Abstract
Oxidative stress and inflammation have not been well-characterized in extreme pediatric obesity. We compared levels of circulating oxidized LDL (oxLDL), C-reactive protein (CRP), and interleukin-6 (IL-6) in extremely obese (EO) children to normal weight (NW) and overweight/obese (OW/OB) children. OxLDL, CRP, IL-6, body mass index (BMI), blood pressure, and fasting glucose, insulin, and lipids were obtained in 225 children and adolescents (age 13.5 ± 2.5 years; boys 55%). Participants were classified into three groups based on gender- and age-specific BMI percentile: NW (<85th, n = 127), OW/OB (85th-<1.2 times the 95th percentile, n = 64) and EO (≥1.2 times the 95th percentile or BMI ≥35 kg/m2, n = 34). Measures were compared across groups using ANCOVA, adjusted for gender, age, and race. Blood pressure, insulin, and lipids worsened across BMI groups (all p<0.0001). OxLDL (NW: 40.8 ± 9.0 U/L, OW/OB: 45.7 ± 12.1 U/L, EO: 63.5 ± 13.8 U/L) and CRP (NW: 0.5 ± 1.0 mg/L, OW/OB: 1.4 ± 2.9 mg/L, EO: 5.6 ± 4.9 mg/L) increased significantly across BMI groups (all groups differed with p<0.01). IL-6 was significantly higher in EO (2.0 ± 0.9 pg/mL) compared to OW/OB (1.3 ± 1.2 pg/mL, p<0.001) and NW (1.1 ± 1.0 pg/mL, p<0.0001) but was not different between NW and OW/OB. Extreme pediatric obesity, compared to milder forms of adiposity and normal weight, is associated with higher levels of oxidative stress and inflammation, suggesting that markers of early cardiovascular disease and type 2 diabetes mellitus are already present in this young population.
Keywords: Circulating Oxidized LDL Cholesterol, Inflammation, Extreme Pediatric Obesity
Introduction
Extreme pediatric obesity, defined as an age- and gender-specific BMI ≥1.2 times the 95th percentile or BMI ≥35 kg/m2 (1;2), is estimated to affect 3–6% of US children, has increased in prevalence by 300% since 1976 and by 70% since 1994 (2;3), and disproportionately affects certain minority groups (4). Approximately 60% of extremely obese children and adolescents have two or more risk factors for cardiovascular disease (5). In addition, obesity in childhood (6–8), especially extreme pediatric obesity (5), tracks strongly into adulthood.
Oxidative stress and inflammation are independently associated with the development of cardiovascular disease (9–14) and type 2 diabetes mellitus in adults (15–20). OxLDL, a marker of oxidative stress, is associated with obesity (21;22), insulin resistance (23), metabolic syndrome (24–27), and cardiovascular disease (9–11) in adults. Recently, we have demonstrated that overweight and obese children and adolescents have higher levels of oxLDL compared to normal-weight peers and that oxLDL is associated with insulin resistance, after adjustment for body fatness (28). CRP and IL-6 are markers of inflammation that have been shown to independently predict cardiovascular disease and type 2 diabetes mellitus in adults (12–15;17–20).
Although the relationships of oxidative stress and inflammation with obesity have been well-described in adults, less is known in the pediatric population (28–32). More specifically, these associations have not been well characterized across categories of pediatric obesity. The purpose of this study was to compare levels of oxLDL, CRP, and IL-6 in EO versus NW, OW, and OB children and adolescents.
Methods and Procedures
Study Design and Participants
This cross-sectional study included 225 children and adolescents (mean age=13.5 ± 2.5 years; boys n=124, 55%) who were categorized (following testing) into three adiposity groups based on age and gender-specific BMI percentiles (normal weight: NW <85th percentile, n = 127; overweight and obese: OW/OB 85th-<1.2 times the 95th percentile, n = 64; and extremely obese: EO ≥1.2 times the 95th percentile or BMI ≥35 kg/m2, n = 34). The current definition of EO is approximately equivalent to a BMI ≥99th percentile. The majority (n = 195, 87%) of these participants were recruited from a healthy sibling control group participating in cross-sectional study of cardiovascular risk in childhood cancer survivors. Thirty (13%) participants were recruited from the University of Minnesota Amplatz Children Hospital Pediatric Weight Management Clinic. The protocol was approved by the University of Minnesota Institutional Review Board and consent/assent was obtained from parents/participants.
Measurement of Clinical Variables
Height and weight were obtained using a standard stadiometer and electronic scale, respectively. BMI was calculated as weight in kilograms divided by height in meters squared. Waist and hip circumference were measured to the nearest 0.5cm, taken in duplicate and the mean values were used in the analyses. Seated blood pressure was obtained after five minutes of quiet rest, on the right arm using an automatic sphygmomanometer. Blood samples were collected after a 10 hour fast. Lipid profile, glucose, and insulin assays were conducted with standard procedures at the Fairview Diagnostic Laboratories, Fairview-University Medical Center (Minneapolis, MN), a Centers for Disease Control and Prevention–certified laboratory. Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as previously described (33).
Measurement of Oxidative Stress and Inflammation Blood Markers
Blood plasma for oxLDL (Mercodia, Inc., Winston-Salem, NC, USA), CRP (Immundiagnostik AG), and IL-6 (Quantikine HS, R& D Systems, Inc., Minneapolis, MN, USA) was stored frozen at −70 C until assayed at the University of Minnesota Cytokine Reference Laboratory (CLIA licensed) using ELISA. Oxidized LDL, measured in this fashion, is a reflection of both minimally and fully oxidized LDL particles. The intra- and inter-assay coefficients of variation were as follows: oxLDL 5.5–7.3 and 4.0–6.2, respectively; CRP 5.5–6 and 11.6–13.8, respectively; IL-6 6.9–7.8 and 6.5–9.6, respectively.
Statistical Analysis
Descriptive statistics, including means and standard deviations, were performed for all variables across the three BMI groups. ANCOVA was performed to assess differences in cardiovascular risk factors, oxLDL, CRP, and IL-6 across BMI groups. Tukey post-tests were conducted to determine which groups differed for oxLDL, CRP, and IL-6. We performed a secondary analysis of oxLDL, CRP, and IL-6 (using ANCOVA with Tukey post-tests) using four BMI groups by further separating the OW/OB group into two groups (OW = 85th -<95th percentile, n=43; OB = 95th -<1.2 times the 95th percentile, n=21). Linear regression was used to evaluate the relationship between oxLDL and fasting insulin and HOMA-IR. All analyses were adjusted for age, gender, and race. OxLDL analyses were performed with and without adjustment for LDL-cholesterol, which is the substrate for oxLDL. Statistical significance was considered p<0.05. Data are presented as mean ± standard deviation.
Results
Clinical characteristics and oxidative stress and inflammation markers, by BMI group, are shown in Table 1. There were no significant differences in age, gender, race, and level of fasting glucose between the groups; however, ethnicity differed among the groups. OxLDL was significantly correlated with fasting insulin (r = 0.23, p<0.001) and HOMA-IR (r = 0.21, p<0.01); however, these relationships disappeared after adjustment for BMI. By design, BMI and waist circumference significantly increased across BMI groups. Across BMI groups, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, low density lipoprotein-cholesterol (LDL-C), triglycerides, fasting insulin and HOMA-IR were higher and high density lipoprotein-cholesterol (HDL-C) was lower. OxLDL, CRP, and IL-6, by BMI group, are shown in Figure 1. OxLDL was significantly higher in the EO group compared to the NW and OW/OB groups, even after adjustment for LDL-cholesterol. The OW/OB group had significantly higher oxLDL compared to the NW group. With additional adjustment for LDL-cholesterol, this difference was no longer significant (p=0.148). CRP was significantly higher in the EO group compared to the NW and OW/OB groups. CRP was also significantly higher in the OW/OB group compared to the NW group. IL-6 was significantly higher in the EO group compared to the NW and OW/OB groups. No significant difference was observed between the NW and OW/OB groups for IL-6.
Table 1.
Cardiovascular Risk Factors, Oxidative Stress, and Inflammation by BMI Group
| BMI percentile | |||||
|---|---|---|---|---|---|
| Variables | NW (n=127) |
OW/OB (n=64) |
EO (n=34) |
P-value | |
| Age (yrs) | 13.6 ± 2.5 | 13.7 ± 2.4 | 12.8 ± 2.8 | 0.327 | |
| Gender | 50% Male | 63% Male | 59% Male | 0.236 | |
| Ethnicity | 0.004 | ||||
| Hispanic | 2 (2%) | 3 (5%) | 5 (15%) | ||
| Non-Hispanic | 125 (98%) | 61 (95%) | 29 (85%) | ||
| Race | 0.712 | ||||
| White | 120 (95%) | 61 (95%) | 26 (76%) | ||
| Black | 0 (0%) | 2 (3%) | 1 (3%) | ||
| American Indian | 1 (1%) | 00 | 2 (6%) | ||
| Asian/Pacific Islander | 3 (2%) | 1 (2%) | 1 (3%) | ||
| Other/more than one race | 3 (2%) | 61 (95%) | 4 (12%) | ||
| BMI (kg/m2) | 19.2 ± 2.5 | 25.1 ± 2.3 | 37.7 ± 6.1 | <0.0001 | |
| BMI Z-Score | -0.05 ± 0.7 | 1.5 ± 0.3 | 2.6 ± 0.3 | <0.0001 | |
| Waist Circumference (cm) | 65.1 ± 8.0 | 78.8 ± 8.4 | 115.8 ± 18.5 | <0.0001 | |
| SBP (mmHg) | 107.8 ± 9.2 | 114.0 ± 9.2 | 121.3 ± 12.3 | <0.0001 | |
| DBP (mmHg) | 57.1 ± 7.5 | 58.1 ± 6.7 | 66.5 ± 10.3 | <0.0001 | |
| Total Cholesterol (mg/dL) | 142.0 ± 23.2 | 148.7 ± 26.8 | 163.2 ± 21.5 | <0.0001 | |
| HDL-Cholesterol (mg/dL) | 49.5 ± 10.7 | 42.7 ± 7.7 | 39.8 ± 10.8 | <0.0001 | |
| LDL-Cholesterol (mg/dL) | 78.9 ± 20.3 | 87.2 ± 23.8 | 99.0 ± 16.4 | <0.0001 | |
| Triglycerides (mg/dL) | 72.0 ± 40.0 | 94.1 ± 44.0 | 121.9 ± 58.0 | <0.0001 | |
| Glucose (mg/dL) | 85.1 ± 7.9 | 88.7 ± 7.8 | 92.5± 8.2 | 0.770 | |
| Insulin (mU/L) | 8.1 ± 4.7 | 12.0 ± 7.1 | 20.9 ± 14.0 | <0.0001 | |
| HOMA-IR | 1.7 ± 1.1 | 2.6 ± 1.6 | 4.4 ± 3.1 | <0.0001 | |
| Oxidized LDL (U/L) | |||||
| Unadjusted | 40.8 ± 9.0 | 45.7 ± 12.1 | 63.1 ± 13.8 | <0.0001 | |
| Adjusted | 42.4 ± 0.8 | 44.7 ± 1.1 | 59.3 ± 1.6 | <0.0001 | |
| CRP (mg/dL) | 0.5 ± 1.0 | 1.4 ± 2.9 | 5.6 ± 4.9 | <0.0001 | |
| IL-6 (pg/mL) | 1.1 ± 1.0 | 1.3 ± 1.2 | 2.0 ± 0.9 | <0.0001 | |
Data are presented as mean ± SD (adjusted oxLDL values are LS mean ± standard error)
Figure 1.
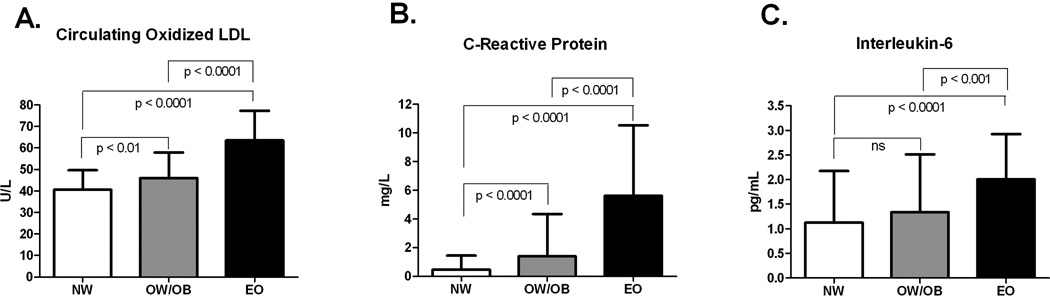
Circulating oxidized LDL (panel A), C-Reactive Protein (panel B) and Interleukin-6 (panel C) in NW, OW/OB, and EO children and adolescents
In the secondary analysis (four-group classification), oxLDL was significantly different across groups (p-trend <0.0001); oxLDL was significantly higher in the EO group (63.1 ± 13.8 U/L) compared to the NW (40.5 ± 9.0 U/L), OW (43.3 ± 9.8 U/L), and OB (51.2 ± 14.3 U/L) groups, even after adjustment for LDL-cholesterol (all p<0.0001, see Figure 2). OxLDL was significantly higher in the OB group compared to the NW and OW groups (p<0.001, p<0.05, respectively), but these differences were no longer significant after adjustment for LDL-cholesterol. CRP was significantly different across groups (p-trend <0.0001); CRP was significantly higher in the EO group (5.6 ± 4.9 mg/L) compared to the NW (0.5 ± 1.0 mg/L, p<0.0001), OW (0.93 ± 2.4 mg/L, p<0.0001) and OB (2.2 ± 3.7, p<0.001) groups. CRP was significantly higher in the OB group compared to the NW and OW groups (both p<0.001). CRP was also significantly higher in the OW group compared to the NW group (p<0.05). IL-6 was significantly different across groups (p-trend <0.001); IL-6 was significantly higher in the EO group (2.0 ± 0.9 pg/mL) compared to the NW (1.1 ± 1.0 pg/mL, P<0.0001), OW (1.4 ± 1.3 pg/mL, P<0.001), and OB (1.3 ± 1.0 pg/mL, p<0.05) groups. No significant difference was observed between the NW, OW and OB groups for IL-6.
Figure 2.
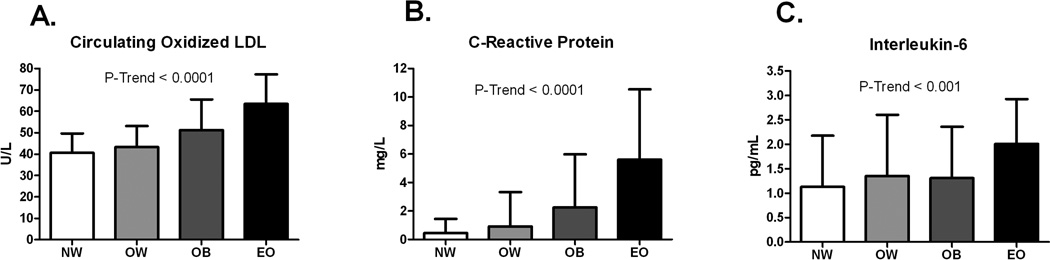
Circulating oxidized LDL (panel A), C-Reactive Protein (panel B) and Interleukin-6 (panel C) in NW, OW, OB, and EO children and adolescents
In other studies, extreme pediatric obesity has been defined as a BMI ≥99th percentile. We performed a separate analysis using this definition for EO and found almost identical results to those we report here.
Discussion
The primary finding of this study is, as expected, that EO children and adolescents, compared to their NW, OW, and OB peers, have elevated levels of oxidative stress and inflammation. In general, oxLDL, CRP, and IL-6 demonstrated linear associations with increasing adiposity across categories of overweight and obese BMI values. We observed markedly elevated CRP in the EO group (mean = 5.6 mg/L) - higher than levels reported in other studies of metabolic syndrome (34) and severe obesity (35) in children and adolescents, but not as high as levels reported in a sub-analysis of morbidly obese youth with a mean BMI = 44 kg/m2 (36). Our results are in line with the findings of Weiss et al. (35) and Kapiotis et al. (36) who reported increasing levels of CRP and IL-6 across categories of obesity in children and adolescents. We extend these findings by demonstrating that extremely obese youth have higher levels of oxidative stress than those with milder forms of adiposity and normal weight.
To our knowledge, ours is the first study to assess oxLDL in extreme pediatric obesity. Oxidative modification of LDL cholesterol is thought to be fundamentally involved in the development of atherosclerosis since oxLDL expedites the maturation of macrophages and eventual conversion to foam cells in the arterial wall (37). Elevations of oxLDL may precede the development of insulin resistance in the context of obesity, suggesting that it may be associated with the pathogenesis of type 2 diabetes mellitus (38). We have previously shown that oxLDL is associated with multiple measures of adiposity (waist circumference, percent body fat, BMI, abdominal visceral fat and abdominal subcutaneous fat). Moreover, oxLDL was associated with insulin resistance measured with the insulin clamp technique, after adjustment for body fat percentage, in children and adolescents (28). The current results extend our previous findings of an association of oxLDL with obesity by providing evidence of a linear relationship between adiposity and oxLDL across a wide spectrum of adiposity, including extreme obesity. In the current study, the relationship between oxLDL and fasting insulin and HOMA-IR was lost when additional adjustments were made for BMI. However, it should be noted that fasting insulin and HOMA-IR are only surrogate measures of insulin resistance. Therefore, results from this study are not directly comparable to those of our previous study, which utilized the insulin clamp technique to quantify insulin resistance.
The findings from the current study, along with others (5;35;36;39), suggest that extreme pediatric obesity is a condition associated with high risk of developing cardiovascular disease and type 2 diabetes mellitus at a young age, even compared to less extreme forms of adiposity such as overweight and obesity. This is especially worrisome since trends suggest that extreme obesity is the fastest growing category of pediatric obesity (3) and afflicts between 3–6% of the pediatric population (1–3). An important consideration is that obesity in childhood tracks strongly into adulthood – approximately 88% of extremely obese youth will have an adult BMI ≥35 kg/m2 (5). Lifestyle modification is the foundational approach to treating pediatric obesity. However, many youth, particularly the extremely obese (40), are unable to reduce their weight to an acceptable level with lifestyle modification alone. Therefore, from a clinical perspective, many of these individuals may benefit from aggressive management of body weight and adverse risk factors potentially beyond lifestyle modification. Studies evaluating medical and surgical therapies for the treatment of extreme pediatric obesity will provide additional insight regarding which strategies are most effective in reducing the risk of developing early cardiovascular disease and type 2 diabetes.
Our study has some limitations including a somewhat heterogeneous population. The EO group was primarily recruited from a pediatric weight management clinic and the other groups from a sample of healthy siblings who participated in a study of cardiovascular risk in pediatric survivors of childhood cancer. Therefore, the results may not be generalizable to the broader pediatric population. We were only able to conduct basic bias control by adjusting for age, gender and race. Tanner staging was not performed on all participants; therefore, we were not able to adjust our analyses for pubertal status. Physical activity and dietary patterns most likely varied among the adiposity groups; however, these data were not collected.
In conclusion, results from the current study provide evidence that extreme pediatric obesity, compared to normal weight, overweight, and obesity, is associated with high levels of oxidative stress and inflammation and an adverse cardiovascular and metabolic risk factor profile. A growing body of literature suggests that extremely obese children and adolescents may require aggressive weight and risk factor management to reduce their risk for cardiovascular disease and type 2 diabetes.
Acknowledgements
Funding for this study was provided by the University of Minnesota Vikings Children’s Fund (A.S.K.), National Institutes of Health: NCI/NIDDK: 1RO1CA113930-01A1 (J.S.), and GCRC: M01-RR00400, General Clinical Research Center Program, NCRR/NIH. Statistical analysis was provided by Jae Choi (Macalester College, St. Paul, MN) and Philippe Gaillard, Ph.D. (University of Minnesota Clinical and Translational Science Institute, Biostatistical Design and Analysis Center).
Footnotes
Disclosures: The authors have no relevant conflicts of interest.
Reference List
- 1.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009 Nov;90(5):1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 2.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of Extreme Obesity in a Multiethnic Cohort of Children and Adolescents. J Pediatr. 2010 Mar 18; doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and Trends of Severe Obesity Among US Children and Adolescents. Acad Pediatr. 2009 Jun 26; doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen KA, Weedn AE, Crawford PB. Disparities in peaks, plateaus, and declines in prevalence of high BMI among adolescents. Pediatrics. 2010 Sep;126(3):434–442. doi: 10.1542/peds.2009-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007 Jan;150(1):12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996 Feb;45(2):235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 7.Steinberger J, Moran A, Hong CP, Jacobs DR, Jr, Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr. 2001 Apr;138(4):469–473. doi: 10.1067/mpd.2001.112658. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997 Sep 25;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 9.Holvoet P, Vanhaecke J, Janssens S, Van de WF, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998 Oct 13;98(15):1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 10.Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001 May;21(5):844–848. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 11.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007 Sep;194(1):245–252. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004 Mar 23;109(11):1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 13.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004 Dec 16;351(25):2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 15.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010 Apr;33(4):804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogeveen RC, Ballantyne CM, Bang H, Heiss G, Duncan BB, Folsom AR, et al. Circulating oxidised low-density lipoprotein and intercellular adhesion molecule-1 and risk of type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Diabetologia. 2007 Jan;50(1):36–42. doi: 10.1007/s00125-006-0533-8. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004 Mar;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 19.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003 Mar;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 20.Thorand B, Lowel H, Schneider A, Kolb H, Meisinger C, Frohlich M, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003 Jan 13;163(1):93–99. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005 Dec;90(12):6454–6459. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 22.Weinbrenner T, Schroder H, Escurriol V, Fito M, Elosua R, Vila J, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006 Jan;83(1):30–35. doi: 10.1093/ajcn/83.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Ho RC, Davy K, Davy B, Melby CL. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism. 2002 Nov;51(11):1478–1483. doi: 10.1053/meta.2002.35577. [DOI] [PubMed] [Google Scholar]
- 24.Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004 Apr;53(4):1068–1073. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]
- 25.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008 May 21;299(19):2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapointe A, Couillard C, Piche ME, Weisnagel SJ, Bergeron J, Nadeau A, et al. Circulating oxidized LDL is associated with parameters of the metabolic syndrome in postmenopausal women. Atherosclerosis. 2007 Apr;191(2):362–368. doi: 10.1016/j.atherosclerosis.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 2006 Dec;14(12):2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 28.Kelly AS, Jacobs DR, Jr, Sinaiko AR, Moran A, Steffen LM, Steinberger J. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr Diabetes. 2010 Jan 19; doi: 10.1111/j.1399-5448.2009.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demirel F, Bideci A, Cinaz P, Camurdan MO, Biberoglu G, Yesilkaya E, et al. Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007 Jul;67(1):129–134. doi: 10.1111/j.1365-2265.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 30.Jarvisalo MJ, Lehtimaki T, Raitakari OT. Determinants of arterial nitrate-mediated dilatation in children: role of oxidized low-density lipoprotein, endothelial function, and carotid intima-media thickness. Circulation. 2004 Jun 15;109(23):2885–2889. doi: 10.1161/01.CIR.0000129304.98566.D8. [DOI] [PubMed] [Google Scholar]
- 31.Kelishadi R, Cook SR, Amra B, Adibi A. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis. 2009 Jun;204(2):538–543. doi: 10.1016/j.atherosclerosis.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in First Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes. 2008 Oct 15; doi: 10.1111/j.1399-5448.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005 Apr;28(4):878–8781. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004 Jun 3;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 36.Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006 Nov;26(11):2541–2546. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 37.Yui S, Sasaki T, Miyazaki A, Horiuchi S, Yamazaki M. Induction of murine macrophage growth by modified LDLs. Arterioscler Thromb. 1993 Mar;13(3):331–337. doi: 10.1161/01.atv.13.3.331. [DOI] [PubMed] [Google Scholar]
- 38.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009 Jul;32(7):1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia, and respiratory compromise. J Pediatr. 2004 Jun;144(6):766–769. doi: 10.1016/j.jpeds.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009 Oct;124(4):1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]


