Abstract
Purpose
To explore norms of decision making regarding life-sustaining treatments (LSTs) at two academic medical centers (AMCs) that contribute to their opposite extremes of end-of-life ICU use.
Methods
We conducted a 4-week mixed methods case study at each AMC in 2008-2009 involving direct observation of patient care during rounds in the main medical ICU, semi-structured interviews with staff, patients, and families, and collection of artifacts (e.g., patient lists, standardized forms). We compared patterns of decision making regarding initiation, continuation, and withdrawal of LST using tests of proportions and grounded theory analysis of fieldnote and interview transcripts.
Results
We observed 80 patients (26 [32.5%] ≥ 65) staffed by 4 attendings, and interviewed 23 staff and 3 patients/families at the low-intensity AMC (LI-AMC), and observed 73 patients (26 [35.6%] ≥ 65) staffed by 4 attending physicians and interviewed 26 staff and 4 patients/families at the high-intensity AMC (HI-AMC). LST initiation among patients > 65 was similar, except feeding tubes (0% LI-AMC vs. 31% HI-AMC, p=.002). The LI-AMC was more likely to use a time-limited trial of LST, followed by withdrawal (27% vs. 8%, p=.01) and to have a known outcome of death (31% vs. 4%, p<.001). We identified qualitative differences in goals of LST, the determination of “dying,” concern about harms of commission versus omission, and physician self-efficacy for LST decision making.
Conclusions
Time-limited trials of LST at the LI-AMC and open-ended use of LST at the HI-AMC explain some of the AMCs’ nationally-profiled differences in end-of-life ICU use.
Keywords: terminal care, palliative care, intensive care, utilization, physician decision making, qualitative research, case study, variation, Medicare, national health policy
Despite decades of documentation in the literature[1-5] and important implications for the efficiency and equity of health care[6-8], the non-health causes of variations in volume and intensity of medical treatment remain elusive. The many factors correlated with volume and intensity range from regional supply[9-15] and market characteristics[16, 17] to structural hospital [18-24] and physician characteristics.[25-33]
Recent U.S. discourse has focused on variations in end-of-life treatment intensity between academic medical centers (AMCs) with similar resources and repute as a cause for alarm.[34, 35] Differences in use and withdrawal of life-sustaining treatments (LSTs) in the intensive care unit (ICU) likely contribute to these variations[36-40], yet little is known about the norms of decision making underlying these differences.
This paper describes LST decision making in the medical ICU of two U.S. tertiary care AMCs in the same state and health care system that are oft-cited archetypes of AMCs on opposite ends of the spectrum of end-of-life treatment intensity.
METHODS
Sample
AMC sample
We used 2001-2005 Dartmouth Atlas Medicare claims measures [41] to purposively sample[42] a low-intensity (LI) AMC and high-intensity (HI) AMC in the same state and health care system (Table 1, top panel), then confirmed differences in LST use in the ICU using 2004-2007 Medicare claims (Table 1, bottom panel).
Table 1.
End-of-life Intensive Care and Life Sustaining Treatment Use among Chronically-ill Medicare Fee-for-Service Decedents 2001-2005 and 2004-2007, by Academic Medical Center
| Cohort and Measure | Low intensity | High intensity |
|---|---|---|
| Chronic illness decedents 2001-2005, N* | 1 420 | 1 657 |
| ICU days in the last 6 months of life | 3.4 | 11.7 |
| High-intensity bed, days | 2.9 | 4.8 |
| Intermediate-intensity bed, days | 0.5 | 6.9 |
| Died with ICU services, % | 23.3 | 37.9 |
|
| ||
| Chronic illness decedents with a terminal admission, 2004- 2007, N† |
789 | 814 |
|
| ||
| Intensive care unit (ICU) admission, % | 50.6 | 74.0 |
| ICU length of stay, mean; median, d | 4.0, 1.0 | 10.1, 3.0 |
| Life-sustaining treatments, % | ||
| Intubation and mechanical ventilation | 34.1 | 44.8 |
| Hemodialysis | 10.4 | 14.4 |
| Tracheostomy | 1.9 | 10.6 |
| Gastrostomy feeding tube placement | 1.4 | 3.1 |
Downloaded from www.dartmouthatlas.org, accession date August 4, 2008
Calculations performed by special request using Dartmouth Atlas Medicare data (Yunjie Song, December 21, 2009)
Provider sample
At each AMC, we recruited via e-mail the attending physicians who were scheduled to be on service during our site visit and consented them to shadow observation and interview. We recruited other clinicians for interview, including nurses, residents, fellows, and consultants involved in the care of sampled patients, via direct contact in the unit, and recruited clinical and administrative leaders involved in policymaking via telephone or e-mail.
Patient sample
Under a waiver of informed consent at each AMC we observed rounds and other clinical decision making for all adult patients staffed by shadowed attending physicians for 8 hours/day for 4 consecutive weeks. Exclusion criteria included age < 21, prisoners, legal concerns (e.g., assault), or opting out. We sampled 5 patients (or their proxies) to complete an interview if the patient met three additional inclusion criteria: 1) age ≥ 65; 2) English-speaking; 3) ≥1 or more life-limiting chronic illnesses [43, 44], maximizing heterogeneity by race and chronic illness.
Data Collection
A nurse (JAT), a medical sociologist (KLR), and a physician (AEB) took field-notes daily during observation of rounds, ICU team and family meetings, and bedside clinical care to document verbal and non-verbal communication, focusing on the processes of LST decision making. We conducted informal interviews with health care providers, patients, or patients’ family members in the unit as the need arose. We dictated these notes daily, then transcribed and edited them for clarity. We conducted formal audio-recorded semi-structured health care provider, clinical leader, and administrative leader, patient, and family member interviews. Finally, we collected artifacts at each institution related to decision making, including institutional policies, standing order sets related to LSTs, and informational brochures designed for patient/family members.
Analyses
We followed the “editing” approach by Crabtree and Miller designed for qualitative analysis in the medical setting.[45] To begin, a multidisciplinary team of study investigators (AEB, KLR, JAT, RMA) conducted iterative close readings of field-notes, interview transcripts, and policy artifacts to identify emergent concepts, categories, and relationships in the data and to develop a comprehensive coding scheme. Two study investigators, including the nurse (JAT) and a qualitative methods expert (SLZ) who was not involved in data collection and was blinded to AMC identity and end-of-life treatment intensity, separately coded one-quarter of the transcripts using Atlas.ti 5.2 (Scientific Software, Berlin, Germany ). Intercoder kappa scores were 0.76 and above, indicating “substantial agreement.”[46] The nurse investigator used the final coding scheme to code 100% of the documents. We then systematically analyzed patterns in the distribution and relationships of emergent concepts and categories within each individual AMC. Finally, we performed “member checking” at each study AMC.
RESULTS
Hospital and ICU
The LI-AMC had 550 licensed beds, of which 60 were ICU beds (9:1 ratio). The study ICU was a 16-bed mixed medical-surgical co-managed/semi-open unit staffed by an anesthesiology or pulmonary critical care attending on one-week rotations.
The HI-AMC had 425 beds, of which 108 were ICU beds (4:1 ratio). The study ICU was a 24-bed medical closed ICU staffed by two pulmonary critical care attending physicians who split the unit during 2-week rotations.
Subjects
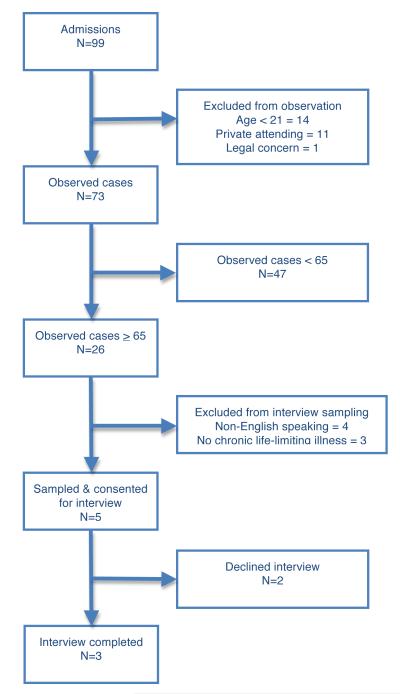
At the LI-AMC we shadowed 4 (100%) attending physicians, conducted semi-structured formal interviews with 12 providers and 11 clinical and administrative leaders, observed 80 patients on rounds (of whom 26 (32.5%) were ≥ 65 years of age), and interviewed 3 of 5 purposively sampled patients/proxies (Figure 1A). At the HI-AMC we shadowed 4 (100%) attending physicians, conducted semi-structured formal interviews with 15 providers and 11 clinical and administrative leaders, observed 73 patients on rounds (of whom 26 (35.6%) were ≥ 65 years of age), and interviewed 4 of 5 purposively sampled patients/proxies (Figure 1B). The age-eligible patients at the LI-AMC were less commonly admitted from long-term care facilities, had shorter length of ICU stay, received fewer feeding tubes, typically received time-limited trials of LST, and were more likely to have an observed outcome of death during the observation period (Table 2).
Figure 1. Panels A and B.
These flow diagrams depict all of the patients admitted to the study ICU at the low-intensity hospital (A) and high-intensity hospital (B) during 4 weeks of intensive care study. We excluded patients from study observation if they were < 21, prisoners, or were not rounded on by the consented ICU physician. Patients eligible for additional data collection, including in-depth interviewing included those 65 and older with one or more life-limiting chronic illness and English fluency.
Table 2.
Characteristics of Observed Patients Age 65 and Older, by Academic Medical Center
| Variable | Low intensity | High Intensity | p-value |
|---|---|---|---|
| Patients, N | 26 | 26 | |
|
| |||
| Age, mean (range), years | 75 (65-94) | 75 (67-84) | 0.48* |
|
| |||
| Female sex, n (%) | 12 (46) | 14 (54) | 0.23† |
|
| |||
| Race, n (%) | 0.28# | ||
| White | 18 (69) | 15 (58) | |
| Black | 2 (8) | 2 (8) | |
| Hispanic | 0 (0) | 4 (15) | |
| Asian | 6 (23) | 5 (19) | |
|
| |||
| Admitted from long-term care, n (%) | 0 (0) | 5 (19) | 0.046# |
|
| |||
| ICU admission source, n (%) | 0.09# | ||
| Emergency department | 6 (23) | 8 (31) | |
| Operating room | 8 (31) | 3 (12) | |
| Floor | 9 (35) | 4 (15) | |
| Transfer | 2 (8) | 5 (19) | |
| Other | 1 (4) | 6 (23) | |
|
| |||
| Diagnosis/Trajectory, n (%) | 0.15‡ | ||
| Cancer | 9 (43) | 12 (52) | |
| Organ Failure | 10 (48) | 5 (22) | |
| Frailty/Dementia | 2 (8) | 6 (26) | |
|
| |||
| ICU length of stay, median (SD); median, days | 7.9 (8.8); 4.0 | 11.2 (13.2); 6.0 | 0.23† |
|
| |||
| Life-sustaining treatments, n (%) | |||
| Mechanical ventilation | 13 (50) | 14 (54) | 0.78$ |
| Continuous renal replacement | 4 (15) | 2 (8) | 0.39$ |
| Intermittent renal replacement | 4 (15) | 1 (4) | 0.16$ |
| Feeding tube | 0 (0) | 8 (31) | 0.002$ |
| Vasopressors | 13 (50) | 9 (35) | 0.26$ |
| Transfusion | 3 (12) | 3 (12) | 0.96$ |
|
| |||
| Life-sustaining treatment decision making, n (%) | 0.01# | ||
| No life-sustaining treatment | 6 (23) | 6 (23) | |
| Rapid recovery to discharge | 10 (38) | 6 (23) | |
| Time-limited trial, early withdrawal | 7 (27) | 2 (8) | |
| Open-ended, late withdrawal or non-resolution | 0 (0) | 8 (31) | |
| Other | 3 (11) | 4 (15) | |
|
| |||
| Disposition from ICU, n (%) | <0.001# | ||
| Dead | 8 (31) | 1 (4) | |
| Floor | 14 (54) | 6 (23) | |
| Home | 0 (0) | 4 (15) | |
| Skilled nursing facility | 0 (0) | 3 (12) | |
| Unknown | 4 (15) | 12 (46) | |
ICU – intensive care unit
t-test
Rank-sum test
Chi-squared test
Fisher’s exact test
Two-sample test of proportion
Decision Making Norms
Drawing on the qualitative data, decision making regarding the use of LST differed between the two AMCs in many implicit and explicit ways, including goals of LST, determination of when a patient is “dying,” concern about harms of commission versus omission, and physicians’ self-efficacy for LST decision making (Table 3). 21/80 (26%) of observed cases at the LI-AMC and 55/73 (75%) at the HI-AMC contributed coded data to the identification of these themes, with 10 patients at the LI-AMC and 14 at the HI-AMC who were age ≥ 65 years old and received LST without a rapid recovery to discharge contributing most densely (Table 2).
Table 3.
Life-sustaining Treatment Decision Making Themes, by Academic Medical Center
| Theme | Low intensity | High intensity |
|---|---|---|
| Goals of life sustaining treatment |
The goal of life-sustaining treatment is a bridge to recovery. It is a means to an end. Examples A 67 year-old black man with metastatic cancer who progressed to multi-system organ failure (MSOF) during a trial of continuous veno-venous hemofiltration (CVVH) discontinued by the team. “Well, it was worth a trial at 3 AM for a few days, but now that his liver has really not improved, everything else that could be improving, all of that would hinge on the fact that his liver was failing.” --Fellow [fieldnotes, rounds] An 81 year-old Asian man with metastatic cancer whose family initially chose intubation for pneumonia, sepsis, and respiratory failure, had mechanica ventilation withdrawn one day later when dialysis became necessary. The ICU resident described meeting with the family at the patient’s bedside overnight with the nephrologist. They asked the family to consider the patient’s longer term prognosis and goals of treatment, including whether the patient would want to have regular dialysis if he were to survive the hospitalization. The family felt that the patient would only have wanted life-sustaining treatment if he could be returned to the same state of health he was just prior to admission and that, given his limited life expectancy, the burden of ongoing dialysis if he should survive hospitalization would not be acceptable to him. --Fieldnotes [informal interview with the resident] |
The goal of life-sustaining treatment is meeting narrow physiologic objectives or averting death in the hospital. It can be an end in itself. Example A 77 year old Asian man with acute respiratory distress syndrome (ARDS) who experienced multiple setbacks over a prolonged course had an advance directive that the fellow interpreted narrowly, such that “get better” meant surviving, not being functionally independent. “He has an advance directive [that] states if I remember correctly that in the event that he can’t make decisions for himself his wife and his 2 sons have been designated as the people to make decisions for him, and that he doesn’t want to be a burden to his family if he’s not going to get better he doesn’t want complete life sustaining therapy. So none of these decisions [to use LST] have gone against what his living will states because everything that has happened to him has shown the potential to improve, but if he makes it through this he’s going to have a prolonged course where he will probably need to go to a nursing home or a rehab center before going home if he can get through these acute illnesses.” --Fellow [interview] |
|
| ||
| Determination of “dying” |
A patient is “dying” when they have a terminal underlying condition, such as metastatic cancer, or if they are judged to have a poor quality of life in the event life-sustaining treatment is continued. Example The family of the 51 year old white man with metastatic glioblastoma wanted to continue mechanical ventilation (MV) with the goal of bringing him home for the holidays. The team felt that continued MV was inappropriate. “His family has been told that it is terminal, and that he has a very short period of time to live. He is on a ventilator, and neurologically has no ability to communicate with his family. He has very poor neurologic function, only opens his eyes, doesn’t track or communicate or follow commands, and the ICU team as well as others have felt that, the way they put it, was that continuing to care for this man in an aggressive way was very undignified to him.” --Resident [interview] |
There is conflict and ambivalence about the when a patient is “dying,” although all agree that a patient whose vital signs cannot be maintained despite maximal life-sustaining treatment is dying. Example The attending vacillated between highlighting longer-term outcomes and nearer-term survival when discussing the 77 year-old Asian man with ARDS on rounds/ “I really feel that the patient has really sustained quite a few hits physically and his course has been so up and down. I really don’t feel as though he’s going to be a good candidate for a good quality of life. “ Two days later: “He’s very stable. We can probably rescue him. He has been salvaged even though he’s 77”. --Attending [fieldnotes, round] |
|
| ||
| Harms of commission versus omission |
Critical care physicians use concerns about harms of commission, such as iatrogenic harms, prolonging dying, and treating a patient against their preferences, to rationalize limitation of life-sustaining treatment. Example An 81 year old white man with multisystem organ failure after a complication from elective surgery was terminally extubated soon after he began making physical gestures indicating he didn’t want to be intubated anymore. “This morning he was intubated and he was signaling to us that he wanted us to stop and just get away from him and he was trying to pull out the tube and when we asked him ‘do you want the breathing tube out?’ he would nod ‘yes’, ‘do you want us to stop what we’re doing here, our treatments with you?’ and he nodded ‘yes’.” --Nurse [interview] “At one point he held his fingers up in an X, and I have never actually seen him move that much, and he put his hand up like he did not want the intervention …we opted to extubate him to see if we could talk to him about what kind of intervention he would want. I was there when we extubated and his first words to me were ‘take home, take home.’” --Resident [interview] |
Critical care physicians express concerns about these harms of commission, but these infrequently impact the treatment plan. Concerns about harms of omission, such as missing something treatable or limiting life-sustaining treatment for a patient who might survive, loom larger. Examples A 75 year-old black woman with metastatic breast and ovarian cancer, respiratory and acute renal failure, was started on hemodialysis at the family’s request after hearing favorable survival statistics from the oncologists: “The bad thing is, as her kidneys improve [while she is supported by dialysis] someone will want to do surgery. We’re supposed to help her.” –Attending [fieldnotes, rounds] An 84 year old white woman with dementia and sepsis was inadvertently intubated in the ED despite having a do not intubate (DNI) order. The team completed all initiated treatments over 5 days in the ICU before making her comfort measures only (CMO). “I received a call this morning about getting a bed for a patient who is 84 years old who was admitted to the emergency room DNR, DNI. They ended up intubating the patient and putting a central venous catheter in, and they collapsed her lung. This is what I have to deal with.” –Attending [fieldnotes, rounds] “By the time I got on [the case that morning], she was already extubatable so we extubated her and then made her, again, DNR/DNI. But just, we still had to deal with the chest tube, we still had to finish up antibiotics for pneumonia, so everything that had started, we were finishing.” --Attending [interview] |
|
| ||
| Physician self- efficacy for decision making |
Critical care physicians have a high degree of self-efficacy for decision making regarding life-sustaining treatment. They view family requests for continued treatment as part of the normal trajectory. Example In the case of the 67 year-old black man with metastatic neuroendocrine tumor on CVVH, we observed the team negotiate an agreement not to escalate treatment over the course of several family meetings. The team actively discussed how to manage issues that interfere with families’ “transitioning to end of life,” such as anger. Fellow: “I am not sure how savvy the family is.” Attending: (to the social worker) “This is some place you could help with. The family is angry not believing the situation. He had an embolization in August and they blame that on his downhill slide. He was vital prior to that embolization.” --Fieldnotes, social service rounds |
Critical care physicians externalize the locus of control for decision making to patients, families, and specialists who they believe expect aggressive treatment. They view family requests for continued treatment as a mandate. Example In the case of the 77 year-old Asian man with ARDS, the attending attributed the decision to continue LST to family demands against the patient’s treatment preferences. We did not observe any family meetings. “I have talked to the dad, the patient, and he said this is not what he wants… [but] the wife is not willing to hear this nor is the family… there are certain cultures that believe you should do everything possible for the patient and he fits that mold very, very well.” --Attending [interview] Yet the resident and nurse who spent the most time at the bedside did not perceive the family to be demanding. “For the most part, her [the wife] and her son kind of defer to the doctors.” --Resident [interview] “The family’s really nice, really open, they don’t ask for something that cannot be done.” --Nurse [interview] |
Goals of life-sustaining treatment
At the LI-AMC, providers identified goals of treatment before initiation of a LST. A dominant theme was that an LST must be a “bridge to something;” it was the means to an end (recovery). We frequently observed explicit time-limited trials of LST. When discussing whether honor the family’s request to extend a time-limited trial of continuous veno-venous hemofiltration (CVVH) for a middle-aged Middle Eastern man with multi-system organ failure (MSOF) ineligible for the liver transplant he required to recover, the consulting nephrologist said: “It [CVVH] is a means to no end.” The fellow explained: “The family wanted to continue without a clear endpoint. Since we decided on no transplant we were kind of dialyzing him to infinity and the guy was not going to get any better.” For patients who might plausibly survive the ICU stay with LST, providers directed surrogates to focus on the patient’s long-term treatment goals (Table 3).
At the HI-AMC, in contrast, we frequently observed open-ended use of LST. The goals of LST were to meet narrow physiologic objectives or avert death in the ICU; it was an end in itself. Although we occasionally heard the critical care attendings asking questions about goals of LST on rounds, the answers to these questions by housestaff were framed in the short-term. For example, when discussing the continuation of CVVH for a middle-aged black woman ineligible for the heart-lung transplant she required to recover, the critical care attending said to the residents “She was here when I was on service 3 weeks ago. We can’t go on indefinitely. What’s the endpoint?” to which the fellow replied “Her dry weight.” Housestaff interpreted written advance directives to identify narrow treatment preferences, which substituted for goals. When discussing the 69 year-old white woman with metastatic cancer and limb-threatening cellulitis, sepsis, and respiratory failure, the attending asked “What is the end game?” to which the fellow replied, “There is an advance directive. She wants everything to be done, but only if it’s a temporary measure.” The intern’s note, recopied verbatim daily, read: “Wait a few days to readdress advance directive type issues with the family.” Goals were not readdressed with the family until the patient was intubated, extubated, and reintubated over 20 days.
Determination of dying
At the LI-AMC, many patients were perceived as dying and there was seldom disagreement among team members or consultants. All of the providers agreed that the middle-aged Middle Eastern man ineligible for the liver transplant he required to recover was dying. Similarly, a 67 year-old black man with metastatic neuroendocrine tumor, an 81 year-old Asian man with metastatic gastric cancer, and a 51 year-old white man with advanced glioblastoma were considered as dying because they had poor prognosis solid tumors and at least one organ failure. Determination of dying also included implicit and explicit valuations of quality of life if LST were continued (Table 3).
At the HI-AMC, there was often disagreement between services and even ambivalence on the part of individual critical care attendings regarding the determination of dying. In the case of a 69 year-old white woman with metastatic pancreatic cancer the critical care attending said of the oncologists: “They give this crazy prognosis. I don’t trust these guys.” Although critical care attendings attributed prognostic over-optimism to other services, we also observed instances of their own ambivalence, demonstrated by frequently vacillating between discussing patients’ longer term prognosis from the underlying condition and their shorter term prognosis for survival to discharge (Table 3).
Harms of commission versus omission
At the LI-AMC there was a particular focus on avoiding harms of commission. For example the team expressed concern that CVVH could be doing “more harm than good” for the middle-aged Middle Eastern man ineligible for the transplant he required to recover, since CVVH filter clogging was “bloodletting” and transfusion was impossible due to alloantibodies. For the 51 year-old white man with glioblastoma, poor neurologic function, and failure to wean from mechanical ventilation, there was resistance to performing a tracheostomy because of an implied concern about transforming him from a state of acute critical illness to chronic critical illness. As one critical care attending asked during social service rounds: “Sure, we could trach him, but what then?” For an 81 year-old white man with MSOF after complications of elective surgery at an outside hospital, the team was preoccupied with the potential harm of providing LST against his will (Table 3).
At the HI-AMC, concerns about harms of commission were raised by critical care attendings, but usually only as frustrated complaints about other providers’ decision making (Table 3). Harms of omission loomed larger, as an intern explained: “You know because we have the resources, the chance that we miss something would just make us feel terrible. You know ‘oh we could have done that and then we would’ve known, and then …’” This manifested in the decision to complete treatment for an iatrogenic pneumothorax prior to initiating comfort measures for an 84 year-old white woman with dementia who was inadvertently resuscitated in the ED despite her DNR order (Table 3).
Physician self-efficacy for LST decision making
At the LI-AMC, we observed a high level of self-efficacy for LST decision making among the intensivists, even though they were not technically the primary service. As one explained: “There’s a lot of interest in decision making at the end of life…a lot of attention to engaging patients in thinking about whether aggressive care is the right way to go.” Providers viewed family requests for continued LST in situations of low anticipated benefit as part of the normal and expected evolution of a process that would take time to work through. Typically the intensivists and the primary team worked collaboratively with each other towards a consensus with the patient’s family. Although negotiated solutions were the norm, we observed one stalemate and one unilateral decision to withdraw “medically” against the family’s wishes on day 6 of admission for the middle-aged Middle Eastern man ineligible for transplant.
At the HI-AMC, providers externalized the locus of control to patients, relatives, referring providers, and specialists who they believe expected LSTs. Instead of seeing family’s requests for continued LST of low anticipated benefit as part of a normal process, they perceived it as a treatment mandate. One member of the clinical leadership hypothesized that the source of patients’ expectations were doctors themselves: “When you’re in an environment where it’s also very common to follow a very aggressive mode, a lot of patients will be swept up into that and begin to believe that that’s their goal as well.” Consulting specialists were perceived by the critical care attendings to control LST decision making, even though critical care was technically primary in the closed ICU. As one attending repeatedly said each time he complained about other services’ decision making: “but I’m not going to tell the [service] what to do.” Also, we repeatedly heard providers attribute demands and expectations to families, sometimes based upon cultural stereotypes, that we did not corroborate through observation and interview (Table 3). The degree of control ceded to patients and families disturbed the durable power of attorney for health care of the 84 year-old woman with dementia who was inadvertently resuscitated in the ED: “I was always the one who got to flip the switch. With all the information, do you want to go this way or that way? I got to go this way or that way…It is not something I want to do again.”
Origins of Norms
The origin of the interest in end-of-life decision making at the LI-AMC was attributed to an influential internist and ethicist starting in the 1980s. A homogeneous approach is promoted by retention of trainees as faculty, as one fellow noted: “This is a very inbred institution. You know pretty much every attending actually was a fellow here.” Strong social norms protected against countervailing influences, as described by one resident “I think that the personalities of the people who are extremely aggressive … really are not influential … because [they] haven’t really made any kind of reasonable case either for extensive use of that life-support or for the thought behind it.”
The origin of the approach to end-of-life decision making at the HI-AMC was attributed to the institution’s status as a referral center attracting patients expecting treatment other centers would not provide, such as transplants for patients over 65. Sunk costs motivated continued investment: “We continue very aggressive care to try and sustain their life in hopes you can reverse the process…because you have invested a lot of not only time, a lot of money, a lot of resources in making sure that they got the transplant.” Norms of treatment for complex referral populations created spillover effects for typical elderly admissions: “Because of our patient population here, the physicians who take care of these patients are highly aggressive and is it not our style to pull back and let people go. So typical bread and butter patients are treated very aggressively, right or wrong … it’s just automatic in your training you know, that you just keep going.”
DISCUSSION
In this case study of two AMCs in the same state and health system that are oft-cited archetypes of AMCs on opposite ends of the spectrum of end-of-life treatment intensity, we observed substantial differences in LST decision making in the medical ICU. At the LI-AMC, LST was a means to an end whereas at the HI-AMC, it was an end in itself.
At the LI-AMC, time-limited trials of LST guided by provider-defined treatment goals (e.g., organ function recovery) was the default. When these goals weren’t met, withdrawal often involved negotiation with families who sometimes pressed for continuation, a process that providers perceived as a natural evolution of the encounter that they were confident in managing. Management involved redirection, and occasional circumnavigation, of family preference for the patient to survive the hospitalization to considerations of longer-term survivability and functional outcomes. The origin of these norms may be historical accident. We did not directly identify the sanctions reinforcing these norms, although we did observe the director of adult critical care services on the unit every day asking whether each patient still needed to be in the ICU, likely motivated by scarcity given the 1:9 ICU-to-ward bed ratio. Moreover, variation in approach to end-of-life decision making was minimized by hiring faculty who also trained at the AMC, among whom the norms had been internalized as values.
At the HI-AMC, open-ended LST guided by narrow physiologic objectives and the goal of survival to discharge was the default. These goals arose from specialist input and perceptions regarding patient treatment preferences based on assumptions, stereotypes, and narrow interpretation of written advance directives more often than facilitated conversations about patient values considered best practice.[47, 48] Withdrawal of LST, which was rare, appeared based on “physiologic futility” in the face of inexorable deterioration despite maximal LST, since critical care physicians and specialists didn’t agree that the patient was dying before that. This did not manifest as open conflict, but instead frustrated passivity on the part of critical care providers embodied by frequent complaint about specialist decision making, suggesting a “learned helplessness” based on prior reprisals. The origin of these patterns is unclear, although they may be promoted by the comparatively resource-rich 1:4 ICU-to-ward bed ratio and an organizational identity defined by doing things that others will not.
This is the first study of its kind to systematically compare the norms of LST decision making between 2 hospitals based upon their known end-of-life treatment intensity. Prior studies have found structural factors, such as bedsize, associated with hospital [24, 49] and ICU-level [50] variation and others have serendipitously documented differences in norms of LST decision making between ICUs purposively sampled on other criteria [51-53] In contrast to Cassell’s findings, the closed administrative model of staffing in the HI-AMC was not associated with greater control over LST decision making, perhaps because informal norms maintained the power of specialists over critical care providers despite formal norms regarding the attending of record.
Although our findings are not generalizable to other high- and low-intensity AMCs, they are robust, having followed best practices in qualitative research, including theoretical sampling; multiple coding; data, investigator, and methodological triangulation; and respondent validation. Limitations include exclusively focusing on decision making conditional upon admission to the ICU, although outpatient and ICU admission decision making result in differences in ICU case-mix (see Online Supplement), and conducting relatively few patient/family interviews.
In conclusion, we are the first to describe behavioral norms that underlie differences between 2 high-profile AMCs’ patterns of end-of-life treatment intensity. Future research should expand the AMC sample and explore the mutability of norms in response to policy initiatives designed to reduce variation.
Supplementary Material
ACKNOWLEDGMENTS
Study Advisory Committee: Derek C. Angus, Judith R. Lave, Mary Elizabeth Happ, Megan Crowley-Matoka, Jonathan Skinner, Denise Anthony, Denise Rousseau, Sharyn Sutton; Dartmouth Atlas: Elliott Fisher, Yunjie Song; Institutional contacts and investigators; Michael Gropper, J. Thomas Rosenthal, Rajan Saggar; Other intellectual contributions and research assistance: Margaret Crighton, Courtney Sperlazza, Elan Cohen.
Funding: This work was supported by the National Institute for Nursing Research (R21NR010265).
Footnotes
CONTRIBUTIONS Obtaining funding (AEB), Conception and design (AEB, RMA), data collection (AEB, JAT, KLR), data analysis (AEB, JAT, KLR, SLZ, RMA), manuscript drafting (AEB), critical review and revision of manuscript (JAT, KLR, SLZ, RMA). Dr. Barnato had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure: There are no financial relationships with any organizations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Wennberg JE, Gittelsohn AM. Small area variations in health care delivery. Science. 1973;182:1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 2.Wennberg J, Gittelsohn A. Variations in medical care among small areas. Scientific American. 1982;246:120–134. doi: 10.1038/scientificamerican0482-120. [DOI] [PubMed] [Google Scholar]
- 3.Wennberg JE, Freeman JL, Culp WJ. Are hospital services rationed in New Haven or over-utilised in Boston? Lancet. 1987;1:1185–1189. doi: 10.1016/s0140-6736(87)92152-0. [DOI] [PubMed] [Google Scholar]
- 4.Chassin MR, Brook RH, Park RE, Keesey J, Fink A, Kosecoff J, Kahn K, Merrick N, Solomon DH. Variations in the use of medical and surgical services by the Medicare population. New England Journal of Medicine. 1986;314:285–290. doi: 10.1056/NEJM198601303140505. [DOI] [PubMed] [Google Scholar]
- 5.Wennberg JE. The Quality of Medical Care in the United States: A Report on the Medicare Program. AHA Press; Chicago, IL: 1999. The Dartmouth Atlas of Health Care in the United States 1999. [Google Scholar]
- 6.Skinner J, Fisher E, Wennberg J. The efficiency of Medicare. National Bureau of Economic Research; Cambridge, MA: 2001. [Google Scholar]
- 7.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 8.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 9.Welch WP, Miller ME, Welch HG, Fisher ES, Wennberg JE. Geographic variation in expenditures for physicians’ services in the United States. New England Journal of Medicine. 1993;328:621–627. doi: 10.1056/NEJM199303043280906. see comments. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard RS, Fisher ES, Teno JM, Sharp SM, Reding DJ, Knaus WA, Wennberg JE, Lynn J. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. Journal of the American Geriatrics Society. 1998;46:1242–1250. doi: 10.1111/j.1532-5415.1998.tb04540.x. see comments. [DOI] [PubMed] [Google Scholar]
- 11.Wennberg JE, Freeman JL, Shelton RM, Bubolz TA. Hospital use and mortality among Medicare beneficiaries in Boston and New Haven. New England Journal of Medicine. 1989;321:1168–1173. doi: 10.1056/NEJM198910263211706. [DOI] [PubMed] [Google Scholar]
- 12.Fisher ES, Wennberg JE, Stukel TA, Sharp SM. Hospital readmission rates for cohorts of Medicare beneficiaries in Boston and New Haven. New England Journal of Medicine. 1994;331:989–995. doi: 10.1056/NEJM199410133311506. see comments. [DOI] [PubMed] [Google Scholar]
- 13.Strauss MJ, LoGerfo JP, Yeltatzie JA, Temkin N, Hudson LD. Rationing of intensive care unit services. An everyday occurrence. Jama. 1986;255:1143–1146. [PubMed] [Google Scholar]
- 14.Gatsonis CA, Epstein AM, Newhouse JP, Normand SL, McNeil BJ. Variations in the utilization of coronary angiography for elderly patients with an acute myocardial infarction. An analysis using hierarchical logistic regression. Medical Care. 1995;33:625–642. doi: 10.1097/00005650-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 15.McClellan M. Uncertainty, health-care technologies, and health-care choices. American Economic Review. 1995;85:38–44. [PubMed] [Google Scholar]
- 16.Baker LC. The effect of HMOs on fee-for-service health care expenditures: evidence from Medicare. Journal of Health Economics. 1997;16:453–481. doi: 10.1016/s0167-6296(96)00535-8. [DOI] [PubMed] [Google Scholar]
- 17.Kessler DP, McClellan MC. Is hospital competition socially wasteful? National Bureau of Economic Research; Cambridge, MA: 1999. [Google Scholar]
- 18.Taylor DH, Jr., Whellan DJ, Sloan FA. Effects of admission to a teaching hospital on the cost and quality of care for Medicare beneficiaries. New England Journal of Medicine. 1999;340:293–299. doi: 10.1056/NEJM199901283400408. see comments. [DOI] [PubMed] [Google Scholar]
- 19.Vistnes G. Hospital mergers and antitrust enforcement. Journal of Health Politics, Policy & Law. 1995;20:175–190. doi: 10.1215/03616878-20-1-175. letter; comment. [DOI] [PubMed] [Google Scholar]
- 20.Claxton G, Feder J, Schactman D, Altman S. Public policy issues in nonprofit conversions: an overview. Health Affairs. 1997;16:9–28. doi: 10.1377/hlthaff.16.2.9. [DOI] [PubMed] [Google Scholar]
- 21.Gray BH, McNerney WJ. For-profit enterprise in health care. The Institute of Medicine Study. New England Journal of Medicine. 1986;314:1523–1528. doi: 10.1056/NEJM198606053142335. [DOI] [PubMed] [Google Scholar]
- 22.Silverman EM, Skinner JS, Fisher ES. The association between for-profit hospital ownership and increased Medicare spending. New England Journal of Medicine. 1999;341:420–426. doi: 10.1056/NEJM199908053410606. see comments. [DOI] [PubMed] [Google Scholar]
- 23.Sloan FA, Picone GA, Taylor DH, Chou SY. Hospital ownership and cost and quality of care: is there a dime’s worth of difference? National Bureau of Economic Research; Cambridge, MA: 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lin CY, Farrell MH, Lave JR, Angus DC, Barnato AE. Organizational determinants of hospital end-of-life treatment intensity. Med Care. 2009;47:524–530. doi: 10.1097/MLR.0b013e31819261bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenfield S, Nelson EC, Zubkoff M, Manning W, Rogers W, Kravitz RL, Keller A, Tarlov AR, Ware JE., Jr. Variations in resource utilization among medical specialties and systems of care. Results from the medical outcomes study. JAMA. 1992;267:1624–1630. comment. [PubMed] [Google Scholar]
- 26.Selby JV, Grumbach K, Quesenberry CP, Jr., Schmittdiel JA, Truman AF. Differences in resource use and costs of primary care in a large HMO according to physician specialty. Health Serv Res. 1999;34:503–518. [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson LC, Danis M, Garrett JM, Multran E. Who decides? Physicians’ willingness to use life-sustaining treatment. Archives of Internal Medicine. 1996;156:785–789. doi: 10.1001/archinte.156.7.785. [DOI] [PubMed] [Google Scholar]
- 28.McCrary SV, Swanson JW, Perkins HS, Winslade WJ. Treatment decisions for terminally ill patients: physicians’ legal defensiveness and knowledge of medical law. Law Med Health Care. 1992;20:364–376. doi: 10.1111/j.1748-720x.1992.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 29.Swanson JW, McCrary SV. Medical futility decisions and physicians’ legal defensiveness: the impact of anticipated conflict on thresholds for end-of-life treatment. Social Science & Medicine. 1996;42:125–132. doi: 10.1016/0277-9536(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 30.McCrary SV, Swanson JW. Physicians’ legal defensiveness and knowledge of medical law: comparing Denmark and the USA. Scandanavian Journal of Public Health. 1999;27:18–21. doi: 10.1080/14034949950153850. [DOI] [PubMed] [Google Scholar]
- 31.Pearson SD, Goldman L, Orav EJ, Guadagnoli E, Garcia TB, Johnson PA, Lee TH. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10:557–564. doi: 10.1007/BF02640365. [DOI] [PubMed] [Google Scholar]
- 32.Allison JJ, Kiefe CI, Cook EF, Gerrity MS, Orav EJ, Centor R. The association of physician attitudes about uncertainty and risk taking with resource use in a Medicare HMO. Med Decis Making. 1998;18:320–329. doi: 10.1177/0272989X9801800310. [DOI] [PubMed] [Google Scholar]
- 33.Franks P, Williams GC, Zwanziger J, Mooney C, Sorbero M. Why do physicians vary so widely in their referral rates? J Gen Intern Med. 2000;15:163–168. doi: 10.1046/j.1525-1497.2000.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abelson R. The New York times. The New York Times Company; New York, NY: 2009. Weighing medical costs of end-of-life care. [Google Scholar]
- 35.Gawande A. The New Yorker. Conde Nast; New York, NY: 2009. The cost conundrum: What a Texas town can teach us about health care. [Google Scholar]
- 36.Bertolini G, Boffelli S, Malacarne P, Peta M, Marchesi M, Barbisan C, Tomelleri S, Spada S, Satolli R, Gridelli B, Lizzola I, Mazzon D. End-of-life decision-making and quality of ICU performance: an observational study in 84 Italian units. Intensive Care Med. 2010;36:1495–1504. doi: 10.1007/s00134-010-1910-9. [DOI] [PubMed] [Google Scholar]
- 37.Cook D, Rocker G, Marshall J, Sjokvist P, Dodek P, Griffith L, Freitag A, Varon J, Bradley C, Levy M, Finfer S, Hamielec C, McMullin J, Weaver B, Walter S, Guyatt G. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 2003;349:1123–1132. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. American Journal of Respiratory & Critical Care Medicine. 1998;158:1163–1167. doi: 10.1164/ajrccm.158.4.9801108. [DOI] [PubMed] [Google Scholar]
- 39.Cook DJ, Guyatt GH, Jaeschke R, Reeve J, Spanier A, King D, Molloy DW, Willan A, Streiner DL. Determinants in Canadian health care workers of the decision to withdraw life support from the critically ill. Canadian Critical Care Trials Group. JAMA. 1995;273:703–708. see comments. [PubMed] [Google Scholar]
- 40.Sprung CL, Cohen SL, Sjokvist P, Baras M, Bulow HH, Hovilehto S, Ledoux D, Lippert A, Maia P, Phelan D, Schobersberger W, Wennberg E, Woodcock T. End-of-life practices in European intensive care units: the Ethicus Study. Jama. 2003;290:790–797. doi: 10.1001/jama.290.6.790. [DOI] [PubMed] [Google Scholar]
- 41.Wennberg JE, Fisher ES, Goodman DC, Skinner JS. The Dartmouth Atlas of Health Care 2008. The Dartmouth Institute for Health Policy and Clinical Practice; Hanover, NH: 2008. Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008. [PubMed] [Google Scholar]
- 42.Patton MQ. Qualitative Research and Evaluation Methods. Sage; Newbury Park, CA: 2001. [Google Scholar]
- 43.Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. Journal of the American Geriatrics Society. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 44.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. Journal of the American Medical Association. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 45.Miller WL, Crabtree BF. Primary care research: a multi-typology and qualitative road map. In: Crabtree BF, Miller WL, editors. Doing Qualitative Research. Sage Press; London, England: 1992. [Google Scholar]
- 46.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 47.Lilly CM, De Meo DL, Sonna LA, Haley KJ, Massaro AF, Wallace RF, Cody S. An intensive communication intervention for the critically ill. Am J Med. 2000;109:469–475. doi: 10.1016/s0002-9343(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 48.Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. Chest. 2008;134:835–843. doi: 10.1378/chest.08-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morden NE, Chang CH, Jacobson JO, Berke EM, Bynum JP, Murray KM, Goodman DC. End-of-life care for medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 2012;31:786–796. doi: 10.1377/hlthaff.2011.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azoulay E, Metnitz B, Sprung CL, Timsit JF, Lemaire F, Bauer P, Schlemmer B, Moreno R, Metnitz P. End-of-life practices in 282 intensive care units: data from the SAPS 3 database. Intensive Care Med. 2009;35:623–630. doi: 10.1007/s00134-008-1310-6. [DOI] [PubMed] [Google Scholar]
- 51.Zussman R. Intensive Care: Medical Ethics and the Medical Profession. University of Chicago Press; Chicago, IL: 1992. [Google Scholar]
- 52.Cassell J. Life and Death in Intensive Care. Temple University Press; Philadelphia, PA: 2005. [Google Scholar]
- 53.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life--Updated. Crit Care Med. 2003;31:1551–1557. discussion 1557-1559. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.