Abstract
Background & Aims
Retinoic acid (RA), produced by intestinal epithelial cells (IECs) and dendritic cells (DCs) and regulated by transforming growth factor (TGF)-β, controls the enteric immune response by activating regulatory T (Treg) cells and preventing activation of T-helper (Th)17 cells
Methods
We studied the roles of RA in mice that overproduce tumor necrosis factor (TNF) and develop chronic ileitis (TNFΔARE mice). We assessed the frequency and function of CD103+ DCs and Th17 and Treg cells by flow cytometry; we measured expression of cytokines and retinaldehyde dehydrogenase (RALDH) enzymes in ileum samples, DCs, and IECs by real-time PCR. We quantified RA by electrochemical analysis and examined the effect of RA supplementation on TNF-induced ileitis using histologic, co-culture, and suppression assays and flow cytometry
Results
Numbers of CD103+ DCs decreased in the inflamed ilea of mice with chronic disease; RA synthetic machinery (RALDH1,2) was downregulated. Nevertheless, the proportion of CD4+, CD25+, FoxP3+ Treg cells increased, indicating an alternate source for RA. IECs responded to reduced levels of RA by upregulating RALDH3 in vivo and in vitro. Net tissue levels of RA levels remained lower in TNFΔARE than wild-type mice, indicating that epithelial up-regulation of RALDH3 could not maintain adequate concentrations of RA, probably because of loss of IEC mass. RA supplementation significantly attenuated disease by increasing the number and function of CD103+ DCs and Treg cells and reducing Th17 cells
Conclusions
Reduced levels of RA appear to induce IEC to upregulate synthesis of RA. RA supplementation attenuates ileitis through its effects on CD103+ DCs and Treg and Th17 cells. RA supplementation might used to treat patients with Crohn's disease
Keywords: inflammation, immune response, inflammatory bowel disease, tolerance
Introduction
Induction of regulatory T cell (Tregs) by dendritic cells (DC) limits inappropriate inflammatory responses. Failure of this mechanism has been implicated in the development of Crohn's disease (CD), which involves the ileum in 60% of patients. Anti-TNF strategies are initially effective in up to 70% of patients1, yet sustained remission drops at one year2 and the manipulation of other cytokines has been less successful, leaving an unmet need for novel therapeutics3.
A subset of CD4+ T cells is defined by expression of forkhead box P3 (FoxP3)4, 5. The frequency of these Tregs is increased in IBD patients compared with healthy individuals6. Expansion of Tregs has also been reported in murine models of IBD7. As a single DC can imprint a regulatory phenotype to many T cells, enhanced understanding of Treg induction by DC may yield better therapeutics to modulate T cell responses8, 9. A subset of intestinal DC release high levels of retinoic acid (RA) and, in the presence of TGF-β10, 11, promote Treg induction12. This DC subset is defined by expression of integrin CD103 (αE) and displays higher expression of aldh1a2 mRNA, which encodes for retinaldehyde dehydrogenase-2 (RALDH2), an enzyme critical for the conversion of retinal to RA10. CD103+ DC attenuate CD45RBhigh –induced colitis13. Due to the capacity of CD103+ DC to induce a small-intestinal homing (i.e. α4β7+, CCR9+) regulatory phenotype (FoxP3+) on naïve CD4+T cells via RA production, enhancing these pro-regulatory pathways may represent a strategy for the treatment of CD.
However, DC are not the sole source of RA in the gut, and recent work demonstrated that epithelial-derived RA induced CD103 expression on monocyte-derived DC resulting in RA-dependent induction of Tregs by the newly matured CD103+ DC14 Furthermore, in the presence of IL-6, TGFβ can also drive induction of IL-17-producing (Th17) CD4+ T cells15. Thus, TGFβ represents the fulcrum on which the balance between Tregs and effector Th17 cells pivots. IL-6 tips the balance in favor of a Th17 phenotype, blocking Treg induction and promoting colitis16. In contrast, RA drives Treg development and inhibits induction of Th17 cells by IL-610, 17, 18. Colitogenic Th17 cells mediate development of colitis in mice19, 20. Paradoxically, patients with IBD also exhibit increased Th17 cells21 which may reflect increased TGF-β availability.
Deletion of 69 bp within the AU-rich element of the TNF gene in mice (i.e. TNFΔARE) stabilizes TNF mRNA, resulting in TNF overproduction and development of chronic ileitis, reminiscent of human CD in its histological features22, 23. Here we examine the role of epithelial and DC-derived RA in perpetuation of ileitis in the TNFΔARE model, one of only two models that recapitulate the histopathological features of CD. We hypothesized that disease induction and maintenance may be due to dysfunction of RA production. We first assessed the impact of ileitis on CD103+ DC frequency, as well as their associated regulatory FoxP3+ T cells. Secondly, we examined the effect of chronic inflammation on DC function and its implications for net RA synthesis during chronic immune dysregulation. We confirmed that RA supplementation has a therapeutic effect in TNF-mediated Crohn's-like ileitis and examined the mechanisms underlying RA-mediated attenuation of disease. Finally, we evaluated the interplay between epithelial- and DC-derived RA synthesis during chronic inflammation and the net effect on RA availability within the ileum.
Methods
Mice
The B6.129S-Tnftm2Gkl/Jarn strain was previously described24 and kept under specific-pathogen-free conditions. Experimental animals were heterozygous for the ΔARE mutation or homozygous wild-type (WT), which served as controls. CD103−/− mice on the C57BL6/J background were obtained from Jackson Laboratories (Bar Harbor, ME). Fecal samples were negative for Helicobacter, protozoa and helminthes. All procedures were approved by the committee for animal use.
Lymphocyte and epithelial cell isolation
Splenocytes, MLN, and LP mononuclear cells were isolated as previously described25. Intestinal epithelia were dissociated using EDTA and the epithelial cell-enriched fractions frozen at −20°C in Tri-Reagent (Sigma Aldrich, St Louis MO).
Flow cytometry
Cells from indicated compartments were incubated with fluorescently-labeled rat anti-mouse antibodies against: mouse CD11c (N418), MHCII (M5/114.15.2), F4/80 (BM8), integrin αE (CD103, 2E7), CD3 (17A2), CD4 (RM4-5), CD44 (IM7), CD62L (MEL-14), CD25 (PC61.5), IL-17A (17B7) and FoxP3 (FJK16S) or their respective isotype controls for T cell subset evaluation. Additional controls included cells isolated from CD103-deficient mice. FoxP3 staining was performed using the PE-FoxP3 labeling kit (eBioscience, San Diego, CA). Cells were washed and fixed with 2% paraformaldehyde and analyzed using the FACS® Canto system (Beckton-Dickinson Immunocytometry Systems, San José, CA). Post-analyses were performed using FLOWJo software (Tree Star Inc, Ashland, OR).
For detection of IL-17A-producing cells, unfractionated cells were incubated with 20ng/ml PMA, 1μg/ml ionomycin and 15μM monensin for 5 h (1×106 cells/well). Cells were stained with antibodies against CD3, CD4 and AmCyan Live/Dead dye (Invitrogen; Carlsbad, CA) prior to permeabilization (FoxP3 staining kit; eBioscience) and IL-17A staining.
T cell proliferation assay
CD4+/CD25+ Tregs were isolated by negative selection of CD4+ T cells, followed by positive selection of CD25+ cells using the MACS Treg isolation kit (Miltenyi Biotec, Auburn CA). Fifty-thousand (CFSE)-labeled CD4+/CD25neg effector T cells/well were stimulated with anti-CD3 mAb in the presence of irradiated syngeneic APC and varying ratios of purified CD4+/CD25+Treg from vehicle or RA-treated 20-week-old TNFΔARE mice; suppression of proliferation was determined by the CFSE profile of dividing effector cells at 72 h.
DC -T cell co-culture studies
CD11c+ cells isolated using CD11c+ MACS beads (Miltenyi Biotec) from 20-week-old vehicle- or RA-treated TNFΔARE mice were co-cultured with MACS-isolated CD4+/CD25Neg T cells from WT mice (1:10 DC: T cell ratio) in the presence of 2ng/ml TGFβ for 3 days prior to analysis of FoxP3 induction by flow cytometry. In concurrent studies, Tcells were re-stimulated on day 3 with PMA/ionomycin/monensin for 4 h followed by intracellular staining for IL-17A as previously described.
In vivo RA treatment studies
20-week-old TNFΔARE mice received twice weekly intraperitoneal injection of RA dissolved in 1:1 DMSO: Soybean Oil, stored at −20°C and diluted 1:4000 in sterile PBS prior to injection (300 μg/injection) or vehicle for two weeks. Mice were sacrificed on day 15, ilea were harvested for histologic assessment and leukocytes isolated from the spleen, MLN and LP for culture, intracellular staining and flow cytometric analysis.
Cytokine production assays
Unfractionated cells from the LP of 20-week-old TNFΔARE mice treated with either RA or vehicle as described, were stimulated for 24 h with CD3/CD28-coated dynabeads. Culture supernatants were assayed for cytokines using the Quansys Cytokine Assay system (Logan, UT).
RNA isolation, cDNA synthesis and real-time PCR
Ileal tissues placed in RNAlater (Qiagen, Valencia, CA) and stored at RT prior to homogenization and RNA isolation using the RNeasy isolation kit (Qiagen). Freshly isolated DC from WT and TNFΔARE mice were enriched by positive selection (CD11c+ N418, Miltenyi Biotec) and FACS sorted based on CD11chi/MHCII+ and CD103 expression. Primary IEC RNA was obtained using Tri-Reagent (Sigma) per manufacturers directions. MODE-K intestinal epithelial cells were harvested in RLT buffer (Qiagen) and RNA extracted using RNeasy columns (Qiagen). Synthesis of cDNA was performed using Superscript III cDNA synthesis kit (Invitrogen). Quantitative determination of mRNA expression was performed using PowerSybr Green and the AB7300 real-time PCR system (Applied Biosystems, Foster City, CA) using GAPDH as an endogenous control. Expression of RALDH isoforms were measured using QuantiTect primer assays QT01195908-RALDH1, QT00120477-RALDH2 and QT01077867-RALDH3 (Qiagen).
Epithelial cell culture
The murine small intestinal epithelial cell line MODE-K was cultured in DMEM (without sodium pyruvate, Cellgro Manassas, VA, supplemented with 10% FBS, 2mM glutamine, 100IU penicillin and 100μg/ml streptomycin; Invitrogen), as described26. Cells were seeded in plates (Invitrogen) at 0.5×106 cells per well and cultured for 24 h in complete media, followed by 24 h culture under serum-free conditions prior to 48h treatment with vehicle (0.01% ethanol) 1μM RA, 1μM AM580, 10nM TNF (Sigma Aldrich) or 1μM LE540 (Enzo Life Sciences, Farmingdale, NY). An additional group was vehicle-treated for 24 h, washed in PBS and treated with 1μM LE540 for an additional 24 h (NT/LE). All cultures were harvested 72 h later and frozen for RNA extraction.
Retinoic acid detection by electrochemical (EC)-array
Ileal tissues were snap frozen, crushed using liquid nitrogen prior to extraction in 0.25M NH4OAc (pH 4). A liquid chromatographic method optimized for lipid-soluble redoxactive metabolites, was used in conjunction with EC-array detection (technical note ESA, Inc., 10-1176). Briefly, mobile phase A consisted of methanol and 0.2 M ammonium acetate, pH 4.4 at a ratio of 90:10, v/v, respectively. Mobile phase B consisted of methanol, 1-propanol, and 1.0 M ammonium acetate, pH 4.4, at a ratio of 78:20:2, v/v/v, respectively. The gradient timeline was a linear gradient from 0%-80% B in 10 min, a linear gradient from 80%-100% B in 10 min, and a 7 min hold at 100% B before returning to initial conditions. A C18 PolarAdvantage II column (150 mm × 4.6 mm; 5 μm; Dionex, Sunnyvale, CA) was used at flow rate of 0.8-mL/min at 37°C. Electrochemical array potentials: 200, 400, 500, 700, 800, −1000, −1000, 500 (8 channels).
Scanning electron microscopy morphologic analysis
Ilea were harvested and processed for electron microscopic analysis according to established protocols27. Briefly samples were fixed, dehydrated in ethanol, critical point dried, coated with a thin layer of AuPd, and imaged with a JEOL JSM-6400 scanning electron microscope at an accelerating voltage of 15 kV.
Statistics
Statistical analyses were performed using the Student t test with Graphpad Prism Data Analysis software (GraphPad Software, La Jolla, CA). Data were expressed as mean ± standard error of the mean (SEM). Statistical significance was set at a P < than 0.05.
Results
CD103+ DC deficiency in the MLN and ileal lamina propria during chronic disease
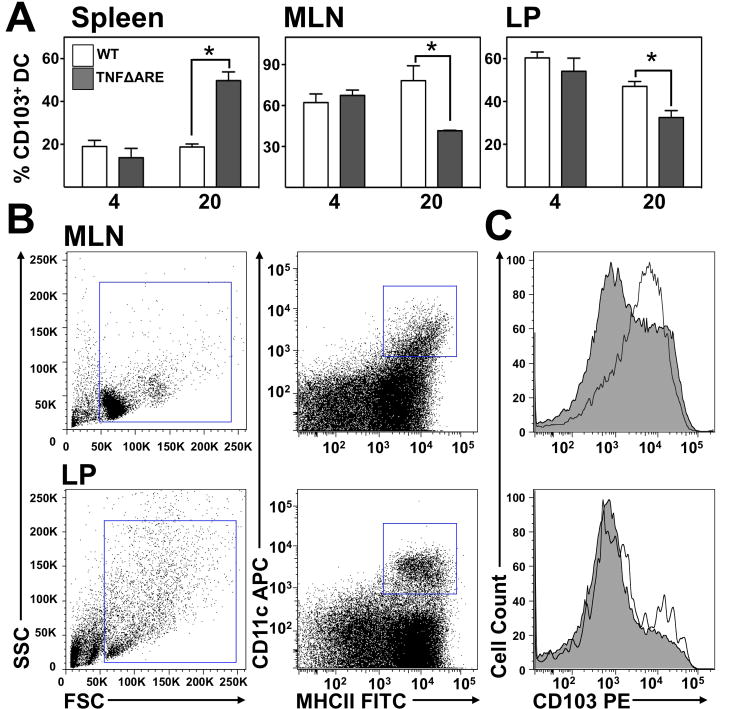
We analyzed the frequency of CD103+ DC in TNFΔARE mice compared with WT littermates. CD103+ DC frequency was unchanged in the spleen, MLN and LP at 4-weeks-of-age of TNFΔARE mice (Figure 1A). By contrast, in 20-week-old mice the proportion of CD103+ DC (gated as shown in Figure 1B & C) increased in the spleen of TNFΔARE mice (19±2% to 50±4%; P<0.01). However, there was a significant decrease in CD103+ DC in the MLN (79±11% to 42±0.4%; P<0.05) and LP of TNFΔARE mice (47±2% to 33±26%; P<0.05) compared with WT littermates. Thus, chronic ileal inflammation reduced the CD103+ DC frequency in ileum and draining lymph node.
Figure 1. Proportion of CD103+DC decreased during late disease in TNFΔARE LP.
(A) Flow cytometric analysis of CD103+ DC in the spleen, MLN and LP of 4- and 20-week-old TNFΔARE mice compared with WT littermates. Mean±SEM of n≥3 individual mice for each genotype and time-point; *P<0.05. (B) Representative plots showing the gating strategy for identifying CD103+ DC from the MLN and LP of 20-week-old WT mice, with overlaid histograms for CD103 on DC from a CD103-deficient mouse of matched age. (C) Representative histograms of CD103 expression on DC from MLN and LP of 20-week-old WT and TNFΔARE mice.
Downregulation of RALDH2 in DC of TNFΔARE mice
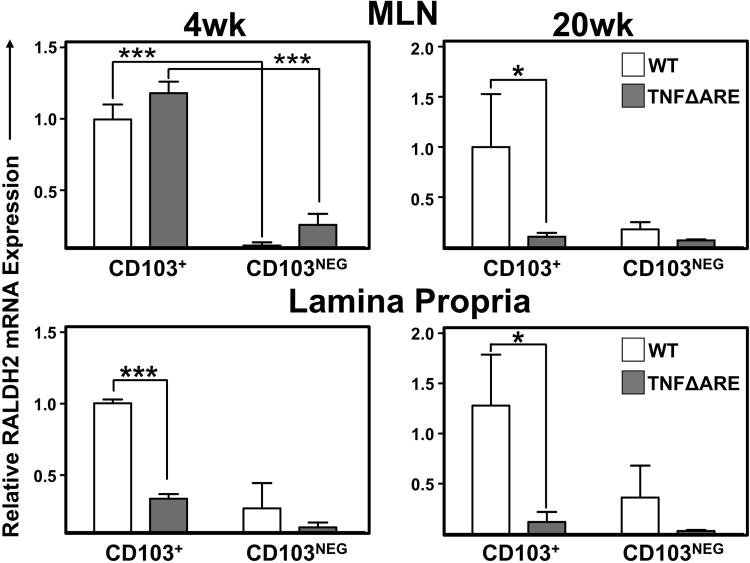
To examine whether chronic inflammation might also affect the RA synthetic machinery of CD103+ DC, we compared the expression of RALDH2 (Aldh1a2) in CD103+ and CD103neg DC subsets isolated from 4- and 20-week-old WT and TNFΔARE mice (Figure 2). In DC isolated from MLN of 4-week-old mice, there was significantly higher expression of RALDH2 mRNA in CD103+ DC compared with CD103neg DC of WT (1±0.1 vs. 0.1±0.02; P<0.001) and TNFΔARE mice (1±0.1 vs. 0.3±0.1; P<0.001). By contrast, in 20-week-old mice, RALDH2 mRNA expression was significantly decreased in CD103+ DC from TNFΔARE mice compared with WT mice (1±0.5 vs. 0.1 ±0.04; P<0.05). Furthermore, LP-derived CD103+ DC from TNFΔARE mice expressed significantly less RALDH2 mRNA compared with WT littermates at both 4- (1±0.03 vs. 0.3±0.03; P<0.001) and 20-weeks-of-age (1±0.5 vs. 0.1 ±0.1; P<0.05). Similarly, analysis of RALDH1 in DC from 20-week-old mice also demonstrated a significant decrease in RALDH1 mRNA expression in both CD103+ (1±0.1 vs 0.3±0.1; P<0.01) and CD103neg DC (0.4±0.1 vs 0.04±0.01; P<0.01; Supplemental figure 1). Taken together, these results confirm that CD103+ DC are the main producers of RA and indicate that inflammatory signals decrease both the number of tolerogenic DC and their pro-regulatory RA synthesis within the chronically-inflamed ileum.
Figure 2. Decreased RALDH2 expression in CD103+and CD103NegDC from 4 and 20-week-old TNFΔARE mice compared to WT littermates.
RALDH2 mRNA expression in isolated CD103+ and CD103Neg CD11chi/MHCII+ DC from MLN and LP of 4- and 20-week-old WT and TNFΔARE mice. Mean±SEM from 3 separate experiments requiring pooled organs from 4-11 mice/strain/experiment. *P<0.05, ***P<0.001.
Increased IL-17A, TGFβ, IL-6 and IL-23 mRNA in LP of 20-week-old TNFΔARE mice
Expression of TGFβ was significantly higher in ileal tissue of 20-week-old TNFΔARE compared with WT littermates (0.5±0.04 vs 2.5±0.2; P<0.01; Supplemental figure 2). Similarly expression of IL-6 was higher in TNFΔARE mice at both 4-(0.6±0.1 vs. 4.6±1.3; P<0.01) and 20-weeks-of-age (0.8±0.2 vs. 36±6; P<0.001). Real-time RT-PCR analysis of IL-17 expression demonstrated higher expression of IL-17 in 20-week-old TNFΔARE compared with littermate controls (2±0.3 to 17.7±2; P<0.001). Finally examination of IL-23 mRNA demonstrated increased expression in TNFΔARE tissue compared with WT at 4-(0.7±0.1 to 1.4±0.2; P<0.01) and 20-weeks-of-age (1±0.3 to 1.8±0.1; P<0.05). The absolute counts of Th17 cells in the MLN and LP of TNFΔARE mice were increased (Supplemental figure 3). Thus there is an increase in Th17 cells in TNFΔARE mice, likely mediated by increased levels of cytokines responsible for induction (i.e. TGFβ, IL-6) and maintenance (i.e. IL-23) of Th17 cells.
CD4+/CD25+/FoxP3+ T cells increased in TNFΔARE mice at 20-weeks-of-age
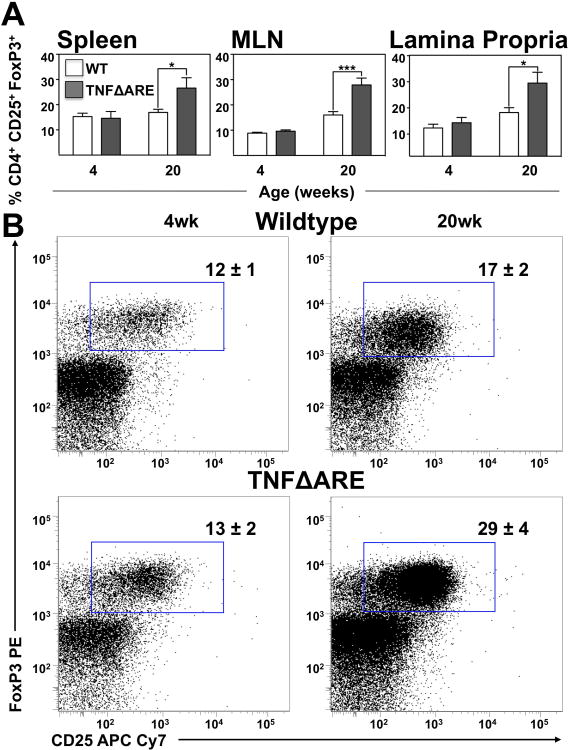
We then examined CD4+/CD25+/FoxP3+ Treg frequency during early and late disease. While there was no change in 4-week-old mice, at 20-weeks-of-age there was an unexpected increase in Tregs in the spleen (17±1% to 27±4%; P<0.05; Figure 3A), MLN (16±1% to 28±2%; P<0.001) and LP of 20-week-old TNFΔARE mice (17±2% to 29±4%; P<0.05; gated as shown Figure 3B). Thus a counterintuitive Tregs expansion in inflamed ileum, suggested that there could be an alternate source of RA.
Figure 3. Increased percentage of Tregs in the spleen, MLN and LP of 20-week-old TNFΔARE mice compared to WT littermates.
(A) The percentage of CD4+/CD25+/FoxP3+ cells in the spleen, MLN and ileal LP of TNFΔARE mice compared to WT mice at 20-weeks-of-age. Mean±SEM for n≥5 individual mice/strain for each time-point; *P<0.05. (B) Representative dot plots showing expression of CD4+/CD25+/FoxP3+ T lymphocytes from the LP of 20-week-old TNFΔARE mice and WT littermates.
Epithelial RALDH machinery is up-regulated in 20-week-old TNFΔARE mice
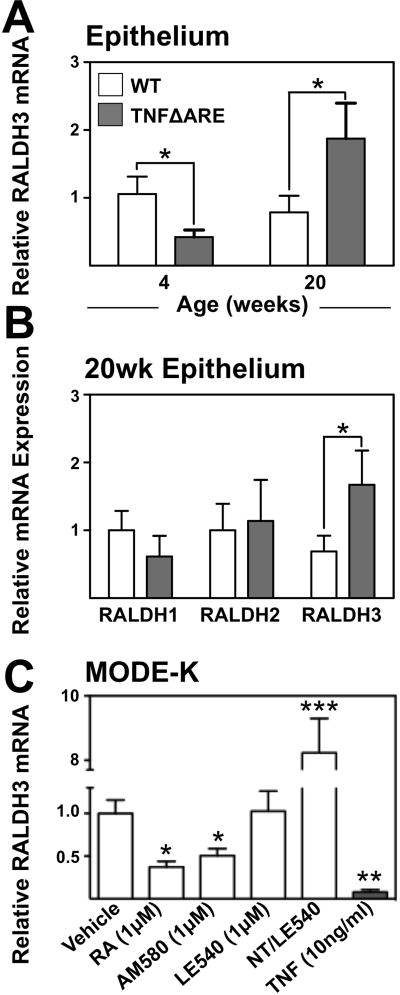
Given that expansion of Tregs was inconsistent with downregulation of DC RA machinery, we examined whether epithelial-derived RA could compensate for the decrease in DC-derived RA production. Real-time PCR analysis demonstrated decreased epithelial-associated RALDH3 (Aldh1a3) mRNA in TNFΔARE compared with WT mice at 4-weeks-of-age (1±0.2 to 0.4±0.1). This was reversed in 20-week-old mice (1±0.3 to 3±1; Figure 4A). Analysis of RALDH expression from IEC demonstrated that RALDH3 mRNA was significantly up-regulated (1±1 to 2±0.5; Figure 4B) while others were unchanged.
Figure 4. Dynamic changes in RALDH expression in IEC likely mediated by TNF overproduction and RA deficiency.
(A) Quantification of RALDH3 mRNA from IEC fractions from 4- and 20-week-old TNFΔARE mice compared with WT by real time RT-PCR. Mean±SEM, n=7/strain, *P<0.05 (B) Expression of RALDH1, 2 and 3 mRNA transcripts in IEC fractions from 20-week-old mice, Mean±SEM, n=7/strain. *P<0.05. (C) Analysis of RALDH3 mRNA expression from MODE-K cells cultured for 48 h treated in triplicate with 1μM RA, 10ng/ml TNF, 1μM LE540 or 1μM AM580 stimulation or untreated for 24 h followed by treatment with 1 μM LE540 for 24h (NT/LE). Mean±SEM of 4 individual experiments, *P≤0.05, ***P≤0.001.
To further examine regulation of RALDH3 mRNA in murine IEC, ileum-derived MODE-K cells were treated for 48h with indicated compounds and RALDH3 mRNA expression assessed. Culture with either RA (0.4±0.1; P<0.05) or the RARα preferential agonist AM580 (0.5±0.1;P<0.05) significantly down-regulated RALDH3 mRNA (Figure 4C). RAR antagonism with LE540 had no effect on RALDH3 expression alone; however, incubation for 24h without antagonist was followed by withdrawal of RA signal by addition of LE540 for 24h this resulted in significant fold-induction of RALDH3 mRNA compared to vehicle-treated cells (8±1; P<0.001). Finally, we examined the effect of TNF on RALDH3 transcripts. Treatment with TNF significantly decreased expression of RALDH3 (0.1±0.03;P<0.01).
Thus, pro-inflammatory cytokines reduces RALDH3 mRNA expression and both in vivo and in vitro data support that epithelial cells sense available RA and up-regulate their RA synthetic machinery to maintain intestinal RA bioavailability.
RA supplementation attenuated ileitis in vivo
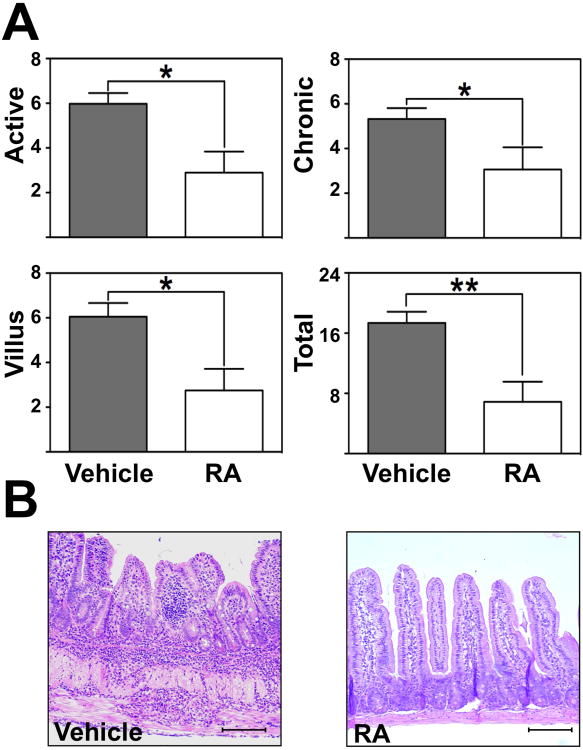
We then examined whether RA supplementation could affect the severity of ileitis during late disease. Twenty-week-old mice received RA for 2 weeks (Figure 5). RA supplementation significantly decreased inflammatory indices compared with vehicle-treated controls (active inflammation; the degree of granulocyte infiltration; 3±0.1 vs. 6±0.5, P<0.05; chronic inflammation; assessment of lymphocyte and monocyte infiltration; 3±1 vs. 5±0.5, P<0.05; villus distortion 3±1 vs. 6±1, P<0.05; total 7±3 vs. 17±2; P<0.01; Figure 5A). Intestinal inflammation was scored by a trained pathologist (PJ) in a blinded fashion as previously described28. Histological hallmarks of ileitis such as leukocyte infiltration, goblet cell hyperplasia and muscularis hypertrophy were noticeably decreased (Figure 5B).
Figure 5. Retinoic acid supplementation significantly attenuated chronic ileitis in vivo.
(A) Inflammatory indices from ilea of 20-week-old TNFΔARE mice treated with RA i.p. twice weekly for 2 weeks or vehicle were assessed as described28. Mean±SEM, n=8/treatment group, *P<0.05, **P<0.01. (B) Representative H&E micrographs of vehicle and RA-treated ilea of 20-week-old TNFΔARE mice (10× magnification, bars=100μm).
A decrease in IL-17A+ T cells, increased CD103+ DC and enhanced suppression by Tregs contributes to the RA-mediated attenuation of ileitis
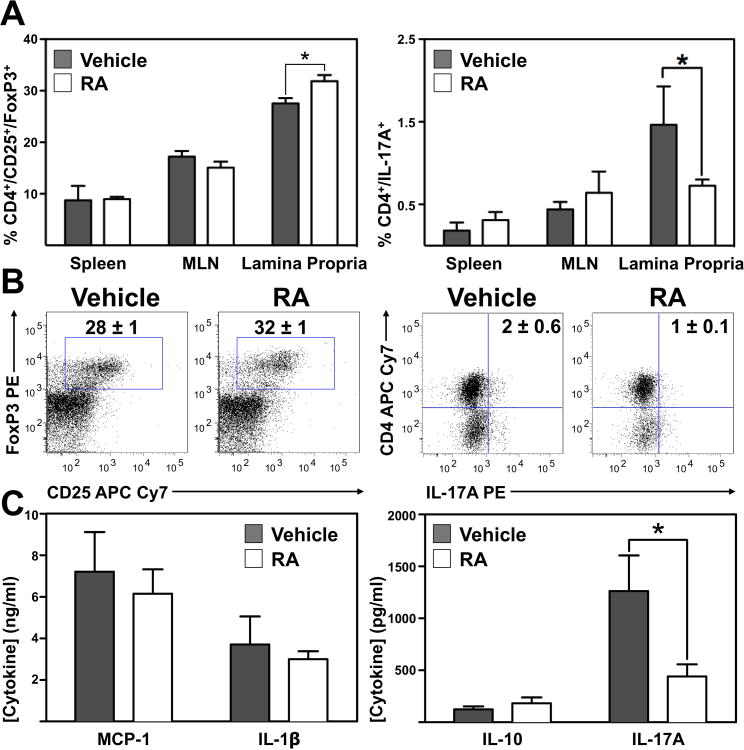
To address the anti-inflammatory mechanism of RA, TNFΔARE mice were treated with vehicle or RA for 2 weeks demonstrating a modest but significant increase in CD4+/CD25+/FoxP3+ T cells (28±1% to 32±1%; P<0.05) and concomitant decrease in proportion of CD4+/IL-17A+ cells (2±1% to 1±0.1%; P<0.05) from the LP of RA-treated mice (Figure 6A, B). Furthermore, supplementation of RA increased the frequency of CD103+ DC in the ileal LP of TNFΔARE mice (Supplemental figure 4) and enhanced the suppressive function of Tregs from RA-treated mice (Supplemental figure 5). Furthermore DC from the LP of RA-treated mice showed an enhanced capacity to induce Tregs, while decreasing induction of Th17 cells when co-cultured with T cells in vitro (Supplemental figure 6).
Figure 6. RA shifts regulatory and effector balance of T cells in the inflamed ileum.
(A) Frequency of CD4+/CD25+/FoxP3+ Tregs and CD4+/IL-17+ Th17 cells in indicated organs isolated from vehicle- or RA-treated 20-week-old TNFΔARE mice. (B) Representative dot plots display the CD4+/CD25+/FoxP3+ T cell expression and CD4+/IL-17+ cells from vehicle-and RA-treated mice. (C) MCP-1, IL-1β, IL-10 and IL-17A from 24 h cultures of unfractionated CD3/28 stimulated lymphocytes, measured using the multiplex cytokine analysis system from Quansys Biosciences. Mean±SEM for n≥3 mice/group. *P≤0.05.
MCP-1 and IL-1β from supernatants of cultured CD3/28-stimulated LP leukocytes from vehicle and RA-treated mice were unchanged. Similarly, while there was a trend toward higher levels of IL-10, this was not statistically significant. However, consistent with the decreased proportion of CD4+/IL-17A+ T cells from the LP of RA-treated mice demonstrated by flow cytometry, release of IL-17A was significantly lower from LP cells of RA-treated mice compared with those of vehicle-treated mice (1300±300pg/ml vs. 400±100pg/ml; P<0.05; Figure 6C). Thus, although there was only a modest increase in the number of Tregs after RA supplementation, there was a decrease in Th17 cells, an increase in CD103+ DC and an enhanced suppressive capacity by Tregs. Thus, the anti-inflammatory effect of RA is mediated not only by an increase in the number of Tregs but also by enhanced Treg function, increased CD103+ DC and decreased Th17 cells (Supplemental figure 7).
Deficiency in RALDH mRNA and net RA levels within the terminal ilea
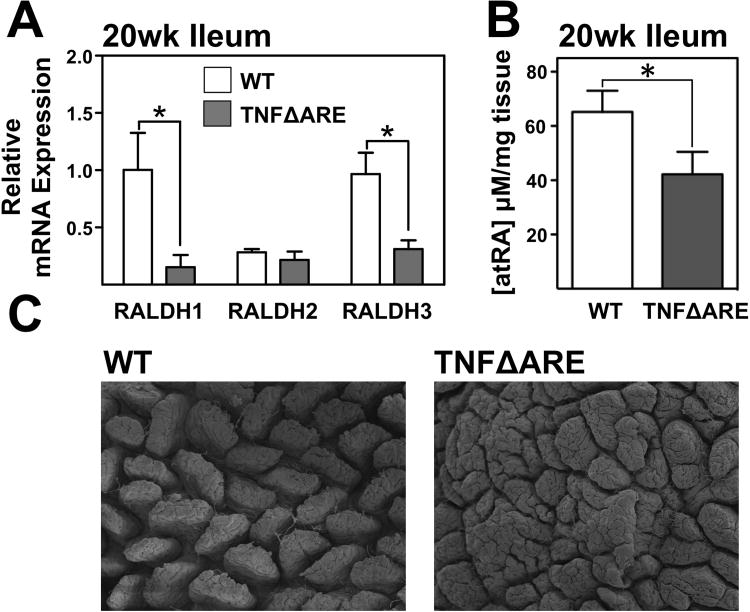
Despite the apparent compensatory RA production mechanism of epithelial cells, the beneficial effect of RA supplementation would suggest that a deficiency in intestinal RA was still present during ileitis. Therefore, we analyzed the net effect of inflammation on expression of RALDH isoforms in whole ileal tissue RNA. Comparison of 20-week-old WT and TNFΔARE whole ileal tissue demonstrated a significant decrease in RALDH1 (1±0.3 to 0.2±0.1; P<0.05) and RALDH3 mRNA (1±0.2 to 0.3±0.1) while RALDH2 mRNA was not significantly altered (Figure 7A).
Figure 7. RA deficiency in whole intestinal tissue of inflamed mice.
(A) Expression of RALDH1,2, and 3 mRNA in ileal tissue from 20-week-old TNFΔARE mice compared with WT littermates. Mean±SEM, n=7/genotype *P≤0.05. (B) Coulometric analysis of whole tissue extracts from WT and TNFΔARE mice demonstrated a significant reduction in RA levels in inflamed mice ilea compared with WT controls. Mean±SEM, n=5/genotype, * P<0.05. (C) Representative scanning electron micrographs of ilea from 20-week-old WT and TNFΔARE mice (80× magnification).
To confirm whether, despite of the attempted IEC compensation, there was net decrease in intestinal RA levels, we measured whole tissue levels of all-trans retinoic acid by electrochemical analysis. This revealed a significant decrease in tissue RA levels in 20-week-old TNFΔARE ilea (42±8 μM/mg; P<0.05; Figure 7B compared with WT mice (65±8 μM/mg). Thus, up-regulation of the IEC RA synthesis assists in maintaining RA levels within the intestine, although not to par with WT mice. This is likely due to the loss of IEC mass caused by the prominent blunting of the villous surface evidenced by the scanning electron microscopy images (Figure 7C). Thus, although individual IEC up-regulate their RA synthesis, IEC loss results in a net decrease in total tissue RALDH3 mRNA transcripts. However, due to the net RA deficit and increased IL-6 and TGFβ, there is preponderance of Th17 cells in ilea of TNFΔARE mice (Supplemental figure 2) and this balance is tilted towards regulation after exogenous RA supplementation (Supplemental figure 6).
Discussion
We show that loss of pro-regulatory CD103+ DC coupled with impaired RA synthesis contributes to the pathogenesis of ileitis. Counter-intuitively, we observed an increased Treg frequency in 20-week-old inflamed mice, which appeared insufficient to attenuate inflammation and led us to believe that an alternate source of RA might be operational during ileitis. We found that while IEC up-regulated RA synthetic machinery, whole tissue levels of RA remained lower in inflamed mice compared with WT. The decreased tissue levels of RA led us to explore whether RA supplementation mice would have an effect of on the severity of ileitis. Indeed, this manipulation attenuated chronic ileitis by driving CD103+ DC and Treg expansion and function and also by increasing Th17 cell frequency. The need for RA supplementation may reflect a failed endogenous compensatory mechanism involving up-regulation of RALDH3 by IEC, as part of an apparent RA-sensing mechanism in mice with reduced tolerogenic DC frequency and RA producing capacity.
CD103+ DC preferentially induce a gut-homing regulatory phenotype on naïve T cells in mice10, 11, 29 and humans30 in a RA/TGFβ-dependent manner. Induction of gut-homing Tregs tilts the balance towards regulation and away from Th17 responses. CD103+ DC deficiency in our model may contribute to the perpetuation of ileitis, as antibody blockade of CD103+ DC exacerbated murine colitis, supporting a protective function for this population29, 31. The decreased frequency coupled with a downregulation of enzymes critical for RA production by DC10, supports a reduction in available RA and is consistent with findings in colitis models32, 33. However, the Treg expansion seen in this study, as in other models of chronic murine ileitis7 and acute colitis34 was inconsistent with such a reduction, suggesting that an alternative source of RA might play a role or that the increase in TGFβ seen in the tissue of these mice may be adequate to increase expression of FoxP3.
A reduced frequency of RA-producing DC and downregulation of RA machinery in DC would limit induction of Tregs and supplementation of RA may attenuate inflammation as seen in other murine models17, 35. However, while RA treatment promoted Treg induction, secretion of IL-10 was mostly unchanged, suggesting that an alternative anti-inflammatory mechanism was involved. RA supplementation increased CD103+ DC and Tregs numbers and function while decreasing IL-17 secretion from intestinal tissue and a concomitant decrease in the proportion of IL-17 secreting CD4+ T lymphocytes, indicating that RA was not only pro-regulatory but also anti-inflammatory. Given the already expanded Treg population present in TNFΔARE mice, the decrease in Th17 cells may be an additional anti-inflammatory mechanism through which RA supplementation operates.
Many studies have demonstrated a role for IL-17-secreting cells in intestinal inflammation both in humans36 and mice20, 37. Expansion of the Th17 population has been demonstrated previously in the TNFΔARE model38. The suppression of the Th17 pathway by RA treatment reflects a tipping of the TGFβ-governed balance between regulatory and Th17 cells and manipulation of this balance has potential not only in chronic murine ileitis where there is a traceable deficiency in RA machinery, but also in human IBD.
It is clear from the our results and that of others33 that intestinal inflammation leads to a loss of frequency and function of pro-regulatory DC. Since ileitis results in expansion of Tregs, our work suggests that the expansion of Tregs is insufficient alone to attenuate inflammation. While RA supplementation did further increase the number of Tregs in the LP of TNFΔARE mice, this increase was modest, however coupled with enhanced suppression, increased CD103+ DC and decreased Th17 cells it appeared to be sufficient to restore homeostasis and attenuates ileitis.
An actual deficit in RA bioavailability in inflamed intestine has not been demonstrated previously, thus we chose to address this and better understand the sources of RA in the inflamed intestine. We also broadened our studies to include IEC due to their ability to act as an alternative source of RA. Intestinal epithelial cells have been shown to induce tolerogenic DC in an RA-dependent manner ex vivo14. We now additionally show that IEC RA machinery is inversely related to extracellular RA concentration, suggesting that these cells may attempt to offset a DC deficiency during inflammation and help regulate intestinal RA production completing a DC:IEC crosstalk to maintain local RA levels. RA-dependent regulation of RA metabolic enzymes has been shown in an embryonic carcinoma cell line previously39. Furthermore, capacity of IEC to react to environmental conditions and respond by directing DC function has been demonstrated with thymic-stromal lymphopoietin14. Nevertheless, this is the first time that alteration of RALDH expression in response to local RA concentration and TNF has been shown in intestinal epithelial cells. While TNF appears to negatively affect RALDH expression in vitro, the upregulation of RA machinery following RA withdrawal in cultured epithelial cells coupled with the upregulation of RA machinery in freshly isolated cells from intestinal tissue with a deficit in available RA suggest that the RA availability signal may supersede the TNF signaling in vivo. It is possible that the deficiency in DC-mediated RA production in these mice drives RALDH expression in IEC in an attempt to compensate for the deficiency as it is supported by the in vitro data, though the decreased IEC mass caused by blunting of villus architecture may render this mechanism inadequate. Thus, despite an upregulation of RA machinery by epithelial cells, the deepithelialization due to chronic ileitis coupled with decreased RALDH mRNA expression by CD103+ DC leads to an overall deficit in RA that leads to decreased expression of CD103+ DC in the MLN and LP. While an overall decrease in available RA was detectable, traceable to a downregulation in DC and fewer IEC, the possibility that secondary lymphoid tissue stromal cells might supply the RA required for the maintenance of Treg levels deserves further exploration.
The ability of retinoic acid supplementation to promote the induction of Tregs with a gut-homing phenotype while also limiting the expansion of effector Th17 cells has significant appeal for the treatment of IBD. While blocking TNF (infliximab) and intestinal homing (natalizumab) have met with success in the treatment of IBD, RA supplementation may provide a new alternative therapy in cases where classical therapeutics fail. Furthermore, all-trans retinoic acid has already been approved for oral use in acute promyelocytic leukemia, facilitating its rapid adaptation to treat chronic intestinal inflammatory conditions.
Supplementary Material
Acknowledgments
We thank Joshua D. Wermers for assistance with flow cytometry.
Grant Support: US PHS DK080212-01A2, CCFA 2826 to JR-N. and CCFA 2652 to CBC.
Abbreviations used in this paper
- DC
dendritic cell
- RA
retinoic acid
- CD
Crohn's disease
- FoxP3
forkhead box P3
- RALDH
retinoic acid aldehyde dehydrogenase
- Tregs
regulatory T cells
- IBD
inflammatory bowel disease
- ΔARE
ΔAU-rich element
- MLN
mesenteric lymph node
- LP
lamina propria
- IEC
intestinal epithelial cell
- MACS
magnetic cell sorting
Footnotes
All authors contributed to the acquiring, analyzing, interpreting data, drafting and proofreading the manuscript
Financial Disclosures: No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Relevant Bibliography
- 1.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–13. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 6.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamias G, Okazawa A, Rivera-Nieves J, et al. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–18. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 9.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 10.Coombes J, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 14.Iliev ID, Spadoni I, Mileti E, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–9. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 15.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 16.Dominitzki S, Fantini MC, Neufert C, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–5. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 17.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 18.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–70. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–9. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 22.Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 23.Rivera-Nieves J, Bamias G, Vidrich A, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–82. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 24.Ho J, Kurtz CC, Naganuma M, et al. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera-Nieves J, Ho J, Bamias G, et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518–29. doi: 10.1053/j.gastro.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Vidal K, Grosjean I, evillard JP, et al. Immortalization of mouse intestinal epithelial cells by the SV40-large T gene. Phenotypic and immune characterization of the MODE-K cell line. J Immunol Methods. 1993;166:63–73. doi: 10.1016/0022-1759(93)90329-6. [DOI] [PubMed] [Google Scholar]
- 27.Santos RL, Tsolis RM, Zhang S, et al. Salmonella-induced cell death is not required for enteritis in calves. Infect Immun. 2001;69:4610–7. doi: 10.1128/IAI.69.7.4610-4617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns RC, Rivera-Nieves J, Moskaluk CA, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 29.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–12. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Strauch UG, Grunwald N, Obermeier F, et al. Loss of CD103+ intestinal dendritic cells during colonic inflammation. World J Gastroenterol. 16:21–9. doi: 10.3748/wjg.v16.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103(+) dendritic cells. Eur J Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimesaat MM, Fischer A, Siegmund B, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS ONE. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang SG, Wang C, Matsumoto S, et al. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–402. e1–6. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin-17 and interferon-{gamma} production in inflammatory bowel disease. Gut. 2009 doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 37.Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Büning J, von Smolinski D, Tafazzoli K, et al. Multivesicular bodies in intestinal epithelial cells: responsible for MHC class II-restricted antigen processing and origin of exosomes. Immunology. 2008;125:510–21. doi: 10.1111/j.1365-2567.2008.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonneveld E, van den Brink CE, Tertoolen LG, et al. Retinoic acid hydroxylase (CYP26) is a key enzyme in neuronal differentiation of embryonal carcinoma cells. Dev Biol. 1999;213:390–404. doi: 10.1006/dbio.1999.9381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.