Type 2 diabetes mellitus is caused by a combination of genetic and environmental factors that result in decreased insulin function at sites of insulin action and a reduced ability of pancreatic beta cells to elevate insulin secretion in response to increased blood glucose levels. The variant genes that cause susceptibility to diabetes were virtually unknown until the advent of genomewide association studies. In 2007, one such study identified associations between type 2 diabetes and six different chromosomal loci.1 The company deCODE Genetics subsequently confirmed these associations and identified an association with variant CDKAL1.2 Subsequent studies increased the number of implicated loci to about 40. Unfortunately, this plethora of loci has not yielded a proportionate improvement in our understanding of disease mechanisms, partly because genomewide association studies often implicate genes (or nongenic regions) of unknown function, such as CDKAL1.
Occasionally, discoveries from disparate fields merge to provide new conceptual frameworks that improve our mechanistic understanding of disease. A recent example is provided in two studies, by Arragain et al.3 and Wei et al.,4 that link CDKAL1 with protein translation and show how this link may be relevant to type 2 diabetes.
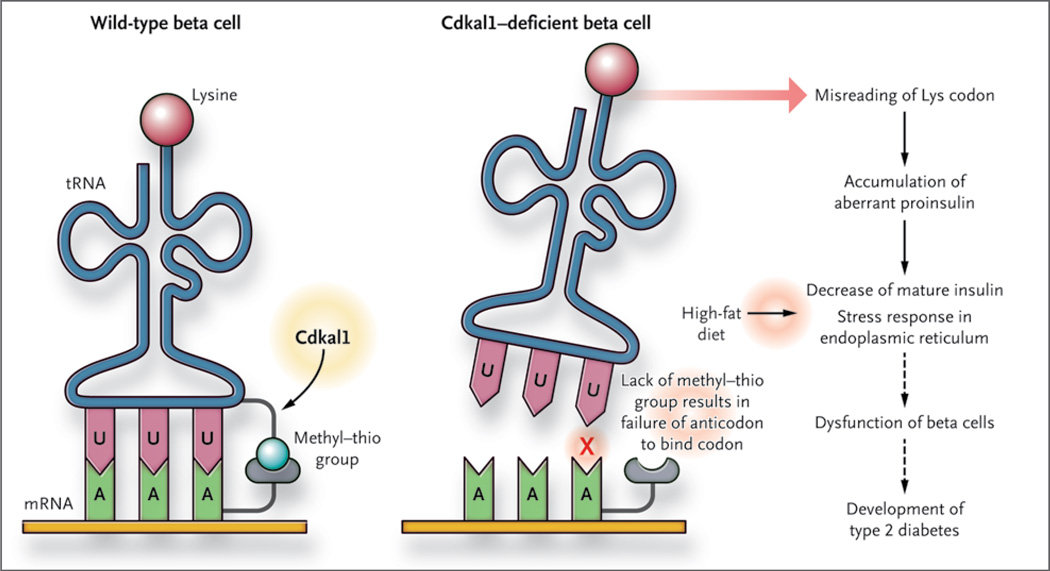
It was previously established that some bacterial and eukaryotic transfer RNAs have a specific moiety (the methyl–thio group ms2t6) attached to the adenosine residue that is adjacent to the anticodon (Fig. 1). The presence of this moiety on transfer RNAs is essential for faithful translation from messenger RNA to protein. Arragain et al. report that CDKAL1 is an enzyme (a methylthiotransferase) and that it adds the moiety to the adenosine residue. In the research group’s most recent study, Wei et al. report that CDKAL1 targets a specific transfer RNA — tRNALys(UUU) (Fig. 1) — which adds a lysine residue during protein synthesis (i.e., during translation from messenger RNA to protein) (Fig. 2). In other words, CDKAL1 seems to have a global effect on protein production by ensuring faithful translation of the AAA codon (encoding lysine) through its mediation of a highly specific event (the modification of a specific amino acid residue of tRNALys[UUU]). The investigators went on to abrogate Cdkal1 in the beta cells of mice and observed a diminished insulin response to an intra-peritoneal injection of glucose. These events were exacerbated in mice that were fed a high-fat diet for several weeks.
Figure 1. Tinkering with Transfer RNA.
Type 2 diabetes is associated with variant CDKAL1. Wei et al.4 recently ascribed a function to this gene: it critically modifies a specific transfer RNA (tRNA) by catalyzing the addition of a methyl–thio moiety (ms2t6) to a residue adjacent to the anticodon (in pink). Mice deficient in beta-cell Cdkal1 incorporate fewer lysine residues into proinsulin, which may contribute to the susceptibility of these mice to type 2 diabetes.
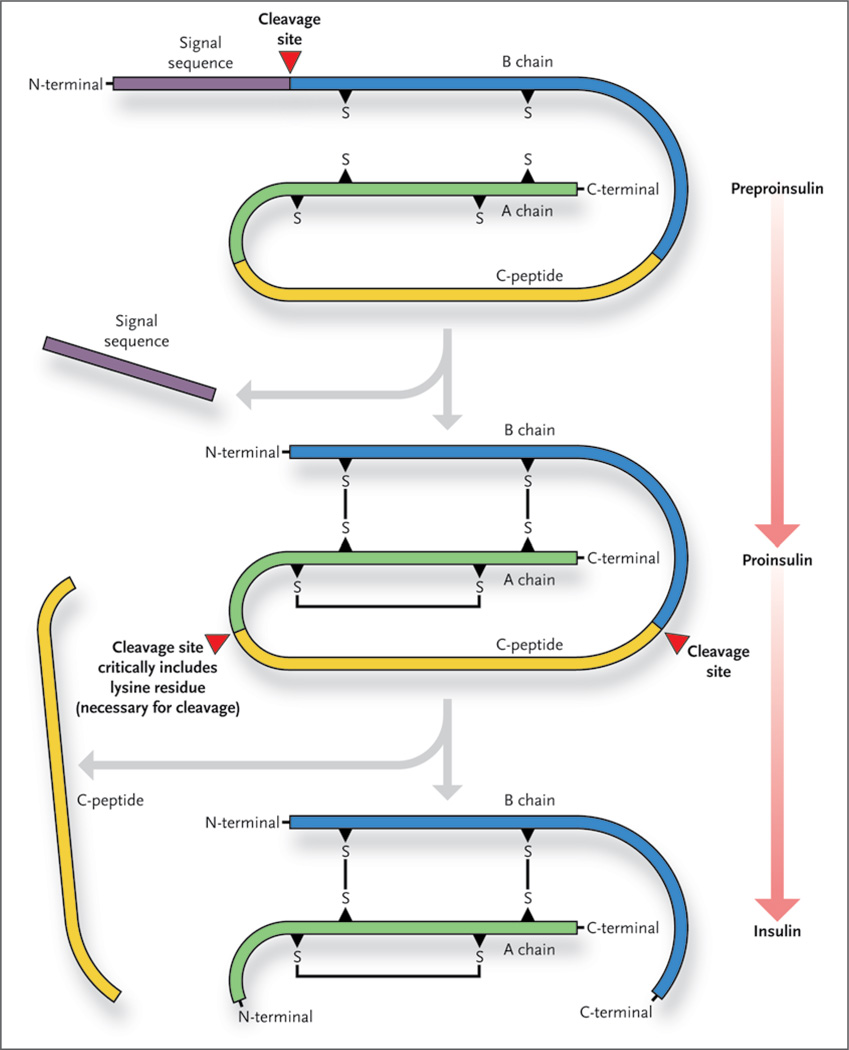
Figure 2. Interfering with Insulin Production.
The production of mature insulin takes place within the beta cell and depends on the cleavage of the preproinsulin and proinsulin molecules. The cleavage site at the junction of the A chain and the C-peptide contains a lysine residue, which is critical for cleavage.
How does an abrogation of Cdkal1 in the beta cell result in a feeble insulin response? One of the most attractive hypotheses is the apparent failure of the mutant beta cell to process the proinsulin protein into insulin. The authors found that proinsulin in the mutant cells had a lower lysine content than proinsulin in wild-type cells. They also found that levels of C-peptide (a by-product of proinsulin processing) were lower in the islets and serum of the mutant mice. Linking these two observations is the fact that lysine makes up the cleavage site between C-peptide and the A chain of insulin (Fig. 2). Thus, a deficiency of lysine content in proinsulin would be predicted to result in a molecule that is resistant to cleavage at the junction between the C-peptide and the A chain.
However, it is clear that the general health of the mutant beta cell is also compromised. For example, the authors observed an increase in expression of stress molecules in the endoplasmic reticulum. Such stress is likely to be caused by an increased number of proteins (including insulin) that are misfolded because they are deficient in lysine. Moreover, there is scanty cell-surface expression of the Glut2 receptor on the mutant beta cell. (Glut2 transports extracellular glucose into the cell, which sets off a chain of events that culminate in the mobilization of insulin-containing granules.) All, some, or none of these events may be pivotal to the diabetic phenotype of the mutant mice.
The authors have provided much-needed insight into how variant CDKAL1 may cause susceptibility to type 2 diabetes. Several questions may be addressed through future study. The authors propose that a failure to incorporate lysine results in the misfolding of proinsulin and prevents proteolytic processing. Is this indeed the case, and if it is, what amino acids (if any) replace lysine? If a general deficiency of lysine incorporation into protein causes protein misfolding, perhaps the cellular response to protein misfolding affects the production of insulin. There are now a number of examples in which protein misfolding in the beta cell prevents proinsulin processing and appropriate trafficking between the endoplasmic reticulum and the Golgi apparatus, thus causing stress and a disruption in mitochondrial structure. Because CDKAL1 is strongly expressed in the endoplasmic reticulum, its own misfolding (assuming that such occurs) may affect other folding, processing, or quality-control events that may cause an accumulation of unfolded protein and beta-cell death. In a broader aspect, there are now many associations between oxidative stress and beta-cell failure. It is interesting to consider that CDKAL1, owing to its composition, may be exquisitely sensitive to oxidation. As the molecular mechanisms of CDKAL1 are revealed, researchers may consider small-molecule mediators to prevent the progression of type 2 diabetes in patients who carry CDKAL1 risk variants.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 2.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 3.Arragain S, Handelman SK, Forouhar F, et al. Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2-methylthio-N6-threonylcarbamoyladenosine in tRNA. J Biol Chem. 2010;285:28425–28433. doi: 10.1074/jbc.M110.106831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei FY, Suzuki T, Watanabe S, et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121:3598–3608. doi: 10.1172/JCI58056. [DOI] [PMC free article] [PubMed] [Google Scholar]