targeted Disruption of Murine ENH
To generate a floxed allele targeting construct, a 682 bp PCR product containing exon 3 of ENH was cloned into a plasmid containing a Neomycin cassette and a diphtheria toxin A (DTA) cassette. A 4.1-kb upstream fragment and a 4.4-kb downstream fragment were cloned into the vector as the 5′-arm or 3′-arm, respectively. The targeting vector was electroporated into R1 mouse ES cells derived from 129-SV/J mice (UCSD Transgenic and Gene Targeting Core, La Jolla, CA). All subsequent procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of UCSD.
An expanded Methods section including all experimental procedures is available in the Online Data Supplement at http://circres.ahajournals.org
Results
Multiple ENH Splice Isoforms in the Mouse Heart
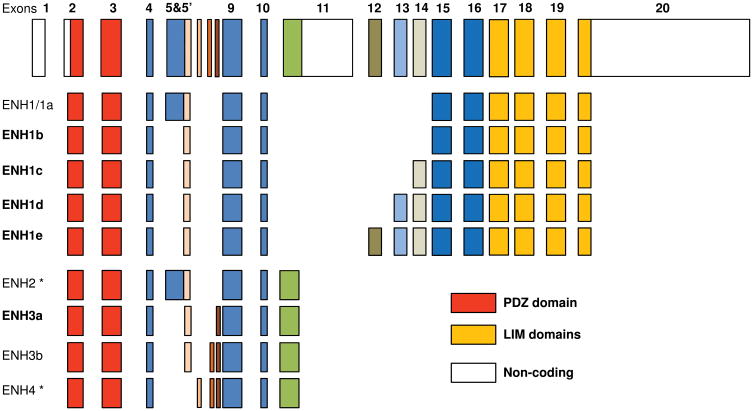
To explore ENH function in the mouse and to ensure that all splice variants of ENH were accounted for in ENH-null mice, we first characterized the splice variants of ENH mRNA in the mouse heart. Four mouse splice isoforms for ENH have been previously reported in the Ensembl database (Pdlim5-201 to Pdlim5-204) and three isoforms in NCBI database (ENH1 to 3). After alignment we found the coding sequence of pdlim5-201 (1776 bp) is similar to ENH1 (NM_019808) (ENH1/1a in Fig. 1), which is the ENH long isoform containing three C-terminal LIM domains. Pdlim5-202 (720 bp) is similar to ENH3 (ENH3/3b in Fig. 1) (NM_022554) and pdlim5-203 (1014 bp) is similar to ENH2 (NM_019809). Pdlim5-204 (645 bp) is not listed in the NCBI database and is homologous to the newly identified human isoform ENH4.28 ENH2, ENH3/3b and ENH4 are ENH short isoforms without the three LIM domains. Using primers for the full-length ENH short isoforms (ENH2-4), we failed to amplify ENH2 and ENH4 from mouse heart cDNA (Online Figure I A and I B). However, we identified ENH3 (we renamed ENH3 as ENH3b, Fig 1) and a new splice isoform with a deletion of exon 7, a small 15-bp exon in the mouse heart which we named ENH3a (Fig. 1) (Online Figure I A). It should also be pointed out that we confirmed that ENH2 and ENH4 were expressed in skeletal muscle by RT-PCR and sequencing analysis (Online Figure I B).
Fig.1.
ENH genomic structure and splice isoforms. Colored boxes are used to represent the 20 exons which encode the murine ENH gene and blank boxes are non-coding regions. The translational start site is in the exon 2. Two stop codons for ENH gene are in exon 11 and exon 20 respectively. The N-terminal exons in red encode PDZ domain. The C-terminal exons in yellow encode three LIM domains. ENH1b is encoded using short exon 5′ instead of exon 5 in ENH1/1a, which was reported as ENH1 in NCBI database (NM_019808). Exons 12-14 are newly identified exons. ENH1c includes the short exon 5′ and exon 14. ENH1d includes exon 5′, exon 13 and exon 14. ENH1e includes exon 5′ and exons 12-14. ENH4 includes the small 17-bp exon 6. ENH3b was renamed from ENH3 (NM_022554) and ENH3a does not include the small 15-bp exon 7. (*) ENH isoforms (ENH2 and ENH4) are only expressed in skeletal muscle.
To determine whether there are more ENH splice long isoforms, we performed RT-PCR analysis with primers in exon 9 and exon 16. By sequencing distinct PCR products, we discovered three previously unidentified exons, 12-14 (Fig. 1 and Online Figure I C, D), containing 120 bp, 144 bp and 132 bp respectively (Online Table I). RT-PCR analysis with primer pairs in exons 3-16 identified four novel ENH long isoforms (ENH1b, c, d, and e), and we renamed ENH1 as ENH1a (Fig. 1 and Online Figure I D). As summarized in Fig 1, there are, in total, 9 splice isoforms for ENH in the mouse heart, among which, there were 5 long isoforms with three LIM domains and 4 short isoforms without LIM domain. Short isoforms ENH2 and ENH4 are skeletal muscle-specific isoforms.
Generation of ENH-null Mice
To investigate the in vivo biological role of ENH, we generated global ENH-null mice by ablating the third exon of the murine ENH gene. The detailed results and figure are shown as Supplement Results and Online Figure II in the Online Data Supplement Material.
Dilated Cardiomyopathy in ENH-null Mice
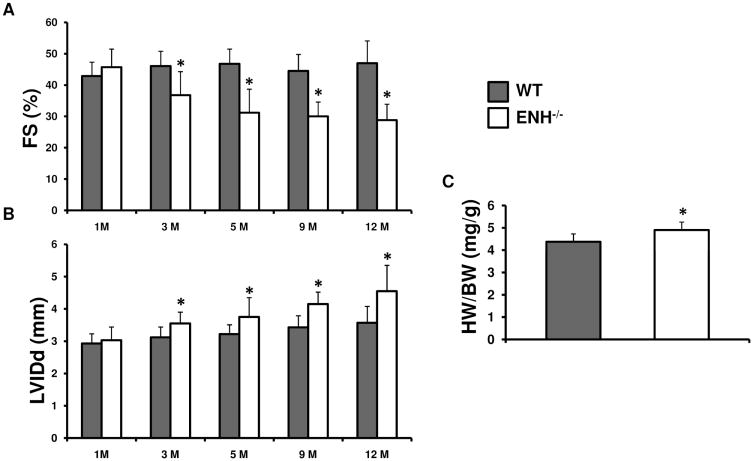
ENH−/− mice are viable and are born at the expected Mendelian ratios. ENH−/− mice are fertile and are indistinguishable from WT littermates. We did not observe any mortality in ENH−/− mice up to 2 years of age when the experiment was terminated. We first explored the heart performance by echocardiography for ENH−/− mice and WT control mice at various time points from 1- to 12-months (Fig. 2). The left ventricular systolic function of ENH−/− mice was impaired beginning at 3-months as shown by a slight, but significant, enlargement of the left ventricle at end-diastole (LVIDd) compared with WT mice beginning at 3-months (Fig. 2A) and a decrease in fractional shortening (FS(%)) (Fig. 2B). However, there was no significant difference in wall thickness as indicated by both interventricular septum and left ventricular posterior wall thickness at end-diastole (data not shown). The heart weight to body weight ratio was mildly, but significantly increased in ENH−/− mice at 3-months (Fig. 2C). In summary, the echocardiographic data shows ENH−/− mice develop typical dilated cardiomyopathy beginning at 3-months with progressive deterioration but do not suffer from sudden cardiac death.
Fig. 2.
Dilated cardiomyopathy assessed by echocardiography in ENH−/− mice. ENH−/− mice (n=8, blank) and WT mice (n=8, grey) were measured at 1-, 3-, 5-, 9- and 12-month-of-age. A, Reduced fractional shortening (FS) in ENH−/− hearts (* P < 0.05). B, Enlarged left ventricles as shown by left ventricular internal dimension (LVIDd) at end diastole in ENH−/− hearts (* P < 0.05). C, The ratios of heart weight to body weight (HW/BW) (mg/g) for ENH−/− mice (male, n=5) and WT mice (male, n=5) at 3-months. (* P < 0.05)
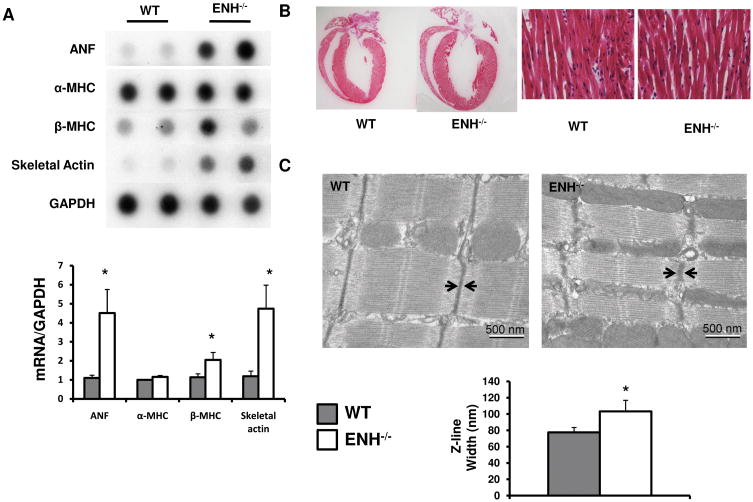
We further assessed the dilated cardiomyopathy in ENH−/− mice by measuring the mRNA levels of cardiac fetal genes (Fig. 3A).34 The expression of ANF and skeletal α-actin were dramatically increased in the ENH−/− hearts beginning at 1-month compared with age matched WT mice. The fetal gene β-MHC was also increased significantly in ENH−/− mouse hearts compared with controls. Thus, fetal gene expression was observed before the onset of dilated cardiomyopathy as measured by echocardiography.
Fig. 3.
Characterization of the dilation and widened Z-lines in ENH−/− mouse hearts. A, mRNA levels for cardiac fetal genes (ANF, α-MHC, β-MHC and skeletal α-actin) was shown by dot-blot analysis of 1-month-old ENH−/− male mice and WT controls. GAPDH was used as a RNA loading control. Quantification of RNA levels normalized to WT levels of the dot densities was shown in the bottom panel (* P < 0.05). B, Representative morphology of an ENH−/− heart and WT heart at 3-months following H&E stain. High-power field was shown in the right panel (20X). C, The ultrastructure of cardiac muscle from the left ventricles of 3-month-old WT and ENH−/− mice was shown by electron microscopy. The quantitative width (distances between two arrows) of Z-lines is shown in the bottom panel (* P < 0.05).
Although chamber dilation and fetal gene expression patterns were significantly increased in ENH−/− mouse hearts, we did not observe any myofibrillar disarray or fibrosis in 3- month-old ENH−/− mice (Fig. 3B and data not shown).
Since ENH has been shown to localize to the Z-line,25 we performed transmission electron microscopy (TEM) to examine Z-line organization (Fig. 3C). In ENH−/− mice, Z-lines were still intact but significantly wider compared with control mice (103.2 ± 13.6 nm versus 77.4 ± 6.1 nm; P ≤ 0.003, respectively). The length of the sarcomeres and the width of the M-lines in ENH−/− mice were comparable to that in control mice. These data showed deletion of ENH impaired the compact and ordered features of the Z-line structure.
Impaired heart contractility in ENH-null Mice
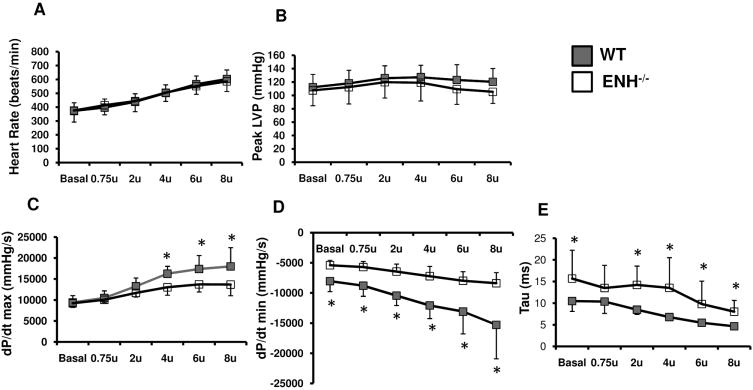
5-month-old male ENH−/− and WT mice (n=8) underwent hemodynmic assessment. Contractile function of the heart was assessed by LV micromanometry (peak dP/dt) at rest and during stepped increases of dobutamine (beta-adrenergic) stimulation. Both groups showed similar heart rates (Fig. 4A) and peak left ventricular systolic pressure (LVSP) (Fig. 4B) under both basal conditions and after application of variable doses of dobutamine. The peak postivie dP/dt (dP/dt max) was slightly, but significantly, decreased in ENH−/− mice under high dobutamine concentrations (beginning at 4 μg/kg/min) (Fig. 4C). Isovolumic relaxation, as assessed by minimum dP/dt, was dramatically impaired in ENH−/− mice compared with WT controls at basal and various doses of dobutamine (Fig. 4D). Also, the calculated Tau values in ENH−/− mice were significantly longer than those in control mice (Fig. 4E). These data suggested ENH−/− mice failed to fully respond to dobutamine stimulation and exhibited myocardial inotropic and lusitropic dysfunction.
Fig. 4.
Impaired contractility in ENH−/− hearts. 5-month-old male ENH−/− (n=8) and WT mice (n=8) were subjected to hemodymic measurements. A and B, ENH−/− and WT mice had the same heart rates and maximum left ventricular pressure. C, ENH−/− mice showed lower dP/dt maximum (dP/dt max) when stimulated with 4-8U (U=μg/kg/min) of dobutamine (* p < 0.05). D, ENH−/− mice had lower dP/dt minimum (dP/dt min) under basal conditions and with various doses of dobutamine (* p < 0.05). E, Tau, a load independent measure of relaxation, was increased in EHN−/− mice under basal conditions and following various doses of dobutamine ranging from 2-8U (* p < 0.05).
Dilated Cardiomyopathy in Cardiac-specific ENH-null Mice
To determine whether the DCM phenotype observed in the ENH global knockout mice was the result of the requirement for ENH in a cardiomyocyte autonomous fashion, we specifically deleted ENH in cardiomyocytes. The Troponin T-Cre mouse (cTnT-Cre), in which expression of Cre was controlled by the rat cardiac Troponin T promoter, was used to generate cardiac-specific ENH knockout mice.35 To assess ENH protein expression in cardiac specific knockout hearts, the ENH antibody from Abnova was used. Both ENH long and short isoforms were dramatically downregulated in the ENH cardiac specific knockout hearts (Online Figure III A). As seen in the global ENH−/− mice, the cardiac specific ENH−/− mice (cENH−/−) developed dilated cardiomyopathy beginning at 3-months (Online Figure III B, C). The left ventricular function was significantly impaired in cENH−/− hearts compared with control mice measured by FS (%) (Online Figure III B). The left ventricular chamber size was enlarged significantly in cENH−/− hearts (Online Figure III C) as indicated by LVID at both end diastole and end systole compared with cTnT-Cre carrier mice. There was no difference in ventricular wall thickness between cENH−/− mice and cTnT-Cre mice (data now shown). Furthermore, as in global ENH−/− hearts, the mRNA levels of cardiac fetal genes (ANF, BNP, β-MHC and skeletal α-actin) were dramatically increased in cENH−/− hearts (Online Figure III D, E). The dilated cardiomyopathy in the cENH−/− mouse suggested that the observed dilated cardiomyopathy and impaired left ventricular function in the global ENH−/− mice were due to the loss of ENH in cardiomyocytes.
Increased Systolic Dysfuntion in ENH-null Mice Following Biomechanical Stress
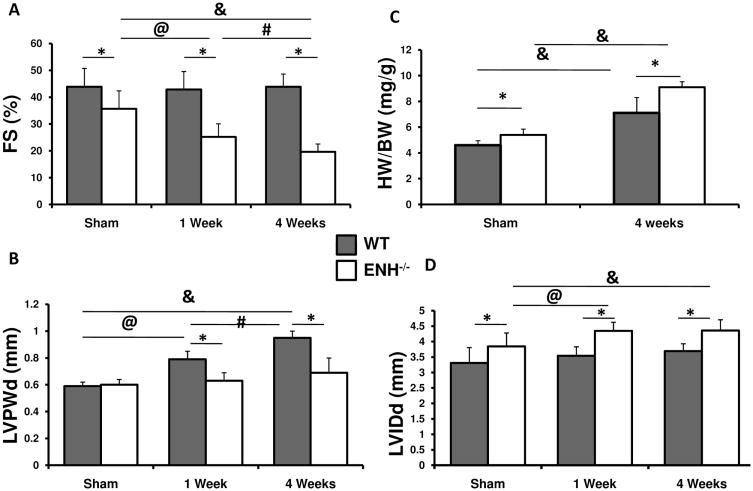
To assess cardiac function of ENH−/− mice following biomechanical stress, we subjected mice to transverse aortic constriction (TAC). Significant decreases in left ventricular systolic function were observed beginning at one week post-TAC in ENH−/− mice when compared with WT mice and sham operated ENH−/− controls (Fig. 5A). Left ventricular diastolic dimension (LVIDd) was increased in TAC-banded ENH−/− mice when compared to sham operated or WT controls (Fig. 5B). This was accompanied by, a blunted increase in wall thickness following pressure overload in ENH−/− mice when compared with WT controls as reflected by the LV posterior wall thickness (LVPWd) (Fig. 5B) and interventricular septum thickness (IVSd) (data not shown). However, both ENH−/− and WT mice displayed significant increases in HW/BW ratio (Fig. 5C). Although ENH−/− mice displayed a blunted LV posterior wall thickness, dilation was increased in TAC-banded ENH−/− mice when compared to sham operated or WT controls (Fig. 5D).
Fig. 5.
Increased Systolic Dysfuntion in ENH-null Mice Following Biomechanical Stress. 2-month-old WT or ENH−/− mice underwent either sham operation (n=3) or TAC surgery (n=8). A, Systolic heart function, as shown by percent fractional shortening ((FS (%)), was assessed by echocardiography. B, Left ventricular posterior wall thickness (LVPWd) at end of diastole is shown for sham, 1-, and 4-week post-TAC. C, Ratios of heart weight to body weight (HW/BW) (mg/g) are shown for sham and 4-week post-TAC surgery. D, Left ventricular internal dimension (LVIDd) at end of diastole is shown for sham, 1-, and 4-week post-TAC. *, p < 0.05 between WT and ENH−/− groups. @, p < 0.05 between sham and 1-week post-TAC. #, p < 0.05 between 1-week and 4-week post- TAC. &, p < 0.05 between sham and 4-week post-TAC.
Decreased Levels of Cypher Short Isoform and Calsarcin-1 in ENH-null Heart
It has been shown that multiple intracellular signals are involved in sarcomeric protein deficiency-induced cardiomyopathy.36 To determine molecular mechanisms underlying the DCM phenotype observed in ENH−/− mice, we first checked stress related signaling pathways including, PKD, PKC, ERK, JNK, P38, Akt, and Calcinurin-NFAT in ENH-null mouse myocardium. No differences were observed in total or phosphorylated PKD, PKC, ERK, JNK, P38, and Akt between ENH−/− and WT controls (Online Figure IV A, B). There was also no difference in calcineurin activity assayed by nuclear translocation of NFATc4 and the expression of its target gene, modulatory calcineurin-interacting protein (MCIP), between ENH−/− and WT controls (Online Figure V).
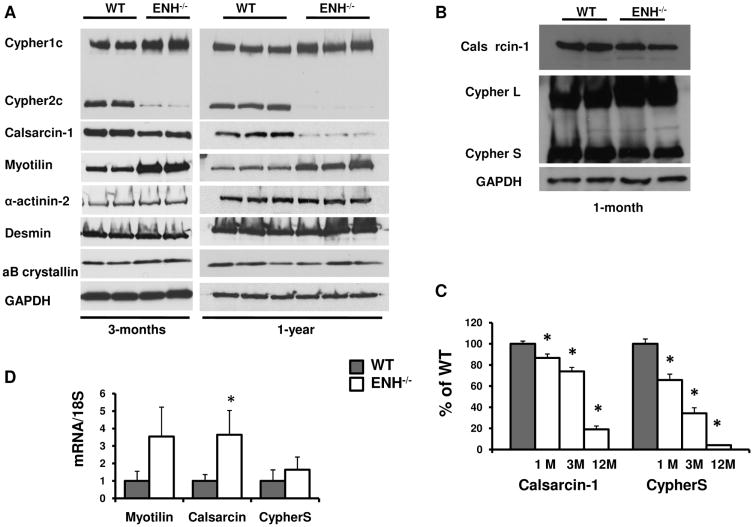
We then performed western blot analysis for various Z-line proteins in ENH−/− mice (Fig. 6A). The CypherS and Calsarcin-1 were specifically downregulated in ENH−/− mice at 3-months, and both proteins were nearly depleted at 12-months (Fig. 6A, 6C). Interestingly, a small but significant decrease in CypherS and Calsarcin-1 was also detected in ENH mutants at 1-month, prior to the onset of dilated cardiomyopathy (Fig. 6B, 6C). We have also found that with increased stress consequent to TAC, ENH mutants exhibit rapid loss of Calsarcin and CypherS (Online Figure VI). This observation is correlated with more severe defects in cardiac contractile function in ENH mutants relative to control littermates (Fig. 5).
Fig. 6.
Loss of Cypher short isoform and Calsarcin-1 proteins in ENH−/− hearts. A, CypherS and Calsarcin-1 were downregulated in 3-month-old ENH−/− mice (left panel) and almost depleted in 12-month-old mice (right panel). Myotilin was upregualted in both 3-month-old and 12-month-old ENH−/− mice. α B-crystallin, α-actinin 2 and Desmin were not different between WT and ENH−/− mice at either age analyzed. GAPDH was shown as loading control. B, CypherS and Calsarcin-1 were slightly downregulated in 1-month ENH−/− mice compared with age-matched WT controls. C, The downregulation of CypherS and Calsarcin-1 in ENH−/− hearts is age dependent. D, The mRNA of Myotilin, Calsarcin-1 and CypherS were assessed by real-time RT-PCR. 18S RNA was used as internal RNA standard. The ratio to 18S RNA was further normalized to WT. *, P < 0.05.
In ENH−/− mice, Myotilin was upregulated. Amounts of other Z-line/Z-line associated proteins including α-actinin-2, Desmin, α B-crystallin and were not changed (Fig. 6A). In contrast to the protein level, at the mRNA level, calsarcin-1 was significantly upregulated in ENH−/− mice compared with controls (Fig. 6D). While not significant, mRNA expression of myotilin and cypherS was increased in ENH−/− hearts (Fig. 6D), suggesting that the loss of CypherS and Calsarcin-1 protein was due to a post-translational mechanism and not due to decreased mRNA synthesis.
An ENH-Cypher-Calsarcin-1 Protein Complex
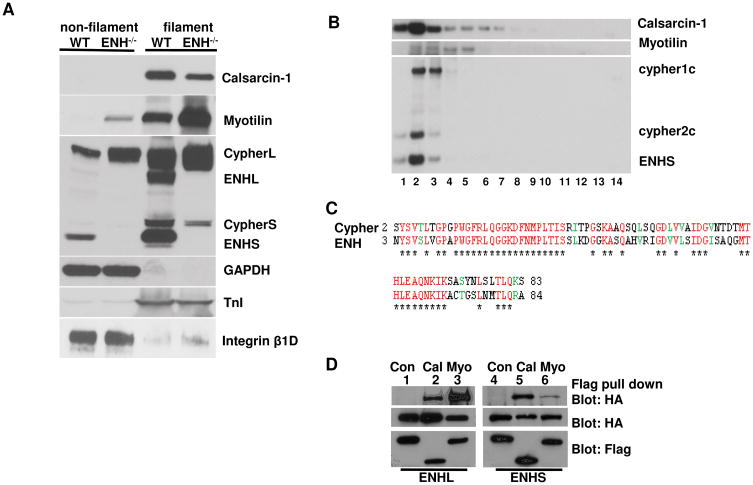
To explore why CypherS and Calsarcin-1 proteins were specifically downregulated in ENH−/− mice, we used a biochemical method to isolate myofibrillar proteins from 3-month-old adult mouse ventricles. As expected, cytosolic protein GAPDH and membrane protein Integrin β1D were dominantly localized in the non-filament fraction and sarcomeric protein Tropoin I was dominantly localized in the filament fraction. These results for control proteins showed that this mild detergent washing method did specifically isolate the myofibril proteins from the heart (Fig. 7A). We found the ENH short and long isoforms were dominantly localized in the filament fraction as previously reported.25 CypherL was found in both the filament and non-filament fractions, and CypherS was found dominantly in the filament fraction. The Z-line proteins Calsarcin-1 and Myotilin were also dominantly localized in the filament fraction in agreement with previously reported immunofluorescent data.37, 38 In ENH−/− hearts, Myotilin was highly increased in the filament fraction and could also be detected in the non-filament fraction (Fig. 7A).
Fig. 7.
ENH-Cypher-Calsarcin protein complex. A, Calsarcin-1, Myotilin, CypherS and ENH long isoforms were dominantly localized in the filament fraction as shown by biochemical isolation and western blot analysis. CypherL and ENH short isoforms are in both filament and non-filament fractions. GAPDH (cytosolic protein), Integrinβ1D (membrane protein) and Troponin I (TnI, filament protein) were used as fractionation controls. B, ENH, CypherS, and Calsarcin are localized in the same fractions as shown by sucrose gradient sediment analysis. A total of 14 fractions were collected (from low to high of sucrose concentrations) and resolved by SDS-PAGE before western blot analysis. C, The ENH PDZ domain shares high similarity in amino acid residues with the Cypher PDZ domain. Identical amino acid residues are shown in red star underneath and similar amino acid residues are shown in green for both the Cypher (2-83) and the ENH PDZ domains (3-84). D, ENH interacted with Calsarcin-1 and Myotilin in vitro. FLAG-tagged Calsarcin-1 (lanes 2 and 5) or Myotilin (lanes 3 and 6) were coexpressed with HA-tagged ENH1 (ENH long isoform, lanes 1-3) or ENH3 (ENH short isoform, lanes 4-6) in HEK 293 cells. Exogenous protein expression was verified by immunobolting with FLAG or HA antibodies. ANTI-FLAG M2 Affinity Gel was used to purify FLAG-tagged proteins and interacting proteins were visualized by immunoblotting with a HA antibody. FLAG-tagged control proteins (protein kinase A RI subunit) (lanes 1 and 4) were used as a control and did not bind to HA-tagged ENH proteins.
In order to identify any possible protein complexes containing either Cypher or ENH, we performed a sedimentation assay. In sedimentation experiments, proteins which tightly interact with each other co-migrate in the sucrose gradient and are localized in identical fractions. Our fractionation experiment showed that Calsarcin-1, CypherS, and ENH co-localize in fractions 1-3 in WT muscle (Fig. 7B). When taken together with the loss of Calsarcin-1 and CypherS in the ENH−/− mouse, this data indicates these three proteins are likely localized within the same core complex. Interestingly, the fractionation patterns of both CypherL and Myotilin are similar, and both of these proteins are increased upon deletion of ENH. These data suggests CypherL and Myotilin may form a separate protein complex and may partially compensate for the loss of the ENH/ CypherS/Calsarcin-1 protein complex in the ENH−/− mouse.
We, and others, have reported that the Myotilin and Calsarcin-1 contain PDZ binding motifs at the C-terminus which interact with the PDZ domain of Cypher. 16, 39 Fig. 7C shows 61% of identity (71% of similarity) between the Cypher PDZ domain and the ENH PDZ domain. We confirmed the interaction between ENH, Myotilin, and Calsarcin-1 in vitro (Fig. 7D). Flag-tagged Calsarcin-1 interacted with both HA-tagged ENH1/1a and ENH3/3b, while a flag-tagged unrelated control protein, PKARIα, did not bind to either ENH isoform. Similar to Calsarcin-1, flag-tagged Myotilin interacted with both HA-tagged ENH1/1a and ENH3/3b. Along with the sedimentation assay results, these findings suggest that the filament proteins ENH, Cypher and Calsarcin-1 form a protein complex through direct protein interactions.
Calsarcin has been shown to interact with Filamin C which directly interacts with β1 integrin 40 and is associated with both γ and δ sarcoglycan 41. Thus, the ENH-CypherS-Calsarcin complex at the Z-line may play a pivotal role in linking Z-lines to extracellular matrix via Filamin C. Loss of this complex may disrupt a critical structural function of the Z-line in establishing continuity between sarcomeres and the extracellular matrix to effect optimal force generation. To investigate this, we examined expression of Filamin C and components of structural complexes known to link sarcomeres to extracellular matrix, the Integrin and the Dystrophin glycoprotein complexes. Expression of Filamin C, β1D Integrin, Dystrophin, Syntrophin, and γ Sarcoglycan were upregulated in ENH-null mice (Online Figure VII).
Discussion
Since the identification of the first PDZ-LIM domain protein (Enigma), a total of ten PDZ-LIM domain protein members have been identified in mammals. 10 Based on sequence similarity and binding affinity, four subgroups have been identified within the PDZ-LIM protein family: Actinin-associated LIM protein (ALP), Enigma, LIM kinases, and LIM-domain only 7 (LMO7). 10 Enigma, Cypher, and the closely related Enigma homolog (ENH) protein are members of the Enigma subfamily. Previously, using global and cardiac-specific knockout mouse models, we have demonstrated that Cypher is not necessary for sarcomerogenesis but is essential to maintain integrity of Z-line structure during muscular contraction. 15, 16 In the current report, we examined cardiac phenotypes of both global and cardiac-specific ENH knockout mice.
Prior to beginning our study, four ENH splice isoforms had been identified: ENH 1-4. In the current report, we have identified five additional previously undescribed ENH isoforms expressed in heart. All isoforms expressed in the mouse heart contain exon 3 which encodes part of the PDZ domain. For this reason, we chose to target exon 3 for deletion. Western blot results using different sources of antibodies demonstrated that ENH was absent in knockout mice. ENH knockout mice developed dilated cardiomyopathy characterized by enlargement of the left ventricle, impaired systolic and diastolic heart function, widened Z-lines, and elevation of fetal gene expression. Cardiac-specific knockout mice showed a similar dilated cardiomyopathy phenotype. In addition, we found that increased biomechanical stress consequent to TAC banding further exasperated the dilated cardiomyopathic phenotype. Together, these results suggest that ENH plays an important role in cardiac muscle structure and function.
Dilated cardiomyopathy is usually accompanied by activation of intracellular signaling pathways.42 In ENH−/− mice, we failed to find activation of common cardiac stress pathways such as PKC, PKD, ERK, JNK, p38, Akt, and calcineurin-NFAT pathways at 3-months. By this age, ENH−/− mice showed enlarged hearts and widened Z-lines. Thus, the dilated phenotype observed with ENH mutants may reflect a structural requirement for ENH in the Z-line.
ENH, Cypher, and Calsarcin-1 directly interact with α-actinin-2 at the Z-line.12, 25, 37 Our results from myofibril isolation, sucrose fractionation, and in vitro pull down assays demonstrate that ENH, CypherS, and Calsarcin-1 are components of a protein complex localized at the Z-line. In ENH knockout hearts, CypherS and Calsarcin-1 were specifically downregulated at the protein level but not at the mRNA level. Thus, deletion of ENH caused instability and loss of the CypherS/ENH/Calsarcin-1 protein complex. Calsarcin has been shown to interact with Filamin C which directly interacts with β1 integrin 40 and is associated with both γ and δ sarcoglycan 41. Thus, the ENH-CypherS-Calsarcin complex at the Z-line is likely to play a pivotal role in linking the Z-line to the extracellular matrix via Filamin C. Observed upregulation of Filamin C, Integrin and components of the Dystrophin-glycoprotein complex in ENH mutants are likely to reflect a compensatory mechanism consequent to disruption of the connection between the Z-line and the extracellular matrix. Similar upregulation of these complexes has been shown to act as a compensatory mechanism to strengthen a weakened connection to the extracellular matrix in cardiac specific β1-integrin knockout mice. 43
Taken together, our data suggest that loss of ENH leads to progressive loss of CypherS and Calsarcin resulting in the loss of Z-line structural integrity and consequent perturbation of the connection between adjacent sarcomeres and extracellular matrix. This leads to a loss of optimal force transmission and a significant decrease in fractional shortening and ultimately DCM.
Both Cypher and ENH knockout mice develop postnatal dilated cardiomyopathy, with differences in severity, hinting that there is a redundant, but unique, role for Cypher and ENH in the heart. Interestingly, in lower invertebrates, such as Drosophila, Drosophila ZASP (dZASP) is the only gene representing the entire ALP/Enigma subfamily of PDZ-LIM domain protein. dZASP is essential for Drosophila to form intact sarcomeres.18, 19
There appears to be substantial functional redundancy in proteins localized to the Z-line, as ablation of numerous Z-line proteins, including Calsarcin 1 44, and Myotilin 45, does not result in a significant basal phenotype. Both ENH and Cypher are expressed early in embryonic mouse heart (unpublished data). 15 There is no obvious heart developmental defect in either ENH−/− or Cypher−/− mice, suggesting a possible functional overlap between these two Enigma family members during embryonic development. Future studies with ENH and Cypher double mutant mice are needed to determine the potential role of ENH and Cypher in sarcomerogenesis and heart development.
Our findings that ENH plays an important role in the heart make it a novel disease candidate and suggest that human patients with cardiomyopathy should be screened for possible ENH mutations.
Supplementary Material
Novelty and Significance.
What Is Known?
The Enigma Homolog protein (ENH) is highly homologous to the proteins Cypher and Enigma
ENH has many splice isoforms. The long isoforms is expressed ubiquitously, while the short isoforms is expressed predominantly in cardiac and skeletal muscle
Mutations in Cypher lead to the development of dilated cardiomyopathyin both mice and humans
What New Information Does This Article Contribute?
Identifies previously unknown ENH exons and splice isoforms
Characterizes dilated cardiomyopathy and impaired cardiac function which develops in the absence of ENH
Identifies Z-line protein complex composed of ENH, Cypher short isoform, and Calsarcin-1
The Enigma subfamily of proteins, including Cypher, Enigma and ENH proteins, are localized to a highly ordered structure at the border between neighboring sarcomeres, termed the Z-line. Cypher can play a pivotal role in maintaining striated muscle integrity in both humans and in mouse models. The role of ENH in striated muscle remains largely unknown. To investigate the role of ENH in mammalian cardiac muscle, we characterized and identified novel ENH splice isoforms in the mouse heart and generated global and cardiac-specific ENH knockout mouse lines in which all identified ENH isoforms were ablated. We found disorganized Z-lines and dilated cardiomyopathy in ENH knockout mouse hearts. Furthermore, we also found that ENH forms a protein complex with the Cypher short isoform and Calsarcin-1 at the Z-line, and that the Cypher short isoform and Calsarcin-1 proteins are specifically downregulated in ENH knockout mouse hearts. We conclude that ablation of ENH leads to destabilization of this protein complex and consequent perturbation of the connection between adjacent sarcomeres and extracellular matrix which leads to a loss of optimal force transmission and a significant decrease in fractional shortening and eventually dilated cardiomyopathy.
Acknowledgments
Sources of Funding: The work was supported by National Institutes of Health grants for Drs JC, KLP, SME, and KUK. AKP was supported by an American Heart Association Postdoctoral Fellowship award.
Non-standard Abbreviations and Acronyms
- ENH
Enigma homolog protein
- DCM
dilated cardiomyopathy
- ZASP
Z-band alternatively spliced PDZ motif protein
- WT
WT
- KO
knockout
- cTnT-Cre
cardiac Troponin T Cre
- cENH−/−
cardiac specific ENH knockout
- ALP
actinin-associated LIM protein
- %FS
percentage of fractional shortening
- LVID
left ventricular internal dimension
- LVPW
left ventricular posterior wall thickness
- IVS
interventricular septal thickness
- DTA
diphtheria toxin A
- I.V.
intravenous
- LVP
left ventricular pressure
- TEM
transmission electron microscopy
- NFAT
nuclear factor of activated T-cells
- MCIP
modulatory Calcineurin-interacting protein
- CypherL
Cypher long isoforms
- CypherS
Cypher short isoform
- LV
left ventricle
- MHC
myosin heavy chain
- ANF
atrial natriuretic factor
- BNP
B-type natriuretic peptide
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanger JM, Sanger JW. The dynamic z bands of striated muscle cells. Sci Signal. 2008;1:pe37. doi: 10.1126/scisignal.132pe37. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein MA, Schroeter JP, Michael LH. Role of the z band in the mechanical properties of the heart. FASEB J. 1991;5:2167–2174. doi: 10.1096/fasebj.5.8.2022313. [DOI] [PubMed] [Google Scholar]
- 3.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 4.Ervasti JM. Costameres: The achilles' heel of herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 5.Kruger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46:490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Luther PK. The vertebrate muscle z-disc: Sarcomere anchor for structure and signalling. J Muscle Res Cell Motil. 2009;30:171–185. doi: 10.1007/s10974-009-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank D, Kuhn C, Katus HA, Frey N. Role of the sarcomeric z-disc in the pathogenesis of cardiomyopathy. Future Cardiol. 2007;3:611–622. doi: 10.2217/14796678.3.6.611. [DOI] [PubMed] [Google Scholar]
- 8.Selcen D, Carpen O. The z-disk diseases. Adv Exp Med Biol. 2008;642:116–130. doi: 10.1007/978-0-387-84847-1_10. [DOI] [PubMed] [Google Scholar]
- 9.Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: An intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- 10.te Velthuis AJ, Bagowski CP. Pdz and lim domain-encoding genes: Molecular interactions and their role in development. ScientificWorldJournal. 2007;7:1470–1492. doi: 10.1100/tsw.2007.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng M, Cheng H, Banerjee I, Chen J. Alp/enigma pdz-lim domain proteins in the heart. J Mol Cell Biol. 2010;2:96–102. doi: 10.1093/jmcb/mjp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Ruiz-Lozano P, Martone ME, Chen J. Cypher, a striated muscle-restricted pdz and lim domain-containing protein, binds to alpha-actinin-2 and protein kinase c. J Bioll Chem. 1999;274:19807–19813. doi: 10.1074/jbc.274.28.19807. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner G, Pallavicini A, Formentin E, Comelli A, Ievolella C, Trevisan S, Bortoletto G, Scannapieco P, Salamon M, Mouly V, Valle G, Lanfranchi G. Zasp: A new z-band alternatively spliced pdz-motif protein. J Cell Biol. 1999;146:465–475. doi: 10.1083/jcb.146.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passier R, Richardson JA, Olson EN. Oracle, a novel pdz-lim domain protein expressed in heart and skeletal muscle. Mech Dev. 2000;92:277–284. doi: 10.1016/s0925-4773(99)00330-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Chu PH, Huang C, Cheng CF, Martone ME, Knoll G, Shelton GD, Evans S, Chen J. Ablation of cypher, a pdz-lim domain z-line protein, causes a severe form of congenital myopathy. J cell biol. 2001;155:605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng M, Cheng H, Li X, Zhang J, Cui L, Ouyang K, Han L, Zhao T, Gu Y, Dalton ND, Bang ML, Peterson KL, Chen J. Cardiac-specific ablation of cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum Mol Genet. 2009;18:701–713. doi: 10.1093/hmg/ddn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikh F, Bang ML, Lange S, Chen J. “Z”Eroing in on the role of cypher in striated muscle function, signaling, and human disease. Trends Cardiovasc Med. 2007;17:258–262. doi: 10.1016/j.tcm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jani K, Schock F. Zasp is required for the assembly of functional integrin adhesion sites. J Cell Biol. 2007;179:1583–1597. doi: 10.1083/jcb.200707045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benna C, Peron S, Rizzo G, Faulkner G, Megighian A, Perini G, Tognon G, Valle G, Reggiani C, Costa R, Zordan MA. Post-transcriptional silencing of the drosophila homolog of human zasp: A molecular and functional analysis. Cell Tissue Res. 2009;337:463–476. doi: 10.1007/s00441-009-0813-y. [DOI] [PubMed] [Google Scholar]
- 20.Arimura T, Hayashi T, Terada H, Lee SY, Zhou Q, Takahashi M, Ueda K, Nouchi T, Hohda S, Shibutani M, Hirose M, Chen J, Park JE, Yasunami M, Hayashi H, Kimura A. A cypher/zasp mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase c. J Biol Chem. 2004;279:6746–6752. doi: 10.1074/jbc.M311849200. [DOI] [PubMed] [Google Scholar]
- 21.Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, Perles Z, Sinagra G, Lin JH, Vu TM, Zhou Q, Bowles KR, Di Lenarda A, Schimmenti L, Fox M, Chrisco MA, Murphy RT, McKenna W, Elliott P, Bowles NE, Chen J, Valle G, Towbin JA. Mutations in cypher/zasp in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol. 2003;42:2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Moric-Janiszewska E, Markiewicz-Loskot G. Genetic heterogeneity of left-ventricular noncompaction cardiomyopathy. Clin Cardiol. 2008;31:201–204. doi: 10.1002/clc.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing Y, Ichida F, Matsuoka T, Isobe T, Ikemoto Y, Higaki T, Tsuji T, Haneda N, Kuwabara A, Chen R, Futatani T, Tsubata S, Watanabe S, Watanabe K, Hirono K, Uese K, Miyawaki T, Bowles KR, Bowles NE, Towbin JA. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab. 2006;88:71–77. doi: 10.1016/j.ymgme.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Griggs R, Vihola A, Hackman P, Talvinen K, Haravuori H, Faulkner G, Eymard B, Richard I, Selcen D, Engel A, Carpen O, Udd B. Zaspopathy in a large classic late-onset distal myopathy family. Brain. 2007;130:1477–1484. doi: 10.1093/brain/awm006. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa N, Hoshijima M, Oyasu M, Saito N, Tanizawa K, Kuroda S. Enh, containing pdz and lim domains, heart/skeletal muscle-specific protein, associates with cytoskeletal proteins through the pdz domain. Biochem Biophys Res Commun. 2000;272:505–512. doi: 10.1006/bbrc.2000.2787. [DOI] [PubMed] [Google Scholar]
- 26.Ueki N, Seki N, Yano K, Masuho Y, Saito T, Muramatsu M. Isolation, tissue expression, and chromosomal assignment of a human lim protein gene, showing homology to rat enigma homologue (enh) J Hum Genet. 1999;44:256–260. doi: 10.1007/s100380050155. [DOI] [PubMed] [Google Scholar]
- 27.Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, Gill GN, Kikkawa U. Protein-protein interaction of zinc finger lim domains with protein kinase c. J Biol Chem. 1996;271:31029–31032. doi: 10.1074/jbc.271.49.31029. [DOI] [PubMed] [Google Scholar]
- 28.Niederlander N, Fayein NA, Auffray C, Pomies P. Characterization of a new human isoform of the enigma homolog family specifically expressed in skeletal muscle. Biochem Biophys Res Commun. 2004;325:1304–1311. doi: 10.1016/j.bbrc.2004.10.178. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki T, Walchli S, Fujita T, Ryser S, Hoshijima M, Schlegel W, Kuroda S, Maturana AD. Splice variants of enigma homolog, differentially expressed during heart development, promote or prevent hypertrophy. Cardiovasc Res. 2010;86:374–382. doi: 10.1093/cvr/cvq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeno-Hikichi Y, Chang S, Matsumura K, Lai M, Lin H, Nakagawa N, Kuroda S, Zhang JF. A pkc epsilon-enh-channel complex specifically modulates n-type ca2+ channels. Nat Neurosci. 2003;6:468–475. doi: 10.1038/nn1041. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Lai M, Maeno-Hikichi Y, Zhang JF. Essential role of the lim domain in the formation of the pkcepsilon-enh-n-type ca2+ channel complex. Cell Signal. 2006;18:215–224. doi: 10.1016/j.cellsig.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Maturana AD, Walchli S, Iwata M, Ryser S, Van Lint J, Hoshijima M, Schlegel W, Ikeda Y, Tanizawa K, Kuroda S. Enigma homolog 1 scaffolds protein kinase d1 to regulate the activity of the cardiac l-type voltage-gated calcium channel. Cardiovasc Res. 2008;78:458–465. doi: 10.1093/cvr/cvn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasorella A, Iavarone A. The protein enh is a cytoplasmic sequestration factor for id2 in normal and tumor cells from the nervous system. Proc Natl Acad Sci U S A. 2006;103:4976–4981. doi: 10.1073/pnas.0600168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chien KR, Zhu H, Knowlton KU, Miller-Hance W, van-Bilsen M, O'Brien TX, Evans SM. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- 35.Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283:26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci U S A. 2000;97:14632–14637. doi: 10.1073/pnas.260501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salmikangas P, Mykkanen OM, Gronholm M, Heiska L, Kere J, Carpen O. Myotilin, a novel sarcomeric protein with two ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–1336. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- 39.von Nandelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpen O, Faulkner G. A class iii pdz binding motif in the myotilin and fatz families binds enigma family proteins: A common link for z-disc myopathies. Mo cell biol. 2009;29:822–834. doi: 10.1128/MCB.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gontier Y, Taivainen A, Fontao L, Sonnenberg A, van der Flier A, Carpen O, Faulkner G, Borradori L. The z-disc proteins myotilin and fatz-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J cell sci. 2005;118:3739–3749. doi: 10.1242/jcs.02484. [DOI] [PubMed] [Google Scholar]
- 41.Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG, McNally EM, Watkins S, Kunkel LM. Filamin 2 (fln2): A muscle-specific sarcoglycan interacting protein. J cell biol. 2000;148:115–126. doi: 10.1083/jcb.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Q, Molkentin JD. Redefining the roles of p38 and jnk signaling in cardiac hypertrophy: Dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Elsherif L, Huang MS, Shai SY, Yang Y, Li RY, Chun J, Mekany MA, Chu AL, Kaufman SJ, Ross RS. Combined deficiency of dystrophin and beta1 integrin in the cardiac myocyte causes myocardial dysfunction, fibrosis and calcification. Circ res. 2008;102:1109–1117. doi: 10.1161/CIRCRESAHA.108.173153. [DOI] [PubMed] [Google Scholar]
- 44.Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004;10:1336–1343. doi: 10.1038/nm1132. [DOI] [PubMed] [Google Scholar]
- 45.Moza M, Mologni L, Trokovic R, Faulkner G, Partanen J, Carpen O. Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice. Mol cell biol. 2007;27:244–252. doi: 10.1128/MCB.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.