Transition-metal-catalyzed annulations are widely used in the synthesis of heterocyclic compounds.[1] One of the most efficient methods for the construction of five-membered oxygen-containing heterocycles involves the annulation of diazocarbonyl compounds with alkynes and nitriles. Thus, Davies et al.[2] and Padwa et al.[3] have employed this method[4] for the synthesis of furans (X = CH), and Helquist et al.[5] for the preparation of oxazoles (X = N) [Eq. (1)]. In contrast, analogous transformations of α-imino diazo compounds, which may lead to the formation of pyrrole and imidazole rings, are unknown. Herein we report an efficient, direct, Rh-catalyzed transannulation of pyridotriazoles with alkynes and nitriles that leads to indolizines (X= CH) and imidazopyridines (X = N), respectively [Eq. (2)].
 |
(1) |
 |
(2) |
It has been shown that 2-pyridyl diazo compounds 1[6] transform into their cyclic triazole form 2[7] upon storage [Eq. (3)], and it is also known that some of these cyclic triazoles can still undergo transformations that are characteristic of diazo compounds.[8] This phenomenon has been attributed to the closed/open form equilibrium of N-fused triazoles in solution,[9] which can produce trace to significant amounts of 1. The position of this equilibrium depends on the temperature and the substitution pattern of the triazole.[9b] Thus, introduction of a halogen substituent at C7 (R1 = Cl) shifts the equilibrium to the left, which has been explained in terms of nonbonding repulsion between the lone pair of the halogen and that of the nitrogen in the peri position.[10]
 |
(3) |
To evaluate the feasibility of using triazoles as precursors of Rh carbenoids we investigated the reaction of triazoles 3a and 3b with triethylsilane in the presence of a catalytic amount of rhodium(II) acetate, which is a method developed by Doyle and coworkers[11] for the efficient trapping of Rh carbenoids [Eq. (4)]. Not surprisingly, pyridotriazoles 3a and 3b behave differently under these reaction conditions. Thus, while the 7-H derivative 3a remains unaffected, the 7-chloro-substituted compound 3b is smoothly converted into 4, which is the product of carbenoid insertion into the Si–H bond. These experiments clearly indicate that 7-halo-substituted pyridotriazoles can indeed serve as convenient precursors of Rh carbenoids.
 |
(4) |
Next, to test our hypothesis regarding the annulation of α-imino diazo compounds with alkynes to form a pyrrole ring, we treated triazole 3b with phenylacetylene in the presence of rhodium(II) acetate. This reaction proceeded smoothly to produce a mixture of cyclopropene 5 and indolizine 6a with yields of 68% and 28% of isolated product, respectively [Eq. (5)]. Surprisingly, cyclopropene 5 does not undergo further isomerization into indolizine 6a under these reaction conditions.[12] The ratio of these products remained constant throughout the course of the reaction, thereby suggesting an independent path for the formation of 6a.
 |
(5) |
We found, however, that the selectivity of the transannulation (6 over 5) could be dramatically improved by using rhodium(II) heptafluorobutyrate as catalyst.[13] Thus, transannulation of 3b with a series of aryl and alkenyl alkynes[14] proceeded highly chemoselectively (90:10 to 95:5 vs. cyclopropene) to produce indolizines 6[15] in good yields (Table 1). Electron-rich, electron-deficient, and sterically hindered aryl alkynes were nearly equally effective in this reaction.
Table 1.
Rhodium(II)-catalyzed transannulation of triazole 3b with alkynes.
| Entry | Alkyne | Product | Yield [%][a] |
|---|---|---|---|
| 1 |
|
6a | 78 |
| 2 |
|
6b | 80 |
| 3 |

|
6c | 73 |
| 4 |
|
6d | 85 |
| 5 |
|
6e | 70 |
| 6 |
|
6f | 65 |
| 7 |

|
6g | 57 |
Yield of isolated product. Indolizines 6 were accompanied by 5–10% of the corresponding cyclopropenes 5; these compounds were readily separable by column chromatography.
Inspired by the successful formation of an N-fused pyrrole ring from the transannulation of triazoles with alkynes, we examined the formation of an N-fused imidazole ring in the reaction of 3 with nitriles and found that pyridotriazoles 3 react smoothly with a variety of aryl, alkyl, and alkenyl nitriles in the presence of Rh2(OAc)4 (1 mol%) in toluene at 60°C (Table 2) to afford N-fused imidazopyridines 7 in reasonable to high yields.
Table 2.
Rhodium(II)-catalyzed transannulation of triazoles with nitriles.

| Entry | R1 | R2 | Triazole | R3 | Product | Yield [%][a] |
|---|---|---|---|---|---|---|
| 1 | Cl | CO2Me | 3b | p-Tol | 7a | 89 |
| 2 | Cl | CO2Me | 3b | Ph | 7b | 83 |
| 3 | Cl | CO2Me | 3b | p-Me(O)CC6H4 | 7c | 54 |
| 4 | Cl | CO2Me | 3b | Bn | 7d | 63 |
| 5 | Cl | CO2Me | 3b | nPr | 7e | 75 |
| 6 | Cl | CO2Me | 3b | cPr | 7f | 74 |
| 7 | Cl | CO2Me | 3b | tBu | 7g | 69 |
| 8 | Cl | CO2Me | 3b |
|
7h | 66 |
| 9 | Cl | CO2Me | 3b | CH2SiMe3 | 7i | 70 |
| 10 | Cl | p-CF3C6H4 | 3c | Ph | 7j | 82 |
| 11 | Br | p-CF3C6H4 | 3d | p-Tol | 7k | 73 |
| 12 | OMe | p-CF3C6H4 | 3e | nPr | 7l | 51 |
Yield of isolated product.
Both 3-carbomethoxy-(Table 2, entries 1–9) and 3-aryl-(Table 2, entry 10) pyridotriazoles are equally efficient in this reaction. Moreover, 7-bromo-(Table 2, entry 11) and even 7-methoxy-substituted (Table 2, entry 12) triazoles proved to be good substrates for this transannulation reaction.
We propose the following mechanism for this novel Rh-catalyzed transformation (Scheme 1). First, pyridotriazole 3 undergoes closed/open form equilibrium[9] to produce small amounts of diazo compound 1 which, upon reaction with rhodium(II) carboxylate, generates the Rh-carbenoid species I. A direct nucleophilic attack[18] of alkyne or nitrile 8 on species I produces ylide species II, according to path A, which then cyclizes to form 6 or 7 via cyclic zwitterion III. Alternatively (path B), [2+2] cycloaddition of I and 8 leads to metallacyclobutene IV, which can also be formed by cyclization of II.[19] Rhodacycle IV then undergoes metathesis to produce Rh carbenoid V which, upon 6π-electrocyclization and subsequent reductive elimination, furnishes product 6 or 7. [2+1] Cycloaddition of I with 8 (path C) accounts for the formation of cyclopropene 5 in the presence of rhodium(II) acetate [see Eq. (5)]. As discussed above, 5 does not transform into heterocycle 6 under these reaction conditions.[12]
Scheme 1.
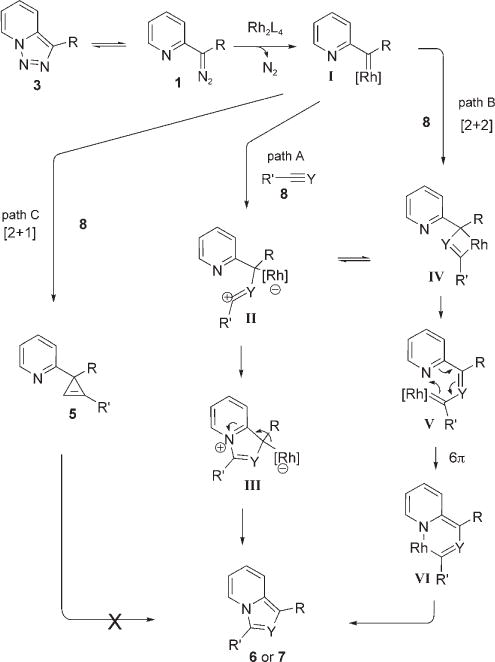
Plausible mechanisms for the Rh-catalyzed transannulation of pyridotriazoles with alkynes and nitriles. Y=N, CR″.
In summary, we have developed an efficient Rh-catalyzed transannulation of pyridotriazoles for the formation of pyrrolo- and imidazopyridines, which are important fused heterocyclic scaffolds.[20] We have also demonstrated that some of these pyridotriazoles can serve as stable[13] and convenient[21] precursors of Rh carbenoids.
Supplementary Material
Footnotes
Financial support from the National Institutes of Health (GM-64444) is gratefully acknowledged.
Supporting Information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For recent reviews, see:; a) Nakamura I, Yamamoto Y. Chem Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]; b) Zeni G, Larock RC. Chem Rev. 2004;104:2285. doi: 10.1021/cr020085h. [DOI] [PubMed] [Google Scholar]; c) Rubin M, Sromek AW, Gevorgyan V. Synlett. 2003:2265. [Google Scholar]
- 2.a) Davies HML, Romines KR. Tetrahedron. 1988;44:3343. [Google Scholar]; b) Davies HML, Cantrell WR, Jr, Romines KR, Baum JS. Org Synth. 1992;70:93. [Google Scholar]
- 3.a) Kinder FR, Padwa A. Tetrahedron Lett. 1990;31:6835. [Google Scholar]; b) Padwa A, Kassir JM, Xu SL. J Org Chem. 1991;56:6971. [Google Scholar]; c) Padwa A, Kinder FR. J Org Chem. 1993;58:21. [Google Scholar]
- 4.For recent applications, see:; a) Gettwert V, Krebs F, Maas G. Eur J Org Chem. 1999:1213. [Google Scholar]; b) Batsila C, Kostakis G, Hadjiarapoglou LP. Tetrahedron Lett. 2002;43:5997. [Google Scholar]
- 5.Connell R, Scavo F, Helquist P. Tetrahedron Lett. 1986;27:5559. [Google Scholar]
- 6.For cyclopropanation with 2-pyridyl diazo compounds, see:; Davies HML, Townsend RJ. J Org Chem. 2001;66:6595. doi: 10.1021/jo015617t. [DOI] [PubMed] [Google Scholar]
- 7.Regitz M. Angew Chem. 1967;79:786. [Google Scholar]; Angew Chem Int Ed Engl. 1967;6:733. [Google Scholar]
- 8.See, for example:; Abarca-González B. J Enzym Inhib Med Chem. 2002;17:359. doi: 10.1080/1475636021000005622. and references therein. [DOI] [PubMed] [Google Scholar]
- 9.a) Regitz M, Arnold B, Danion D, Schubert H, Fusser G. Bull Soc Chim Belg. 1981;90:615. [Google Scholar]; b) L’abbé G. Bull Soc Chim Belg. 1990;99:281. [Google Scholar]; c) L’abbé G, Godts F, Toppet S. J Chem Soc Chem Commun. 1985:589. [Google Scholar]; d) L’abbé G, Luyten I, Toppet S. J Heterocycl Chem. 1992;29:713. [Google Scholar]; e) L’abbé G, Godts F, Toppet S. Bull Soc Chim Belg. 1986;95:679. [Google Scholar]
- 10.Abarca-González B, Ballesteros R, Mojarred F, Jones G, Mouat DJ. J Chem Soc Perkin Trans. 1987;1:1865. [Google Scholar]
- 11.Bagheri V, Doyle MP, Taunton J, Claxton EE. J Org Chem. 1988;53:6158. [Google Scholar]
- 12.For a related transformation of 3-carbonyl cyclopropenes into furans, see refs. [2a] and [3a].
- 13.See the Supporting Information for details.
- 14.The use of aliphatic alkynes resulted in sluggish and incomplete reactions.
- 15.Halo-substituted N-fused heterocycles can be further functionalized by cross-coupling reactions.[16] We have also found that the chloride in 6a can be quantitatively removed by treatment with PdCl2/HSiEt3.[17]
- 16.For a review of coupling reactions of aryl chlorides, see:; Littke AF, Fu GC. Angew Chem. 2002;114:4350. [Google Scholar]; Angew Chem Int Ed. 2002;41:4176. [Google Scholar]
- 17.Boukherroub R, Chatgilialoglu C, Manuel G. Organometallics. 1996;15:1508. [Google Scholar]
- 18.Padwa A, Austin DJ, Price AT, Semones MA, Doyle MP, Protopopova MN, Winchester WR, Tran A. J Am Chem Soc. 1993;115:8669. [Google Scholar]
- 19.Hoye TR, Dinsmore CJ, Johnson DS, Korkowski PF. J Org Chem. 1990;55:4518. [Google Scholar]
- 20.For selected reports of the biological activity of indolizines, see:; a) Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M, Chikazawa Y, Yamada K, Ono T, Teshirogi I, Ohtani M. J Med Chem. 1996;39:3636. doi: 10.1021/jm960395q. [DOI] [PubMed] [Google Scholar]; b) Gundersen LL, Malterud KE, Negussie AH, Rise F, Teklu S, Østby OB. Bioorg Med Chem. 2003;11:5409. doi: 10.1016/j.bmc.2003.09.033. [DOI] [PubMed] [Google Scholar]; for reports on the biological activity of imidazopyridines, see:; c) Kim D, Wang L, Hale JJ, Lynch CL, Budhu RJ, MacCoss M, Mills SG, Malkowitz L, Gould SL, DeMartino JA, Springer MS, Hazuda D, Miller M, Kessler J, Hrin RC, Carver G, Carella A, Henry K, Line-berger J, Schleif WA, Emini EA. Bioorg Med Chem Lett. 2005;15:2129. [Google Scholar]; d) Nakahara S, Kubo A, Mikami Y, Ito J. Heterocycles. 2006;68:515. [Google Scholar]
- 21.The use of 3 does not require special slow-addition techniques as the concentration of 1 in the reaction mixture is always low.[9]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


