Abstract
Objectives
To assess the associations between renal artery calcification (RAC) and mortality in a healthy outpatient cohort without known cardiovascular disease (CVD).
Background
Studies in individuals with known diabetes and kidney disease have suggested that RAC confers additional mortality risk independent of coronary artery calcification, but this has not been explored in healthier populations.
Methods
We assessed RAC by CT scan in 4450 healthy outpatients without known CVD. We used Cox proportional-hazards models to examine the association of RAC with mortality.
Results
The mean age of participants was 57; 42.6% were women. RAC was present in 622 of 4,450 (14%) participants. Over a median follow-up of 8.2 years, 178 deaths occurred. After adjustment for age, gender, diabetes, smoking, cholesterol and family history of CVD, the presence of RAC conferred a more than 60% increased hazard for all-cause mortality (HR 1.63, 95% CI 1.17–2.29). Adjustment for calcification in other vascular beds attenuated this association (HR 1.40, 95% CI 0.99–1.97). Adjustment for hypertension, a potential mediator of the association, did not substantially change the results (HR 1.44, 95% CI, 1.02–2.03). Adding RAC to a model including Framingham risk and CAC improved the predictive ability of the model, from 0.73 to 0.77 (p 0.0002); the net reclassification index was 14.4% for addition of RAC. Results for cardiovascular mortality were not significant, limited by a small number of cardiovascular deaths.
Conclusions
We found that RAC is associated with an increased risk of subsequent all-cause mortality in healthy outpatient individuals, independent of traditional cardiac risk factors. The risk was modestly attenuated by adjustment for vascular calcification in other vascular beds, suggesting partial confounding by systemic calcified atherosclerosis. The effect did not appear to be mediated by hypertension.
Introduction
Among those without clinically apparent cardiovascular disease (CVD), coronary artery calcification (CAC), a marker of subclinical cardiovascular disease, has been established as a risk factor for incident cardiovascular events and mortality (1,2). An increased prevalence of renal arterial disease, including stenosis and calcification, has been noted in persons with coronary artery disease and systemic vascular disease (3,4). Calcification of the renal arteries has also been noted in studies of individuals with overt renovascular disease (5). Despite these known relationships, the association of calcification in the renal vasculature with mortality has not been described. Thus, it is unclear if RAC is simply a marker of systemic vascular calcium burden, or whether it provides independent risk information.
There are reasons to believe that renal artery calcification (RAC) may be an independent risk factor for mortality. RAC is linked with calcification in other vascular beds (6) but is less common than coronary calcification in population based studies. Its presence may thus suggest a different and possibly more severe vascular calcification phenotype. The presence of RAC may also be a marker of atherosclerosis-mediated hypertension with activation of the renin-angiotensin system (7) In support of this link, in asymptomatic individuals RAC has been associated with prevalent hypertension independent of the presence or severity of calcification in other vascular beds (8). Additionally, RAC may be associated with kidney dysfunction, which has well-documented associations with cardiovascular disease and mortality (9). In select populations with diabetes and proteinuric kidney disease, the presence of RAC has been associated with more rapid progression of kidney disease and with mortality (10). To our knowledge, however, no previous study has evaluated the association between RAC and mortality in the general population.
Using data from a large cohort of adults without CVD who underwent whole-body computed tomography (CT) scans primarily for cardiac risk assessment, we investigated the relationship between RAC and all-cause mortality. We hypothesized that RAC would be associated with mortality, independent of traditional cardiovascular risk factors. We suspected that this association would be independent of systemic calcification. Given the association between RAC and hypertension, we hypothesized that hypertension would at least partially mediate this association.
Methods
Subjects
We studied a cohort of community-dwelling individuals (total n = 4950) who were seen for preventive medicine services including whole-body CT scanning at a university-affiliated center in La Jolla, CA between 1999 and 2003. This cohort has been described in detail previously (8,11). In brief, this was a population where subjects either self-referred or were referred on the recommendation of their personal physician as an adjunct to their standard preventive health care. The majority of these individuals (99%) did not have known coronary disease at the time of the visit. The visit included an assessment of standard cardiac risk factors and whole-body CT scans extending from the carotid arteries to the symphsysis pubis. Coronary calcium was scored at the time of the preventive visit and reported to the subject. Calcification in other vascular beds was scored for research purposes, after the visit had taken place.
All patients completed a detailed health history, including self-reported medication use for hypertension, diabetes, or high cholesterol, and had serum lipid and glucose measurements obtained by finger stick using the Cholestec LDX system. Participants were included whether or not they were not fasting at the time of the laboratory tests. We excluded individuals with a history of cardiovascular disease (myocardial infarction, stroke, transient ischemic attack, angina, or coronary revascularization; n = 45) those whose scans did not allow scoring of renal artery calcium data (n =452), and those for whom vital status could not be determined (n=3) leaving 4,450 individuals for this analysis.
Because this study involved de-identified, existing data, a waiver of informed consent was granted by the UCSD Institutional Review Board, which approved the study.
Clinical, laboratory, and history measures
Blood pressure was measured in the right upper extremity after the patient had rested in the seated position for 5 minutes. Hypertension was defined as a systolic blood pressure ≥140, DBP ≥90 and/or reported use of anti-hypertensive medication. Diabetes mellitus was defined by current use of antiglycemic medications or a casual glucose level >200 mg/dL. Individuals with a total:high-density lipoprotein cholesterol ratio >5 and/or those who reported the use of a medication to treat high cholesterol were classified as dyslipidemic. Family history of CHD was defined as a CHD event before age 55 in a first-degree relative. Smoking status was classified as current, former, or never.
Imaging
An Imatron C-150 scanner (GE) was used to obtain computed tomography (CT) scans from the base of the skull to the pubic symphysis. All images were obtained during a single session using 100-millisecond scan time and proceeded caudally from the base of the skull to the symphysis pubis. Each bed was obtained by a distinct scan of the segment in question using the following slice thicknesses: 3 mm for the coronary bed; 6 mm through the neck, abdomen, and pelvis; and 5 mm for the thorax. We obtained measurements of calcified atherosclerosis in the following vascular beds: coronary, carotid, thoracic aorta, abdominal aorta, renal and iliac arteries. The coronary and carotid images were analyzed at the time of the CT scan; other vascular beds were analyzed retrospectively. Calcium scoring in the different vascular beds was performed using the method described by Agatston et al. (12) Calcium in the renal arteries was categorized as arising from the ostium or within the renal arterial segment on each side. We used the sum of the right and left ostial and arterial segments of the renal arteries to calculate a RAC score. Similar sums were used to calculate calcium burden in the other vascular beds. The number of vascular beds other than the renal arteries with calcium present was then tabulated. The reader for renal artery calcium only viewed images that contained the renal arteries and this individual was blinded to the scores for the other beds.
Mortality outcomes
All-cause mortality was determined through August 31, 2009 using Social Security Death Index searches to determine mortality status for all individuals in the cohort. Date and cause of death was obtained from death certificate information.
Statistical Analysis
To examine demographic associations with the presence of RAC, we dichotomized this variable based on presence (score greater than zero) or absence (score equal to zero) of any RAC. Differences in the distributions of baseline characteristics between those with and without RAC were described using t or Chi-square tests, as appropriate.
To examine the effects of the burden of RAC, we further divided those with non-zero RAC scores into quintiles. For graphical modeling, we compared each of these quintiles of RAC to the group with no RAC. Additionally, we examined multivariate models using the continuous RAC score among those with RAC; since the distribution of RAC scores was highly skewed, we log-transformed this score for mortality analyses, setting the log-score of those without calcium to zero.
We examined Kaplan-Meier curves to visually inspect the mortality rates of those with or without RAC. Subsequently, Cox-proportional hazards models were constructed to examine the adjusted association between RAC and all-cause and cardiovascular mortality. Initial models were adjusted for age and gender. A second model was adjusted for most traditional risk factors (former or current smoking, dyslipidemia, diabetes, family history of CVD). A third model adjusted for the CAC score (modeled as log(CAC + 1)). We then adjusted for atherosclerosis in other vascular beds (carotid, thoracic and abdominal aorta, and iliac arteries), using the number of other beds affected divided by the number of other beds with scorable calcium (e.g. percentage of other beds affected) as an adjustment variable. We tested for heterogeneity in the association of RAC on mortality by age and gender.
We postulated that the association between RAC and mortality might be mediated by hypertension, since hypertension can be caused by atherosclerotic renovascular disease, and since hypertension itself is then a risk factor for mortality. Thus, we examined whether hypertension mediated the association between RAC and mortality. We developed nested models, first without, and then with adjustment for hypertension and examined the change in the hazard ratios of RAC.
We calculated hazard ratios and 95% confidence intervals for each estimate. We also tested for proportionality of hazards by visual examination of log(−log) survival curves. All of the P values were 2 tailed, with a P value of <0.05 considered to indicate statistical significance.
Last, we developed receiver-operator characteristic (ROC) curves for prediction of all-cause mortality and cardiovascular mortality. Because our outcome of interest was mortality rather than cardiovascular events, our base model included the calculated Framingham hard CHD score as this best approximates mortality rather than event-based risk; (13) (14) we chose risk cut-offs of 5% and 15% for the low, intermediate, and high groups, respectively, as standard predefined thresholds have not been established for all-cause mortality. Additional models added CAC, RAC or both (modeled as log(CAC+1) and log(RAC+1))to the base model. We additionally calculated the integrated discrimination index (IDI) and net reclassification index (NRI) (15) for adding CAC, RAC, and both to these models and tested whether these improvements were statistically significant.
All of the statistical analyses were conducted using SAS (version 4.3, SAS Institute, Cary, NC).
Results
Of the 4450 person study sample, 622 (14%) had RAC. These individuals were older (mean age 68.8 vs. 54.8), and more likely to have diabetes, hyperlipidemia, and a smoking history than those without RAC (Table 1). Individuals with RAC were also more likely to be have systolic hypertension and to have calcification in other vascular beds; those with RAC had on average 4.3 other vascular beds with calcification present, while those without RAC had 1.9 other vascular beds with calcification present. (6,8)
Table 1.
Participant characteristics by presence/absence of renal artery calcification
| No RAC (N=3828) | RAC present (N = 622) | p value | ||
|---|---|---|---|---|
|
| ||||
| age, mean (SD) | 54.8 (10.3) | 68.8 (8.7) | <0.0001 | |
|
| ||||
| female n(%) | 1656 (43.2) | 243 (39.0) | 0.05 | |
|
| ||||
| Total Cholesterol mean (SD) | 208.2 (42.0) | 202.6 (39.4) | 0.004 | |
|
| ||||
| HDL mean (SD) | 53.2 (17.3) | 51.7 (16.7) | 0.052 | |
|
| ||||
| LDL mean (SD) | 122.1 (36.9) | 116.8 (35.7) | 0.002 | |
|
| ||||
| Systolic BP mean (SD) | 124.3 (16.5) | 131.4 (16.0) | <0.0001 | |
|
| ||||
| Diastolic BP mean (SD) | 78.1 (10.3) | 78.2 (10.4) | 0.79 | |
|
| ||||
| Renal artery calcium score (median, IQR) | 0 | 44 (17,115) | ||
|
| ||||
| Number of other vascular beds with calcium (SD) | 1.9 (1.7) | 4.3 (1.06) | < 0.001 | |
|
| ||||
| CAC score (Agaston units) | 1.03 [0, 62.40] | 218 [17.02, 769.20] | < 0.001 | |
|
| ||||
| Smoker, current n(%) | 360 (9.4) | 63 (10.1) | < 0.001 | |
| former | 1229 (32.1) | 310 (49.8) | ||
| never | 2239 (58.4) | 249 (40.0) | ||
|
| ||||
| Family hx CHD n(%) | 915 (23.9) | 162 (26.0) | 0.25 | |
|
| ||||
| Diabetes n(%) | 70(1.8) | 16 (2.6) | 0.21 | |
|
| ||||
| Hypertension n(%) | 1160 (30) | 316 (50) | <0.0001 | |
|
| ||||
| Medication use for hypertension n(%) | 526(13.7) | 201(32.3) | < 0.0001 | |
|
| ||||
| Medication use for hyperlipidemia n(%) | 461(12.0) | 130(20.9) | < 0.0001 | |
|
| ||||
| BMI mean(SD) | 27.1 (5.1) | 26.9(4.1) | 0.21 | |
|
| ||||
| Framingham CHD risk score (SD) | 5 [2, 12] | 12 [6, 20] | 0.001 | |
|
| ||||
| Deceased n(%) | 96 (2.5) | 82 (13.2) | <0.0001 | |
p for t-test (continuous) or chisq (categorical) as appropriate
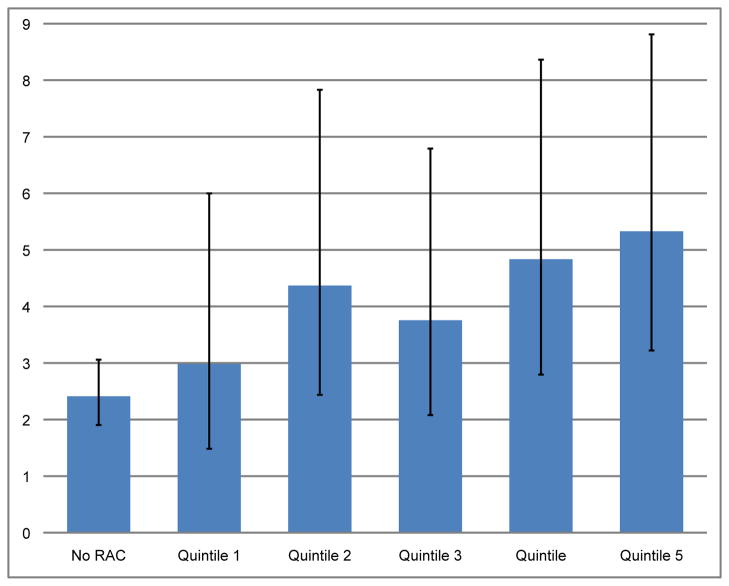
In total, 178 deaths of which 41 were cardiovascular deaths occurred over a median 8.2 years of follow-up; those with greater burden of RAC had lower survival rates (figure 1, log-rank p value for trend, < 0.001). Among those without RAC, the age-adjusted mortality rate was 2.3 per 1000 person years (95% CI, 1.88 to 3.02), and among those with RAC the age-adjusted mortality rate was 4.24 per 1000 person-years (95%CI, 2.97 to 6.07). There was a significant trend toward increasing age-adjusted mortality rates with increasing quintiles of RAC (p < 0.001, figure 2).
Figure 1. Kaplan-Meier curves for mortality at different levels of RAC.
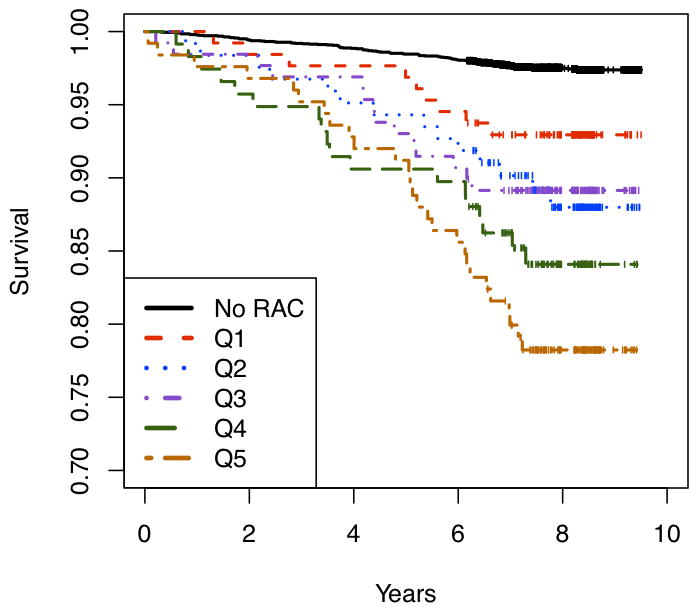
Mortality curves over time for those with no RAC and increasing levels of RAC as follows: 0 = no RAC; quintile 1, 1–13 AU; quintile 2, 14–29 AU; quintile 3, 30–66 AU; quintile 4, 67–133 AU; quintile 5, 134–779 AU. The log-rank p test for trend was < 0.01. (AU = Agatston units)
Figure 2. Age-adjusted rates of all-cause mortality at different levels of RAC.
Yearly mortality rates per 1000 person-years, adjusted for age, corresponding to the categories of RAC as in figure 1.
In models adjusted for age and gender, the presence of RAC was associated with a 76% higher risk of death (Table 3). After further adjustment for demographic and cardiac risk factors the presence of RAC still conferred a more than 60% increase in mortality risk, and remained associated with a nearly 40% increase in risk (HR 1.40, 95% CI, 0.99 to 1.97) after adjustment for vascular calcification in other locations.
Table 3.
Hazard of all-cause mortality and cardiovascular mortality at different levels of renal artery calcification
| All-cause mortality (272 deaths) | |||||
|---|---|---|---|---|---|
| Age-gender adjusted HR (95% CI) | risk adjusted* HR (95% CI) | CAC-adjusted | Fully adjusted*** HR (95% CI) | Putative mediator adjusted **** | |
| RAC (yes/no) | 1.76 (1.26, 2.45) | 1.63 (1.17, 2.29) | 1.49 (1.06–2.11) | 1.40 (0.99, 1.97) | 1.44 (1.02, 2.03) |
| Cardiovascular mortality (41 deaths) | |||||
|---|---|---|---|---|---|
| Age-gender adjusted HR (95% CI) | risk adjusted* HR (95% CI) | CAC-adjusted HR (95% CI) | Fully adjusted** HR (95% CI) | Putative mediator adjusted *** | |
| RAC (yes/no) | 1.42 (0.73, 2.78) | 1.23 (0.61, 2.44) | 1.05 (0.52, 2.10) | 0.95 (0.48, 1.88) | 1.01 (0.50, 2.01) |
adjusted for smoking, hypercholesterolemia, diabetes, and family history of CVD
additionally adjusted for log(CAC + 1)
additionally adjusted for percentage of other beds with vascular calcification
fully adjusted, with additional adjustment for presence of hypertension
In age and sex-adjusted proportional hazards models, each log-increase in RAC score among the 622 participants with any RAC present conferred a modest increase in the hazard of mortality (HR 1.18, 95% CI 0.98, 1.4). Additional adjustment for cardiovascular risk factors and number of other calcified beds further attenuated this association (HR 1.14, 95% CI 0.94–1.38).
When we evaluated the possible mediating effect of hypertension, we found little attenuation of mortality risk by hypertension, regardless of whether hypertension was added prior to or after addition of other adjustment variables. For example, adjusting for hypertension after adjustment for other traditional risk factors (after model 2, table 3) changed the hazard ratio associated with the presence of RAC minimally from 1.63 (1.17–2.29) to 1.66 (1.18–2.34); adding hypertension at the end of the modeling process had a similar minimal effect on the hazard associated with the presence of RAC. The ROC models (figure 3) adding CAC and RAC to a predictive model including the hard FRS demonstrated improvement in the c-statistic, IDI, and NRI with sequential addition of CAC and RAC (table 4). Adding RAC to a model including hard FRS and CAC generated an improvement in c-statistic from 0.73–0.77, (p 0.0002). The NRI for the model including RAC compared to that including FRS and CAC was 14.4% (p for comparison, 0.002).
Figure 3. Receiver-operator curves for prediction of all-cause mortality using Framingham score, CAC, and RAC.
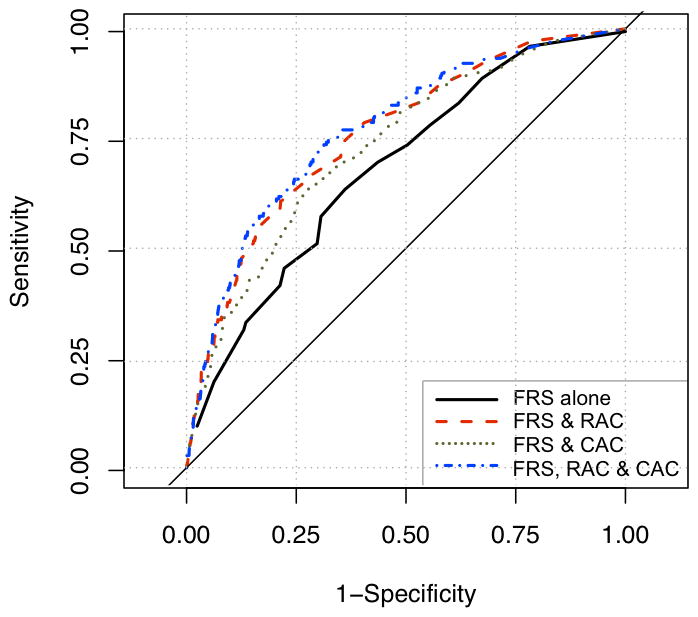
Area under the ROC curve for Framingham score (FRS) alone: 0.69; for FRS plus CAC: 0.73; for FRS, CAC, and RAC together: 0.77. p for comparisons between ROC statistics all < 0.01.
Table 4.
c-Statistics, IDI, and NRI for the assessment of CAC and RAC in addition to FRS in predicting all-cause and cardiovascular mortality
| All-cause Mortality | Cardiovascular Mortality | |||||
|---|---|---|---|---|---|---|
| c-Statistics | IDI | NRI | c-Statistics | IDI | NRI | |
| 1. Base model alone (hard FRS) | 0.69 (0.65–0.73) | / | / | 0.77 (0.705–0.831) | / | / |
| 2. Base plus CAC | 0.73 (0.694–0.77) | 0.016 vs #1 | 20.8% vs #1 | 0.83 (0.774–0.880) | 0.011 | 12.5% vs #1 |
| p values (vs. model 1) | 0.001 | < 0.0001 | < 0.0001 | 0.049 | 0.001 | 0.05 |
| 3. Base plus CAC plus RAC | 0.77 (0.74–0.81) | 0.036 vs #1 0.020 vs #2 |
30.3% vs. #1 14.4% vs #2 |
0.84 (0.79–0.89) | 0.018 vs #1 0.008 vs #2 |
14.2% vs #1 1.7% vs #2 |
| p values (vs model 1) | < 0.0001 | < 0.0001 | < 0.0001 | 0.03 | 0.001 | 0.06 |
| p values (vs. model 2) | 0.0002 | < 0.0001 | 0.002 | 0.134 | 0.002 | 0.75 |
IDI = integrated discrimination improvement; NRI = net reclassification index.
A set of parallel models were conducted for cardiovascular mortality. There were 41 confirmed cardiovascular deaths available for analysis. Table 3 shows Cox-proportional hazard regression models, which demonstrate attenuation of the risk for cardiovascular mortality with relatively wide confidence limits after addition of successive adjustment variables. Similarly, table 4 shows c-statistic, IDI, and NRI analyses for cardiovascular mortality; although the addition of RAC to these models improved these values the improvements did not reach statistical significance.
Discussion
Renal arterial calcification was a relatively common occurrence in this cohort of asymptomatic adults, affecting 14% of the cohort, and was associated with a substantial increase in mortality risk even after adjustment for demographic risk factors and traditional cardiovascular risk factors. RAC was more prevalent in those with vascular calcification in other beds, and adjustment for the presence of other vascular calcification and hypertension attenuated but did not eliminate the association between RAC and mortality. Despite this adjustment, individuals with RAC remained at approximately 50% greater mortality risk.
We considered whether hypertension was a potential mediator of the remaining association between RAC and mortality. In these analyses, hypertension had a trivial effect on the association regardless of when it was added to the models, suggesting that the association between RAC and mortality was independent of hypertension. Prior angiographic studies have demonstrated that RAC is not strongly associated with overt, flow limiting renal arterial disease (15). Our data support the hypothesis that the independent effect of RAC on mortality is not entirely explained by decreased blood flow to the kidney and corresponding increases in blood pressure as mediated by the renin-angiotensin system, but is instead part of a systemic vascular calcification phenotype in which vascular calcification and hypertension may be parallel processes.
In prior studies, RAC was strongly associated with calcification in other vascular beds in those with diabetes (16), in those with overt renovascular disease (17), and in those without clinical cardiovascular disease (6). These findings suggest that RAC is likely part of a systemic process and contributes to mortality risk perhaps by marking the severity of the atherosclerotic disease burden.
We are aware of one small study examining RAC and mortality, which evaluated a cohort of 172 individuals with diabetes and kidney disease (10). In that study, RAC had significant associations with a combined outcome of ESRD and mortality even after adjustment for coronary artery calcification (CAC). The participants in that study did not have measures of calcification in vascular beds other than the coronary and renal beds, and that the prevalence of both RAC and CAC were substantially higher than in our study (31% and 74% respectively). That study reported only a combined end-point, with most events being progression to ESRD, and separate analyses of mortality outcomes were not presented. Our study extends the finding of an independent association of RAC with mortality to a healthy, outpatient population with relatively low prevalence of RAC, and to non-diabetic individuals.
Our findings should be interpreted in the context of existing data regarding non-coronary vascular calcification. While prior studies have shown that CAC is associated with both cardiovascular and all-cause mortality (18,19), findings for non-coronary calcium have not been entirely consistent and appear to depend on the vascular bed and outcome studied. Thoracic calcification, for example, has been associated with total mortality and CVD events (20,21); abdominal aortic calcification has been associated with CVD and CVD-related mortality (22). While our findings were suggestive of a stronger association between renal artery calcification and all-cause mortality rather than cardiovascular mortality, the number of cardiovascular deaths was relatively small and so the lack of significant association seen in this study does not rule out the possibility of an association with CVD mortality. It is also possible that the presence of RAC makes individuals more hemodynamically fragile and less likely to survive non-cardiac illnesses, thus explaining the substantially higher all-cause mortality rates in those with RAC.
The clinical utility of whole-body CT screening, with analysis of RAC as part of a multi-bed analysis, is still uncertain and these cannot yet be recommended as part of routine risk stratification. Whole-body CT scans expose the individual to substantial radiation (23) and incur the added risk of incidental findings requiring further evaluation and invasive management. Our data suggest, however, that determination of RAC may add to the prognostic information contained in such scans above that gleaned from analysis of standard Framingham risk factors and of calcification in other vascular beds.
The strengths of our study include a large sample size, the availability of calcification in multiple vascular beds, and the long follow-up time for mortality. We were able to adjust for standard CVD risk factors as well as detailed measures of vascular calcification in the coronary arteries as well as in other beds.
Limitations include the use of a self-referred cohort of patients rather than a community sample. These participants did not have estimated glomerular filtration (eGFR) or proteinuria measured at the time of the whole-body scan, and so we do not have renal function measures to correlate with RAC. If eGFR or proteinuria are true confounders of the association between RAC and mortality, however, one would have to postulate that decreased kidney function leads to both RAC and mortality. We do not know of supportive data for this hypothesis. RAC might itself cause decreased eGFR which then is a risk factor for mortality (mediation); we cannot assess this using the available data. History of CVD and of CVD risk factors was by self-report and was not confirmed via review of medical charts. We do not have information about the ethnic background of the participants, nor do we have information about peripheral vascular disease, other comorbid diseases, or details of exercise habits. We cannot exclude the possibility of residual confounding because of these limits on the covariates available to us.
In conclusion, we found that RAC was associated with all-cause mortality independent of traditional risk factors. The association was attenuated but was still present after adjustment for vascular calcification in other locations and was minimally attenuated by adjustment for hypertension. Adding RAC to a predictive model of all-cause mortality using the Framingham hard CHD score and the CAC score improved the predictive value of the model. For cardiovascular mortality, our findings were limited by the number of cardiovascular deaths and Cox models did not demonstrate significance after multivariate adjustment.
Further work should examine whether similar associations are seen in groups with a higher prevalence of diabetes and vascular disease, as the findings may differ across populations, and should investigate the associations of RAC with CVD events and CVD mortality in these higher risk populations.
Table 2.
Univariate Hazard Ratios of Mortality
| Variable | Univariate Hazard of Mortality (95% CI) |
|---|---|
|
| |
| Age (per year) | 1.11 (1.10–1.13) |
| Male gender | 0.98 (0.73–1.32) |
| Diabetes Mellitus | 2.47 (1.22–5.0) |
| Hypertension (presence) | 2.17 (1.62–2.91) |
| Hyperlipidemia (presence) | 0.99 (0.73–1.35) |
| Current smoking | 1.65(1.09–2.50) |
| Family history of CVD | 1.09 (0.78, 1.5) |
| BMI (per kg/m2) | 0.99 (0.96, 1.02) |
| Coronary Artery Calcification (presence) | 3.60 (2.44, 5.25) |
Acknowledgments
This study was funded by grants from the American Heart Association (M.A.A).
Footnotes
The authors have no relevant financial or industry-related conflicts of interest to disclose regarding this manuscript.
References cited
- 1.Achenbach S, Nomayo A, Couturier G, Ropers D, Pohle K, Schlundt C, et al. Relation between coronary calcium and 10-year risk scores in primary prevention patients. Am J Cardiol. 2003 Dec 15;92(12):1471–5. doi: 10.1016/j.amjcard.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary Artery Calcium Score Combined With Framingham Score for Risk Prediction in Asymptomatic Individuals. JAMA: The Journal of the American Medical Association. 2004 Jan 14;291(2):210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 3.Harding MB, Smith LR, Himmelstein SI, Harrison K, Phillips HR, Schwab SJ, et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol. 1992 May;2(11):1608–16. doi: 10.1681/ASN.V2111608. [DOI] [PubMed] [Google Scholar]
- 4.Gross CM, Krämer J, Waigand J, Luft FC, Dietz R. Relation between arteriosclerosis in the coronary and renal arteries. Am J Cardiol. 1997 Dec 1;80(11):1478–81. doi: 10.1016/s0002-9149(97)00727-3. [DOI] [PubMed] [Google Scholar]
- 5.Olin JW, Melia M, Young JR, Graor RA, Risius B. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990 Jan;88(1N):46N–51N. [PubMed] [Google Scholar]
- 6.Allison MA, DiTomasso D, Criqui MH, Langer RD, Wright CM. Renal artery calcium: relationship to systemic calcified atherosclerosis. Vascular Medicine. 2006 Nov 1;11(4):232–238. doi: 10.1177/1358863x06073449. [DOI] [PubMed] [Google Scholar]
- 7.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003 Nov 4;139(9):761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 8.Allison MA, Lillie EO, DiTomasso D, Wright CM, Criqui MH. Renal artery calcium is independently associated with hypertension. J Am Coll Cardiol. 2007 Oct 16;50(16):1578–83. doi: 10.1016/j.jacc.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.Chiu Y-W, Adler S, Budoff M, Takasu J, Ashai J, Mehrotra R. Prevalence and Prognostic Significance of Renal Artery Calcification in Patients with Diabetes and Proteinuria. Clinical Journal of the American Society of Nephrology. 2010 Nov 1;5(11):2093–2100. doi: 10.2215/CJN.03730410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison MA, Criqui MH, Wright CM. Patterns and Risk Factors for Systemic Calcified Atherosclerosis. Arterioscler Thromb Vasc Biol. 2004 Feb 1;24(2):331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 12.Agatston A, Janowitz W, Hildner F, Zusmer N, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar 15;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA: The Journal of the American Medical Association. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001 Jul 11;286(2):180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008 Jan 30;27(2):157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 16.Gayard P, Garcier JM, Boire JY, Ravel A, Perez N, Privat C, et al. Spiral CT quantification of aorto-renal calcification and its use in the detection of atheromatous renal artery stenosis: A study in 42 patients. Cardiovasc Intervent Radiol. 2000 Feb;23(1):17–21. doi: 10.1007/s002709910003. [DOI] [PubMed] [Google Scholar]
- 17.Freedman BI, Hsu F-C, Langefeld CD, Bowden DW, Moossavi S, Dryman BN, et al. Renal artery calcified plaque associations with subclinical renal and cardiovascular disease. Kidney Int. 2004 Jun;65(6):2262–7. doi: 10.1111/j.1523-1755.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- 18.Wright JR, Shurrab AE, Cheung C, Waldek S, O’Donoghue DJ, Foley RN, et al. A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis. 2002 Jun;39(6):1153–61. doi: 10.1053/ajkd.2002.33384. [DOI] [PubMed] [Google Scholar]
- 19.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary Artery Calcium to Predict All-Cause Mortality in Elderly Men and Women. J Am Coll Cardiol. 2008 Jul 1;52(1):17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007 May 8;49(18):1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 21.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2011 Mar;215(1):196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong ND, Gransar H, Shaw L, Polk D, Moon JH, Miranda-Peats R, et al. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009 Mar;2(3):319–26. doi: 10.1016/j.jcmg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Wilson PWF, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal Aortic Calcific Deposits Are an Important Predictor of Vascular Morbidity and Mortality. Circulation. 2001 Mar 20;103(11):1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 24.Jablon S, Bailar JC., III The contribution of ionizing radiation to cancer mortality in the United States. Preventive Medicine. 1980 Mar;9(2):219–26. doi: 10.1016/0091-7435(80)90079-1. [DOI] [PubMed] [Google Scholar]