Abstract
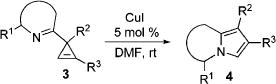
A highly efficient regiodivergent method for the synthesis of N-fused heterocycles via transition-metal-catalyzed rearrangement of 3-iminocyclopropenes has been developed.
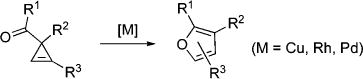
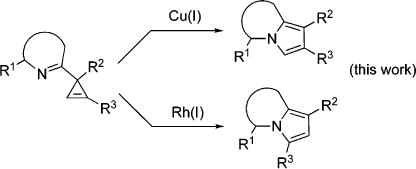
Transition-metal-catalyzed chemistry of cyclopropenes1 benefits from their enormous ring strain.2 The highly reactive double bond enjoys a variety of addition3 and cycloaddition4 reactions,5 while rearrangements allow for construction of various carbo-6 and heterocycles.6c–g,7 Thus, there are several reports on the metal-catalyzed rearrangements of 3-acylcyclopropenes into furans (eq 1).6c–g,7 However, to the best of our knowledge, an analogous metal-catalyzed construction of N-containing heterocycles has no precedents.8 Herein, we wish to report the first example of a regiodivergent Cu- and Rh-catalyzed rearrangement of 3-iminocyclopropenes into N-fused pyrroles, heterocyclic scaffolds endowed with a wide array of important biological properties (eq 2).9
 |
(1) |
 |
(2) |
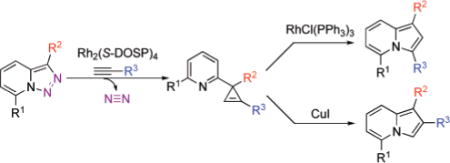
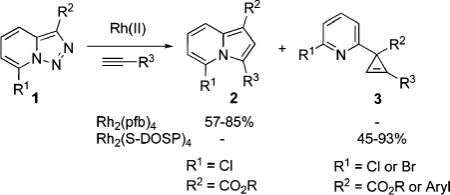
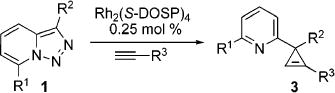
It deserves mentioning that, until recently, there were no convenient approaches toward C-3 imino-substituted cyclopropenes, potentially useful building blocks for organic chemistry.1 Recently, we found10 that 7-halo-substituted N-fused triazoles 1 could be used as surrogates for α-imino diazocompounds11 in the Rh(II)-catalyzed chemoselective reaction with terminal alkynes to produce indolizines 2 or 3-(2-pyridyl)cyclopropenes 3, depending upon catalyst source (eq 3).10 The presence of the halogen substituent in 1 was crucial, as no reaction occurred with triazoles possessing H or alkyl groups at C-7.10 Although the direct Rh(II) perfluorobutyrate-catalyzed transannulation of triazoles provided a rapid and convenient approach toward indolizines,10 it was not without limitations. Thus, only triazoles that possessed strong electron-withdrawing group at C-3 (R2 = CO2R) were efficient in this transannulation reaction. We hypothesized that, potentially, the rearrangement of 3-(2-pyridyl)cyclopropenes 3 could provide alternative routes to indolizines 2 as shown in eq 2.
 |
(3) |
To this end, we tested the generality of the Rh2(S-DOSP)2-catalyzed cyclopropenation of triazoles with alkynes. To our delight, we found that a variety of pyridyl-containing cyclopropenes can easily be synthesized in good yields via this method (Table 1).12 Thus, triazoles 1a–d possessing both electron-rich and electron-deficient aryl substituents at C-3 reacted smoothly with various alkyl-, aryl-, and alkenyl-containing alkynes to afford corresponding cyclopropenes 3 chemoselectively (Table 1). Cyclopropenation of 3-carbomethoxytriazole 1e proceeded uneventfully, producing corresponding cyclopropenes 3 in good to excellent yields (entries 9–12, 14).
Table 1.
Rh2(S-DOSP)4-Catalyzed Cyclopropenation of Pyridotriazoles with Alkynes
| no. | R1 | R2 | R3 | yield,a % | ||
|---|---|---|---|---|---|---|
| 1 | Cl | Ph | 1a | Ph | 3a | 81 |
| 2 | Cl | Ph | 1a | p-OMeC6H4 | 3b | 79 |
| 3 | Cl | Ph | 1a | p-CO2MeC6H4 | 3c | 65 |
| 4 | Br | Ph | 1b | Ph | 3d | 88b |
| 5 | Cl | p-OMeC6H4 | 1c | Ph | 3e | 67 |
| 6 | Cl | p-OMeC6H4 | 1c | o-tolyl | 3f | 45 |
| 7 | Cl | p-CF3C6H4 | 1d | Ph | 3g | 68 |
| 8 | Cl | p-CF3C6H4 | 1d | 1-cyclohexenyl | 3h | 93 |
| 9 | Cl | CO2Me | 1e | p-tolyl | 3i | 93c |
| 10 | Cl | CO2Me | 1e | p-OMeC6H4 | 3j | 67 |
| 11 | Cl | CO2Me | 1e | p-CO2MeC6H4 | 3k | 72 |
| 12 | Cl | CO2Me | 1e | m-CO2MeC6H4 | 3l | 87 |
| 13 | Br | Ph | 1b | n-butyl | 3m | 69 |
| 14 | Cl | CO2Me | 1e | Ph | 3n | 86d |
| 15 | Cl | Ph | 1a | (CH2)3Cl | 3o | 68 |
Isolated yield.
8% ee.
86% ee.
84% ee.
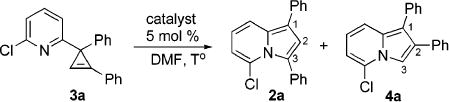
Naturally, having in hand this convenient method for the synthesis of 3-iminocyclopropenes, we evaluated our hypothesis on the cyclopropene into N-fused pyrrole transformation (eq 2). To this end, we tested rearrangement of cyclopropene 3a into indolizines 2a and 4a in the presence of a series of transition-metal catalysts (Table 2). The employment of Pd(II) and Pt(II) chlorides in DMF at room temperature resulted in low yields and moderate regioselectivity of rearrangement (entries 1 and 2). The yield was improved upon switching to Pt(0) complex (entry 3); however, the selectivity remained unsatisfactory. Gratifyingly, we found that the employment of Ir(I) and Rh(I) complexes led to the highly regioselective isomerization of 3a into 2a (entries 6 and 7) in moderate and excellent yields, respectively. Interestingly, when Rh(II) perfluorobutyrate was used as a catalyst, another regioisomer 4a, with the substituent at C-2 position,14,15 was formed as a single product, though in low yield (entry 8). AgSbF6 and Au(III) chloride16 were completely inefficient catalysts for this transformation (entries 9 and 10). However, Cu(I) iodide smoothly isomerized 3a into indolizine 4a in good yield and excellent regioselectivity (entry 11, Table 2).
Table 2.
Metal-Catalyzed Rearrangement of Cyclopropene 3a
| no. | Catalyst | T (°C) | 2a:4a ratioa | yield,b % |
|---|---|---|---|---|
| 1 | PdCl2 | rt | 2:1 | 23 |
| 2 | PtCl2 | rt | 4:1 | 38 |
| 3 | Pt(PPh3)4 | 60 | 5:1 | 86 |
| 4 | NiCl2 | 60 | 0c | |
| 5 | RuCl2(PPh3)3 | 60 | 8:1 | 32 |
| 6 | [Ir(cod)py(PCy3)]PF6 | 60 | > 99:1 | 49 |
| 7 | RhCl(PPh3)3 | rt | > 99:1 | 92 |
| 8 | Rh2(pfb)4 | rtd | < 1:99 | 31 |
| 9 | AgSbF6 | 60 | 0c | |
| 10 | AuCl3 | 60d | 0c | |
| 11 | CuI | rt | < 1:99 | 78 |
NMR ratio.
Combined NMR yield of both isomers.
A mixture of unidentified products formed.
Dichloroethane used as solvent.
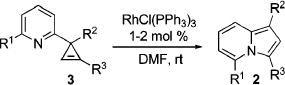
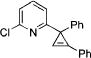
Next, we explored the scope of this novel regiodivergent rearrangement methodology. First, rearrangement of a series of 3-(2-pyridyl)cyclopropenes into 1,3-disubstituted indolizines 2 was tested under Rh(I) catalysis (Table 3). It was found that cyclopropenes possessing both electron-rich and electron-deficient aryl groups17 at position C-3 and at the double bond underwent clean and regioselective rearrangement into indolizines 2. Notably, 1-alkenyl- (entry 8) and Br-containing (entry 4) substrates were found to be equally efficient in this reaction, as well. Thus, the Rh(I)-catalyzed isomerization of cyclopropenes compliments the direct transannulation protocol,10 providing access to a wider selection of 1,3-substituted indolizines possessing not only ester but also various aryl groups at C-3. Attempts to perform the analogous transformation with 3-carbomethoxycyclopropene 3n produced only a small amount of the corresponding indolizine 5 together with furan 6 as major product of this reaction (eq 4). Formation of furan 6 in the presence of Wilkinson’s catalyst is consistent with earlier observations (eq 1).6c–g,7
Table 3.
Rh(I)-Catalyzed Rearrangement of Cyclopropenes
| no. | cyclopropene 3 | product 2 | yielda, % | ||
|---|---|---|---|---|---|
| 1 |
|
3a |
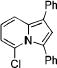
|
2a | 85 |
| 2 |
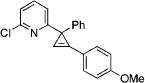
|
3b |
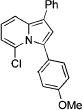
|
2b | 91 |
| 3 |
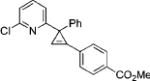
|
3c |
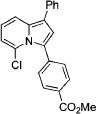
|
2c | 79 |
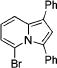
| 4 |
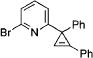
|
3d |
|
2d | 87 |
| 5 |
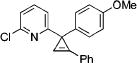
|
3e |
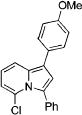
|
2e | 81 |
| 6 |
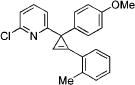
|
3f |
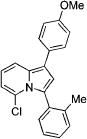
|
2f | 93 |
| 7 |
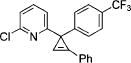
|
3g |
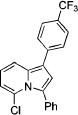
|
2g | 84 |
| 8 |
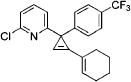
|
3h |
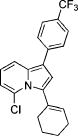
|
2h | 88 |
Isolated yield.
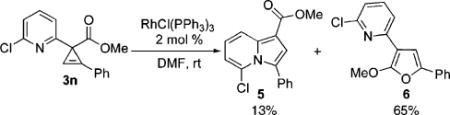 |
(4) |
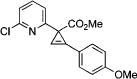
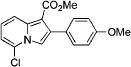
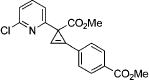
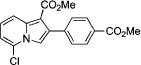
After successful synthesis of 1,3-disubstituted indolizines 2 (Table 3), we turned our attention to regioselective formation of valuble14,15 1,2-subsituted N-fused pyrroles 4 via the Cu(I)-catalyzed rearrangement. To our delight, a variety of 3-carbomethoxy- and 3-arylcyclopropenes reacted smoothly to produce indolizines 4 in good to excellent yields (Table 4). Electron-rich (entries 2 and 3) and electron-deficient (entries 4 and 5) aryl groups and alkyl substituents (entries 7 and 8) at the double bond of cyclopropene were equally well tolerated in this reaction. Gratifyingly, this rearrangement mode worked well with different 3-heteroaryl-cyclopropenes, such as oxazole18 and isoquinoline19 derivatives 3p and 3r, giving access to their fused analogues 4j and 4k in good yields (entries 10 and 11).
Table 4.
Cu(I)-Catalyzed Rearrangement of Cyclopropenes
| no. | cyclopropene 3 | product 4 | yielda, % | ||
|---|---|---|---|---|---|
| 1 | 3a |
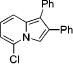
|
4a | 75 | |
| 2 |
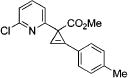
|
3i |
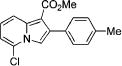
|
4b | 83 |
| 3 |
|
3j |
|
4c | 95 |
| 4 |
|
3k |
|
4d | 73 |
| 5 |
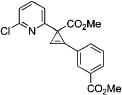
|
3l |
|
4e | 81 |
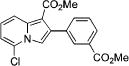
| 6 | 3d |
|
4f | 78 | |
| 7 |
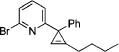
|
3m |
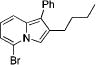
|
4g | 71 |
| 8 |
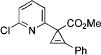
|
3n |
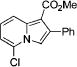
|
4h | 67 |
| 9 |
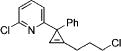
|
3o |
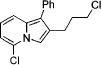
|
4i | 73 |
| 10 |
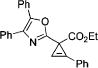
|
3P |
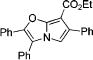
|
4j | 71 |
| 11 |
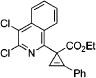
|
3r |
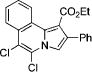
|
4k | 88 |
Isolated yield.
We propose the following mechanistic rationale for the novel regiodivergent rearrangement of imino cyclopropenes 3 into fused pyrroloheterocycles 2 and 4 (Scheme 1). Cyclopropene 3, in the presence of Rh(I) complex, undergoes ring opening to produce the most substituted carbenoid 5.6c–e,7c A nucleophilic attack by nitrogen lone pair on carbenoid center leads to the formation of zwitterion 7. A subsequent elimination of the metal furnishes regioisomer 2. In contrast, when Cu(I) catalyst is used, formation of less substituted carbenoid 6 occurs,6c,f,g,7a cyclization of which via a zwitterion 10 leads to the product 4 selectively. Alternatively, regioisomers 2 and 4 may arise via a reductive elimination of aza metalacycles 8 and 9, respectively, which in turn are formed via a 6π-electrocyclization6e,20 of carbenoids 5 and 6, or directly from cyclopropene 3, upon regioselective oxidative addition of the metal.6e,7c It was also proposed that isomeric carbenoids 5 and 6 could interconvert through the cycloaddition/cycloreversion equilibrium.6d,e We evaluated a possibility of such equilibrium by performing a crossover experiment in the presence of 5 equiv of “external” alkyne. However, no crossover products were detected, thus suggesting independent routes for the formation of 5 and 6.
Scheme 1.
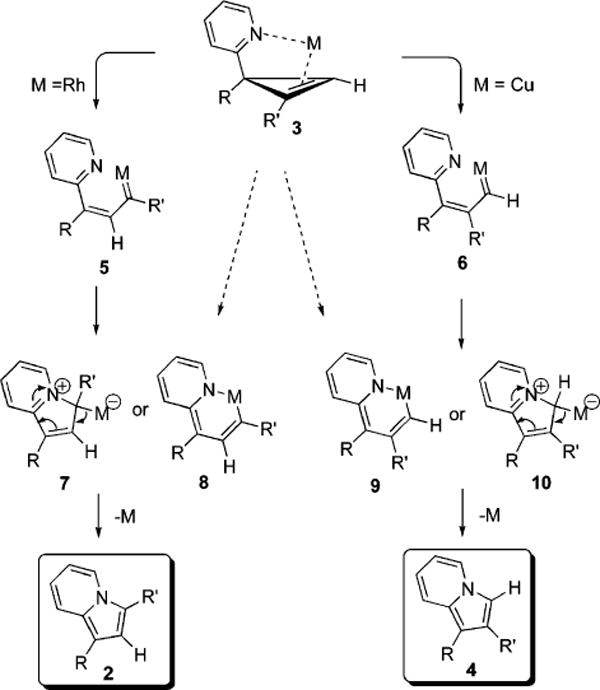
Mechanistic Rationale for Regiodivergent Rearrangement Reactions
In summary, we have developed a highly efficient synthesis of 1,3- and 1,2-disubstituted N-fused pyrroloheterocycles,9,21 including indolizines, pyrrolooxazole, and pyrroloisoquinoline, via a novel regiodivergent transition-metal-catalyzed rearrangement of 3-iminocyclopropenes. We also demonstrated that the latter can conviniently be synthesized from 1,2,3-triazoles.23
Supplementary Material
Acknowledgments
The support of the National Institutes of Health (GM-64444) and the National Science Foundation (CHE-0710749) is gratefully acknowledged.
Footnotes
Supporting Information Available: Preparative procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For recent reviews see:; (a) Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]; (b) Rubin M, Rubina M, Gevorgyan V. Synthesis. 2006:1221. [Google Scholar]; (c) Fox JM, Yan N. Curr Org Chem. 2005;9:719. [Google Scholar]
- (2).For discussion on the strain energy in small rings see for example:; Back RD, Dmitrenko O. J Am Chem Soc. 2004;126:4444. doi: 10.1021/ja036309a. [DOI] [PubMed] [Google Scholar]
- (3).For carbometalations see:; (a) Liao L, Fox JM. J Am Chem Soc. 2002;124:14322. doi: 10.1021/ja0278234. [DOI] [PubMed] [Google Scholar]; (b) Liu X, Fox JM. J Am Chem Soc. 2006;128:5600. doi: 10.1021/ja058101q. [DOI] [PubMed] [Google Scholar]; For hydrometalations see:; (c) Rubina M, Rubin M, Gevorgyan V. J Am Chem Soc. 2002;124:11566. doi: 10.1021/ja027095k. [DOI] [PubMed] [Google Scholar]; (d) Rubina M, Rubin M, Gevorgyan V. J Am Chem Soc. 2003;125:7198. doi: 10.1021/ja034210y. [DOI] [PubMed] [Google Scholar]; (e) Rubina M, Rubin M, Gevorgyan V. J Am Chem Soc. 2004;126:3688. doi: 10.1021/ja0496928. [DOI] [PubMed] [Google Scholar]
- (4).For selected examples see:; (a) Diev VV, Kostikov RR, Gleiter R, Molchanov AP. J Org Chem. 2006;71:4066. doi: 10.1021/jo0600656. [DOI] [PubMed] [Google Scholar]; (b) Ma S, Zhang J, Lu L, Jin X, Cai Y, Hou H. Chem Commun. 2005:909. doi: 10.1039/b413807d. [DOI] [PubMed] [Google Scholar]; (c) Palleria MK, Fox JM. Org Lett. 2005;7:3593. doi: 10.1021/ol051456u. [DOI] [PubMed] [Google Scholar]; (d) Weatherhead-Kloster RA, Corey EJ. Org Lett. 2006;8:171. doi: 10.1021/ol052752+. [DOI] [PubMed] [Google Scholar]; (e) Padwa A, Kulkarni YS, Terry LW. J Org Chem. 1990;55:2478. [Google Scholar]
- (5).For coupling reactions, see:; (a) Yin J, Chisholm JD. Chem Commun. 2006:632. doi: 10.1039/b516021a. [DOI] [PubMed] [Google Scholar]; (b) Chuprakov S, Rubin M, Gevorgyan V. J Am Chem Soc. 2005;127:3714. doi: 10.1021/ja042380k. [DOI] [PubMed] [Google Scholar]
- (6).(a) Padwa A, Blacklock T, Loza R. J Am Chem Soc. 1981;103:2404. [Google Scholar]; (b) Padwa A, Blackbock TJ, Loza R. J Org Chem. 1982;47:3712. [Google Scholar]; (c) Müller P, Pautex N, Doyle MP, Baheri V. Helv Chim Acta. 1990;73:1233. [Google Scholar]; (d) Cho SH, Liebeskind LS. J Org Chem. 1987;52:2631. [Google Scholar]; (e) Padwa A, Kassir JM, Xu SL. J Org Chem. 1997;62:1642. [Google Scholar]; (f) Müller P, Gränicher C. Helv Chim Acta. 1993;76:521. [Google Scholar]; (g) Müller P, Gränicher C. Helv Chim Acta. 1995;78:129. [Google Scholar]
- (7).(a) Tomilov YV, Shapiro EA, Protopopova MN, Ioffe AI, Dolgii IE, Nefedov OM. Izv Akad Nauk SSSR Ser Khim. 1985:631. [Google Scholar]; (b) Davies HML, Romines KR. Tetrahedron. 1988;44:3343. [Google Scholar]; (c) Padwa A, Kassir JM, Xu SL. J Org Chem. 1991;56:6971. [Google Scholar]; (d) Ma S, Zhang J. J Am Chem Soc. 2003;125:12386. doi: 10.1021/ja036616g. [DOI] [PubMed] [Google Scholar]
- (8).For photochemical isomerization see:; Zimmerman HE, Wright CW. J Am Chem Soc. 1992;114:6603. [Google Scholar]
- (9).For selected examples on biological activity of indolizines see:; (a) Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M, Chikazawa Y, Yamada K, Ono T, Teshirogi I, Ohtani M. J Med Chem. 1996;39:3636. doi: 10.1021/jm960395q. [DOI] [PubMed] [Google Scholar]; (b) Gundersen L-L, Malterud KE, Negussie AH, Rise F, Teklu S, Østby OB. Bioorg Med Chem. 2003;11:5409. doi: 10.1016/j.bmc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- (10).Chuprakov S, Hwang FW, Gevorgyan V. Angew Chem Int Ed. 2007;46:4757. doi: 10.1002/anie.200700804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For cyclopropanation with 2-pyridyl diazo compounds see:; Davies HML, Townsend RJ. J Org Chem. 2001;66:6595. doi: 10.1021/jo015617t. [DOI] [PubMed] [Google Scholar]
- (12).Despite the efficiency of Rh2(S-DOSP)4 in enantioselective cyclopropenations,13we observed very low levels of enantioselectivity in synthesis of 3-aryl-substituted cyclopropenes. However, selected examples indicate highly enantioselective cyclopropenation in a case of 3-carbomethoxy derivatives (see Table 1).
- (13).Davies HML, Lee GH. Org Lett. 2004;6:1233. doi: 10.1021/ol049928c. [DOI] [PubMed] [Google Scholar]
- (14).The C-2 site of indolizine is unfunctionalizable position and a substituent must be introduced prior to cyclization (see ref 15).
- (15).For a review see:; (a) Behnisch R, Behnisch P, Eggenweiler M, Wallenhorst T. In: Houben-Weyl. Kreher RP, editor. E6a/2a. Georg Thieme Verlag Stuttgart; New York: 1994. pp. 323–451. [Google Scholar]; See also:; (b) Seregin IV, Gevorgyan V. J Am Chem Soc. 2006;128:12050. doi: 10.1021/ja063278l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For recent review on gold catalysis see:; Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]
- (17).Substrates, possessing alkyl substituents at C-1 position of cyclopropene ring produced only trace amounts of products under these reaction conditions.
- (18).For the synthesis of oxazolyl diazo compound, see ref 11.
- (19).Prepared via cyclopropenation of corresponding triazoloisoquinoline analogously to pyridyl derivatives 3a–o (see the Supporting Information for details).
- (20).Hoye TR, Dinsmore CJ, Johnson DS, Korkowski PF. J Org Chem. 1990;55:4518. [Google Scholar]
- (21).Heterocyclic halides may be further functionalized via cross-coupling reactions.22 We have also shown (see ref 10) that halogen can be efficiently removed from the products.
- (22).For a review see:; Littke AF, Fu GC. Angew Chem Int Ed. 2002;41:4176. doi: 10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- (23).Halogen-free 3-(2-pyridyl)cyclopropenes were not examined in the described rearrangements as they were not available via cyclopropenation of pyridotriazoles (see ref 10).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


