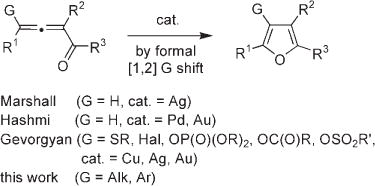
Cycloisomerization of allenyl ketones is an efficient approach for the assembly of the furan ring, an important heterocyclic unit.[1] This transformation in the presence of transition-metal catalysts was first reported by Marshall et al.[2] and later by Hashmi et al.[3] for the synthesis of furans [G = H, Eq. (1)]. Recently, we have developed a set of transition-metal-catalyzed cascade transformations of allenyl ketones involving 1,2-migration of various groups (G = SR,[4] Hal,[5] OP(O)(OR)2, OC(O)R, OSO2R[6]) to produce up to tetrasubstituted furans [Eq. (1)]. Herein, we wish to report a novel metal-catalyzed [1,2]-alkyl shift in allenyl ketones as a key step in the formation of up to fully carbon-substituted furans [Eq. (1)].
 |
(1) |
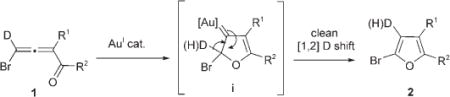
Recently, we reported the Au-catalyzed regiodivergent synthesis of halofurans.[5] It was found that in the presence of AuI catalysts clean hydrogen migration from 1 occurs to form 2 [Eq. (2)]. The absence of H/D-scrambling, in contrast to that observed in the Cu/base-assisted synthesis of pyrroles,[7] supported the clean [1,2]-hydrogen shift to the carbenoid center in intermediate i.[5]
 |
(2) |
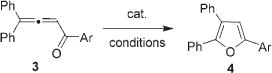
It occurred to us that 1,2-migration of an alkyl/aryl group by this mechanism is also feasible,[8–11] which may allow for the assembly of fully carbon-substituted furans. To this end, we have tested the possible cycloisomerization of allene 3 to give furan 4 in the presence of different catalysts (Table 1). We have found that employment of AuI and AuIII halides gave low yields of furan 4 (Table 1, entries 1 and 2). Gratifyingly, switching to cationic AuI complexes led to formation of 4k in nearly quantitative yield (Table 1, entries 3 and 4). In analogy to gold halides, PtII, PtIV, and PdII salts were inefficient in this reaction (Table 1, entries 5–7). Use of CuI halides resulted in no reaction (Table 1, entry 8), while employment of cationic AgI, CuI, and CuII salts produced 4 in moderate to high yields (Table 1, entries 9–13). Encouraged by these results, we also tested main-group metals in this reaction. Surprisingly, Al, Si, Sn, and In triflates provided moderate to excellent yields of desired furan 4 (Table 1, entries 14, 16–19). Although [Au(PPh3)]OTf, AgOTf, In(OTf)3, Sn(OTf)2, and TIPSOTf were nearly equally efficient in the cascade cycloisomerization of 3 to give 4, In(OTf)3 appeared to be a more general catalyst with respect to the substrate scope.[12]
Table 1.
Optimization of reaction conditions.a
| Entry | Catalystb | mol% | Solvent | T [°C] | Yield [%]c |
|---|---|---|---|---|---|
| 1 | AuBr3 | 5 | toluened | 100 | 23 |
| 2 | AuI | 5 | toluened | 100 | traces |
| 3 | [Au(PPh3)]OTf | 1 | toluened | 100 | 100 (89) |
| 4 | [Au(PPh3)]OTf | 5 | CH2Cl2e | RT | 99 |
| 5 | PtCl2 | 5 | toluenef | 100 | 21 |
| 6 | PtCl4 | 5 | toluenef | 100 | 21 |
| 7 | [PdCl2(PhCN)2] | 5 | toluenef | 100 | 35 |
| 8 | CuX (X = Cl, Br, I) | 5 | toluenef | 100 | 0 |
| 9 | CuOTf·PhH | 5 | toluenef | 100 | 42 |
| 10 | Cu(OTf)2 | 5 | tolueneg | 100 | 95 |
| 11 | AgPF6 | 5 | tolueneg | 100 | 47 |
| 12 | AgOTf | 5 | tolueneg | 100 | (80) |
| 13 | AgOTf | 20 | CH2Cl2e | RT | 70 (62) |
| 14 | Al(OTf)3 | 5 | tolueneg | 100 | 64 |
| 15 | Zn(OTf)2 | 5 | tolueneg | 100 | 39 |
| 16 | TMSOTf | 20 | CH2Cl2e | RT | 82 (62) |
| 17 | In(OTf)3 | 5 | tolueneg | 100 | 91 (81) |
| 18 | Sn(OTf)2 | 5 | tolueneg | 100 | 97 (81) |
| 19 | TIPSOTf | 5 | tolueneg | 100 | 100 (81) |
| 20 | TMSNTf2 | 5 | tolueneg | 100 | 72 |
Entries 1–4: Ar = p-Br-C6H4; entries 5–20: Ar = Ph.
Tf = trifluoromethanesulfonyl, TIPS = triisopropylsilyl, TMS = trimethylsilyl.
Yield determined from NMR spectrum; yield of isolated product in parentheses.
0.05 M, solution of 3.
0.02 M solution of 3.
1 M solution of 3.
0.1 M solution of 3.
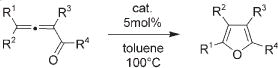
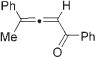
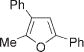
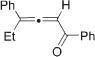
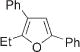
Next, cycloisomerization of differently substituted allenyl ketones 3a–m was examined under the optimized conditions (Table 2). Thus, cycloisomerization of 4,4-diphenyl-substituted allenyl ketones 3b–d proceeded smoothly to provide good to high yields of furans 4b–d (Table 2, entries 2–4). Selective migration of the phenyl over the methyl group occurred in allenyl ketone 3e to give 4e in 72% yield (Table 2, entry 5). Not surprisingly, cycloisomerization of allenyl ketone 3i, possessing two methyl groups, provided the corresponding furan 4i in low yield only (Table 2, entry 8). In contrast to the disfavored methyl-group migration in Table 2, entry 5, migration of the ethyl group competed with the phenyl group in 3f, which resulted in formation of a 2.3:1 mixture of regioisomeric furans 4f and 4g, respectively (Table 2, entry 6). Cyclopentylidene allenyl ketone 3h underwent smooth cyclization with ring expansion[13] to give fused furan 4h in 75% yield (Table 2, entry 7). It was also demonstrated that a variety of functional groups such as methoxy (Table 2, entry 9), bromo (Table 2, entry 10), nitro (Table 2, entry 11), and cyano (Table 2, entry 12) were perfectly tolerated under these reaction conditions.
Table 2.
Lewis acid catalyzed synthesis of furans.
| Entry | Allenyl ketone | Furan | Yield [%]a | ||
|---|---|---|---|---|---|
| 1 |
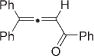
|
3a |
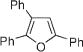
|
4a | 81b |
| 2 |
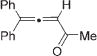
|
3b |
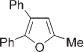
|
4b | 64c |
| 3 |
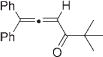
|
3c |
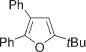
|
4c | 90 |
| 4 |
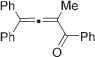
|
3d |
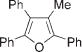
|
4d | 79d |
| 5 |
|
3e |
|
4e | 72 (52)e,f |
| 6 |
|
3f |
|
4f | 88g |
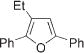
|
4g | (76)h,f,i | |||
| 7 |
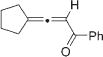
|
3h |
|
4h | 75 |
| 8 |
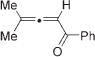
|
3i |
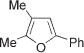
|
4i | 10f |
| 9 |
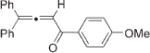
|
3j |
|
4j | 62 |
| 10 |
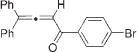
|
3k |
|
4k | 93 (89)h |
| 11 |
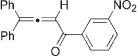
|
3l |
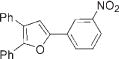
|
4l | 85b |
| 12 |
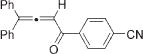
|
3m |
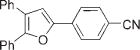
|
4m | 94b |
Yield of isolated product; 0.25–0.8-mmol scale, In(OTf)3 was used unless otherwise mentioned.
5 mol% Sn(OTf)2 was used.
10 mol% In(OTf)3 was used.
20 mol% AgOTf/p-xylene, 140°C, 1 h.
2 mol% [Au(PPh3)]OTf was used.
Yield determined from NMR spectrum.
2.3:1 mixture of 4 f:4g by 1H NMR spectroscopy.
1 mol% [Au(PPh3)]OTf was used.
2.2:1 mixture of 4 f:4g by 1H NMR spectroscopy.
In addition, we have shown that trisubstituted furan 4b can be obtained directly from alkynyl ketone 5b [Eq. (3)]. However, the yield for this one-pot transformation was somewhat lower than that for cycloisomerization of allene 3b (Table 2, entry 2).
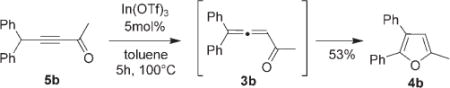 |
(3) |
We propose the following mechanism for the cascade transformation of allenyl ketone 3 into furan 4 (Scheme 1). Cycloisomerization in the presence of oxophilic Lewis acids, such as In, Sn, and Si triflates, follows path A, according to which, the Lewis acid activates the enone moiety (see 6) to form vinyl cation 7.[14] [1,2]-Alkyl shift in 7 produces the regioisomeric vinyl cation 8,[15] which, upon cyclization, transforms into furan 4 and regenerates the Lewis acid catalyst. Alternatively, π-philic catalysts, such as AgI, CuI, and AuI salts, activate the carbon–carbon double bond of allene (see 9) and trigger nucleophilic attack of a carbonyl oxygen lone pair at the terminal carbon of the allene moiety to form cyclic oxonium intermediate 10.[2c,5] [1,5]-Alkyl shift[16] (Scheme 1, path B) to form 11 with subsequent elimination of metal gives 4. The involvement of an electrophilic mechanism (Scheme 1, paths A and B) is supported by the data presented in Table 2. Thus, the migratory aptitude of a phenyl vs. that of a methyl group (> 100:1) is in good agreement with that reported in the literature for rearrangements of cations.[17] Although a mechanism involving [1,2]-alkyl shift in the carbenoid intermediate 12[5,8] (Scheme 1, path C) cannot be completely ruled out at this point, it is considered to be less likely.[18,19]
Scheme 1.
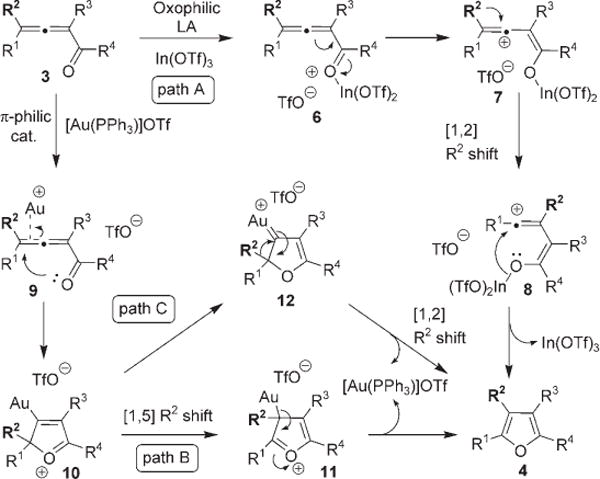
Proposed mechanisms for the synthesis of furans 4.
In summary, we have developed a novel metal-catalyzed method for the synthesis of furans, which proceeds by an unprecedented [1,2]-alkyl shift in allenyl ketones. This method allows for efficient synthesis of up to fully carbon-substituted and fused furans.
Supplementary Material
Footnotes
The support of the National Institutes of Health (GM-64444) is gratefully acknowledged.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For a recent review, see:; Kirsch SF. Org Biomol Chem. 2006;4:2076. doi: 10.1039/b602596j. [DOI] [PubMed] [Google Scholar]
- 2.a) Marshall JA, Robinson ED. J Org Chem. 1990;55:3450. [Google Scholar]; b) Marshall JA, Wang XJ. J Org Chem. 1991;56:960. [Google Scholar]; c) Marshall JA, Bartley GS. J Org Chem. 1994;59:7169. [Google Scholar]; d) Marshall JA, Sehon CA. J Org Chem. 1995;60:5966. doi: 10.1021/jo970360d. [DOI] [PubMed] [Google Scholar]; e) Marshall JA, Wallace EM. J Org Chem. 1995;60:796. [Google Scholar]
- 3.a) Hashmi ASK. Angew Chem. 1995;107:1749. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:1581. [Google Scholar]; b) Hashmi ASK, Schwarz L, Choi JH, Frost TM. Angew Chem. 2000;112:2382. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:2285. [Google Scholar]
- 4.Kim JT, Kel’in AV, Gevorgyan V. Angew Chem. 2003;115:102. doi: 10.1002/anie.200390064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:98. [Google Scholar]
- 5.Sromek AW, Rubina M, Gevorgyan V. J Am Chem Soc. 2005;127:10500. doi: 10.1021/ja053290y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sromek AW, Kel’in AV, Gevorgyan V. Angew Chem. 2004;116:2330. doi: 10.1002/anie.200353535. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:2280. [Google Scholar]
- 7.Kel’in AV, Sromek AW, Gevorgyan V. J Am Chem Soc. 2001;123:2074. doi: 10.1021/ja0058684. [DOI] [PubMed] [Google Scholar]
- 8.For examples of [1,2]-shifts in carbenoids, see:; a) Xiao F, Wang J. J Org Chem. 2006;71:5789. doi: 10.1021/jo0605391. and references therein. [DOI] [PubMed] [Google Scholar]; b) Markham JP, Staben ST, Toste FD. J Am Chem Soc. 2005;127:9708. doi: 10.1021/ja052831g. [DOI] [PubMed] [Google Scholar]; c) Gorin DJ, Davis NR, Toste FD. J Am Chem Soc. 2005;127:11260. doi: 10.1021/ja053804t. [DOI] [PubMed] [Google Scholar]
- 9.For general reviews, see:; One or more CH and/or CC bond(s) formed by rearrangement:; a) Ducrot PH. In: Comprehensive Organic Functional Group Transformations II. Katritzky AR, Taylor RJK, editors. Vol. 1. Elsevier; Oxford, UK: 2005. pp. 375–426. [Google Scholar]; b) Pattenden G. Carbon–Carbon σ-Bond Formation: Rearrangement Reactions. In: Trost BM, Fleming I, editors. Comprehensive Organic Synthesis: Selectivity, Strategy, and Efficiency in Modern Organic Chemistry. Vol. 3. Pergamon; New York: 1991. pp. 705–1043. [Google Scholar]
- 10.While this manuscript was in preparation, two independent works on synthesis of carbocycles involving [1,2]-alkyl shifts to carbenoid center in allenes were reported. See:; a) Funami H, Kusama H, Iwasawa N. Angew Chem Int Ed. 2007;46:909. doi: 10.1002/anie.200603986. [DOI] [PubMed] [Google Scholar]; b) Lee JH, Toste FD. Angew Chem Int Ed. 2007;46:912. doi: 10.1002/anie.200604006. [DOI] [PubMed] [Google Scholar]
- 11.For a synthesis of dehydrofuranones by [1,2]-alkyl shift, analogous to a formal ketol rearrangement, see:; Kirsch SF, Binder JT, Liébert C, Menz H. Angew Chem. 2006;118:6010. doi: 10.1002/anie.200601836. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:5878. and references therein. [Google Scholar]
- 12.See the Supporting Information for details.
- 13.For examples of ring expansions in the synthesis of carbocycles proceeding by a carbenoid mechanism, see Ref. [9].
- 14.For activation of enone moiety by Lewis acids see, for example:; a) Childs RF, Mulholland DL, Nixon A. Can J Chem. 1982;60:801. [Google Scholar]; b) Schwier T, Gevorgyan V. Org Lett. 2005;7:5191. doi: 10.1021/ol0521026. [DOI] [PubMed] [Google Scholar]
- 15.For examples of [1,2]-shifts in vinyl cations, see:; a) Capozzi G, Lucchini V, Marcuzzi F, Melloni G. Tetrahedron Lett. 1976;17:717. [Google Scholar]; b) Jäckel KP, Hanack M. Tetrahedron Lett. 1974;15:1637. [Google Scholar]
- 16.a) Miller B. J Am Chem Soc. 1970;92:432. [Google Scholar]; b) Dolbier WR, Anapolle KE, McCullagh L, Matsui K, Riemann JM, Rolison D. J Org Chem. 1979;44:2845. [Google Scholar]; c) Ode M, Breslow R. Tetrahedron Lett. 1973;14:2537. [Google Scholar]
- 17.Saunders WH, Paine RH. J Am Chem Soc. 1961;83:882. [Google Scholar]
- 18.The observed migratory aptitude trends (Ph vs. Et, and Ph vs. Me) do not correspond to those reported in literature for [1,2]-alkyl migration to a carbenoid center. See, for example:; a) Philip H, Keating J. Tetrahedron Lett. 1961;2:523. [Google Scholar]; b) von der Schulenburg W Graf, Hopf H, Walsh R. Angew Chem. 1999;111:1200. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1128::AID-ANIE1128>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1999;38:1128. [Google Scholar]
- 19.No cyclopropanation product was observed in the cycloisomerization of dimethylallenyl ketone 3i; however, this transformation was reported as a major process in the cycloisomerization of a carbocyclic analogue of 12. See Ref. [9a].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


