Abstract
Desensitization is a clinical procedure whereby incremental doses of a drug are administered over several hours to a sensitive patient until a therapeutic dose and clinical tolerance are achieved. Clinical tolerance may occur in part by attenuating the mast cell response. In the present study, primary human skin mast cells were used to establish and characterize an in vitro model of desensitization. Mast cells in culture were armed with allergen-specific (4-hydroxy-3-nitro-phenylacety and Der p2) and non-specific IgE antibodies, and then desensitized by incremental exposures to 4-hydroxy-3-nitrophenylacety-BSA. This desensitization procedure abrogated the subsequent degranulation response to the desensitizing allergen, to an unrelated allergen, and to IgG anti-FcεRI, but not to C5a, substance P, compound 48/80, and calcium ionophore. Desensitized cells regained their FcεRI-dependent degranulation capability by 24–48 h after free allergen had been removed. Therefore, sensitized human skin mast cells are reversibly desensitized in vitro by exposure to incremental doses of that allergen, which also cross-desensitizes them to an unrelated allergen.
Keywords: Mast cell, desensitization, IgE, tolerance, allergy, Syk, Lyn, Fyn, CD63
Introduction
Mast cells and basophils are key effector cells in IgE-dependent immediate hypersensitivity disorders because they constitutively and uniquely express large amounts of the tetrameric high affinity receptor for IgE and FcεRI. Multivalent allergen activates mast cells through binding to IgE and aggregating IgE:FcεRI complexes. FcεRI-mediated signaling induces the activation of Src family tyrosine kinases Lyn and Fyn followed by the recruitment and activation of tyrosine kinase Syk, eventually leading to mast cell degranulation. Inducing tolerance to such allergens is an important therapeutic goal. Immunotherapy and desensitization are two interventions used by allergists-immunologists to tolerize allergic patients.
Immunotherapy is usually performed with complete allergens, typically proteins. The allergen(s) are administered subcutaneously or sublingually, first during a build-up phase and then a maintenance phase lasting 3–5 years. The resulting clinical tolerance is specific to the antigens used and often persists for years after maintenance immunotherapy ceases [1, 2]. The mechanism(s) remain under investigation, but appear to involve decreased TH2 responsiveness, increased allergen-specific IgG, and upregulation of Treg cells.
In contrast to immunotherapy, desensitization is typically performed on a patient who is allergic to a drug needed for therapy [3–5]. Beginning with a low dose of drug (1,000–1,000,000-fold less than therapeutic), increasing amounts are administered every 15–30 min over several hours until a therapeutic dose is achieved. Desensitization tolerizes the patient to that drug and permits its safe administration, but intolerance returns after discontinuation of the drug, perhaps within days. Whether cross-desensitization to unrelated allergens occurs with desensitization is questionable.
The hypothesis that desensitization targets mast cells and basophils while immunotherapy targets T and B cells may explain some of the different features of these two therapeutic interventions. A potential clue to a mechanism for desensitization emerged when peripheral blood basophils that fail to degranulate to allergen (“non-releaser” phenotype) were found to be deficient in Syk [6], a reversible phenotype after such cells were exposed to IL-3 [6–8]. Reportedly, non-releaser lung mast cells also are Syk-deficient [9]. A key question is whether the “non-releaser” phenotype noted above occurs in basophils and mast cells during clinical desensitization. Desensitization in vitro at a fixed antigen concentration has been associated with depletion of Syk in both basophils [10, 11] and mast cells [11]. Thus, proximal signaling molecules, like Syk, may be involved in antigen desensitization.
In the present study, primary human mast cells derived from fresh surgical skin are used to establish an in vitro model of desensitization and to elucidate cellular and molecular features of this process with intriguing clinical implications. Such mast cells are of the MCTC type (tryptase+, chymase+) [12, 13] and also are the predominant type of mast cells in vascular walls, heart, conjunctiva and bowel submucosa, near bronchial mucus glands [14], and in the bronchial smooth muscle of asthmatics [15].
Methods
Reagents
The non-competing mouse IgG1 anti-FcεRIα mAb, 22E7 (generously provided by J. P. Kochan, Hoffman-LaRoche, Nutley, NJ, USA) [16]; 4-hydroxy-3-nitrophenylacety (NP)-BSA (Biosearch Technologies, Novato, CA, USA); human IgE (Millipore, Billerica, MA, USA); IgE anti-NP (AbD Serotec, Raleigh, NC, USA); Der p2 and Der p2 specific IgE (Indoor Biotechnologies, Charlottesville, VA, USA); biotin labeling kit (Thermo Scientific, Rockford, IL, USA); mouse IgG1 monoclonal anti-biotin (clone BN-34), substance P, compound 48/80, A23187 (Sigma, St. Louis, MO, USA); rabbit IgG anti-Syk, Lyn, Fyn, and β-actin Abs (Cell Signaling Technology Inc, Danvers, MA, USA); Fura-2 AM (Invitrogen, Carlsbad, CA, USA); and C5a and mouse IgG1, κ anti-CD63 mAb (H5C6) (BD Bioscience) were obtained and used as described.
Purification and Culture of Human Skin-Derived Mast Cells
Human skin mast cells were purified and cultured as described previously [17, 18]. Typically, mature mast cells approaching 100% purity were obtained by 4–6 weeks of culture, and 6–16-week-old mast cells were used in the experiments described below.
In Vitro Desensitization of Mast Cells
In single-dose desensitization protocols, human skin mast cells were sensitized with IgE anti-NP at 1 μg/ml overnight. Unbound IgE was removed, and cells were then exposed to a single concentration of NP-BSA (0, 0.000625, 0.00125, 0.005, 0.01, 0.02, 0.039, 0.078, 0.015, 0.31, 0.625, 1.25, 2.5, 5, and 10 ng/ml) for 24 h. These cells were then washed and stimulated with 10 ng/ml of NP-BSA for 30 min. All desensitization and cell activation experiments were conducted at 37°C. For 22E7 mAb desensitization, cells were incubated with a fixed, single concentration of 22E7 at 0.0006, 0.00125, 0.0025, 0.005, 0.01, 0.02, 0.039, 0.078, 0.15, 0.31, 0.625, 1.25, 2.5, 5, 10, and 100 ng/ml for 24 h. Mast cells were then washed and stimulated with 100 ng/ml of 22E7 for 30 min.
In sequential desensitization experiments, human skin mast cells were sensitized by overnight incubation with 10% or 100% NP-specific IgE at a total IgE concentration of 1 μg/ml, and then washed and exposed to increasing concentrations of NP-BSA to achieve accumulated concentrations of 1, 2, 5, 10, 20, 50, 100, 200, and 500 pg/ml followed by 1, 2, 5 and 10 ng/ml at 15-min intervals. Diluent controls also were performed. Fifteen minutes after the last desensitization dose, cells were stimulated with additional NP-BSA (10 ng/ml), 22E7 (100 ng/ml), C5a (100 ng/ml), substance P (4 μg/ml), compound 48/80 (1 μg/ml), or A23187 (1 μM) for 30 min.
For cross-desensitization, mast cells were sensitized by overnight incubation with a mix of NP-specific IgE (10%), Der p2-specific IgE (10%), and non-specific human IgE (80%), the total IgE concentration being 1 μg/ml. Cells were desensitized with sequentially increasing concentrations of NP-BSA as above, and then stimulated with NP-BSA (10 ng/ml), 22E7 (100 ng/ml), or Der p2 (1 μg/ml). Der p2 was added as an aggregate that had been formed by labeling it with biotin (Pierce Biotin labeling kit) and combining it with mouse anti-biotin (clone BN-34) mAb at a 2:1 Der p2: mAb molar ratio.
Mast cell degranulation during the phases of desensitization and activation was assessed by measuring β-hexosaminidase release as described [19]. %Degranulation values were calculated using the formula:
Calcium Flux Measurement
Human skin mast cells (2×106/ml) were loaded with Fura-2 AM at a final concentration of 2 μg/ml for 30 min at 37°C in HBSS buffer. The cells were washed twice in the same buffer and further incubated for 15 min. The cells were then stimulated with 22E7 or C5a. Calcium flux was measured in real time on a Perkin-Elmer LS55 Spectrofluorometer (Perkin-Elmer Laboratories).
Flow Cytometry Analysis
For surface staining, human skin mast cells were first blocked in PBS containing 10% BSA for 30 min at 4°C. The cells were washed in PBS containing 1% BSA (FACS medium) and incubated with the primary antibody for 30 min at 4°C. After washing away unbound primary Ab, the cells were incubated with fluorescent dye-conjugated secondary antibody, washed again, and then analyzed by flow cytometry (FACscan, Becton Dickinson, San Diego, CA, USA).
Western Blotting
Mast cells were sensitized and sequentially desensitized as described above. The cells were then stimulated with 22E7 mAb or buffer for 5 min. The reaction was then stopped by adding 2 vol. of ice-cold PBS. Controls included desensitized and unstimulated cells, non-desensitized and stimulated cells, and nondesensitized and unstimulated cells. Cells were collected, washed in PBS, and lysed with 1% Triton X-100 in borate-buffered saline (lysis buffer) containing protease inhibitors (leupeptin, pepstatin A, aprotinin, phenylmethyl-sulfonylfluoride, sodium pyrophosphate, sodium orthovanadate, sodium fluoride, N-octyl-β-D-glucoside, and soybean trypsin inhibitor). The lysates were centrifuged at 12,000 rpm×10 min to remove large cell debris and were boiled in SDS sample buffer (Invitrogen, Carlsbad, CA, USA). Samples were loaded onto polyacrylamide gels, subjected to electrophoresis, and then transferred to a polyvinylidene fluoride membrane. After blocking in the manufacturer’s blocking buffer (Li-Cor Biosciences, Lincoln, NE, USA), membranes were incubated with primary rabbit anti-Syk, anti-Lyn, anti-Fyn, and anti-β-actin mAbs overnight at 4°C, washed, incubated with IRDye-labeled donkey anti-rabbit Ab, and scanned in an Odyssey Infrared Imaging System (Li-Cor Biosciences). Data were analyzed using Odyssey 3.1 software.
Statistical Analysis
Mean ± SD data are shown throughout. A Student’s t test was used to compare data between two treatment groups, ANOVA to compare data among three or more different treatment groups, followed by post hoc testing as appropriate using SigmaStat (Systat Software Inc., San Jose, CA, USA).
Results
Human Skin Mast Cells Are Desensitized after Exposure to Increasing Doses of Antigen
Mast cells in vivo are armed with IgE antibodies against many different allergens. Accordingly, the percentage of antigen-specific IgE required for effective activation of skin mast cells was assessed. Human skin mast cells sensitized with 0.01–100% NP-specific IgE were challenged with 10 ng/ml of NP-BSA. As shown in Fig. 1a, NP-specific IgE percentages of less than 0.3% failed to induce degranulation, while those equal to or greater than 10% caused maximal degranulation. Therefore, 10% NP-specific IgE was chosen for future experiments unless indicated otherwise. Cells sensitized with 10% NP-specific IgE and stimulated with 0.01 pg/ml to 100 ng/ml of NP-BSA did not degranulate to concentrations below 100 pg/ml, but showed a dose-dependent increase in degranulation at higher concentrations (Fig. 1b). A concentration of 10 ng/ml was used to stimulate mast cells in the following experiments.
Fig. 1.
NP-BSA-induced degranulation of human skin mast cells. a % NP-specific IgE. Human skin mast cells, sensitized with different percentages of NP-specific IgE (total IgE=1 μg/ml), were washed and activated with NP-BSA (10 ng/ml) or 22E7 (1 μg/ml; n=3). b NP-BSA dose–response. Mast cells, sensitized with 10% NP-specific IgE (1 μg/ml total IgE), were stimulated with different concentrations of NP-BSA (n=3). Mean±SD, dagger, p<0.001 compared to non-stimulated cells
To simulate the step-wise, escalating doses of a drug that is administered in vivo to desensitize patients, sequential desensitization experiments were performed in vitro. Human skin mast cells were sensitized overnight with human IgE of which 10% was NP-specific. Sensitized cells were washed and sequentially incubated with increasing concentrations of NP-BSA (from 1 pg/ml to 10 ng/ml) that were approximately 2- to 2.5-fold every 15 min. At the end of this desensitization process, 17±2.8% of the β-hexosaminidase had been released, which was significantly higher than the 4.4±1.1% released by cells in buffer alone. However, further stimulation by FcεRI aggregation did not result in additional degranulation. As shown in Fig. 2a, neither NP-BSA nor 22E7 caused appreciable or significant degranulation above that which occurred during desensitization alone. Compared to non-desensitized cells, NP-BSA- and 22E7-stimulated degranulation values were significantly and substantially lower in the NP-desensitized cells. However, these NP-desensitized cells were still capable of degranulating to non-FcεRI-dependent stimuli. For example, %degranulation values after stimulation of desensitized cells with substance P, with compound 48/80, and with C5a that act through G protein receptor-coupled pathways and with A23187, a calcium ionophore, were not significantly different than values with non-desensitized cells. These results suggest that desensitization affects proximal events in the FcεRI-initiated pathway, whereas G protein-coupled receptor and calcium ionophore degranulation pathways remain available.
Fig. 2.
Desensitization of mast cells with sequential NP-BSA concentrations. Bars: black, degranulation during desensitization or exposure to buffer alone; grey, degranulation during exposure to activating concentrations of stimulants. a Sequential allergen desensitization. Mast cells sensitized with 10% NP-specific IgE (1 μg/ml total IgE) were desensitized with increasing NP-BSA concentrations (“Methods” section; n=4). Degranulation of desensitized mast cells was compared to that of corresponding non-desensitized mast cells. b Degranulation during each step of sequential allergen desensitization (n=3) as performed in a with 10% NP-specific IgE-sensitized (1 μg/ml total IgE) mast cells. Degranulation at each dose of NP-BSA was compared to that at 1 pg/ml. c Degranulation of mast cells sensitized with 100% NP-specific IgE (1 μg/ml) after sequential allergen desensitization (as in a; n=3). 22E7 and NP-BSA-challenged desensitized cells were compared to NP-BSA-challenged non-desensitized cells, and to desensitized and non-desensitized cells challenged with buffer. a–c Asterisk, p<0.05; dagger, p<0.001. d Calculations of %Des and %DegDD using simulated bar values. A, net degranulation during desensitization; C, net degranulation of desensitized cells upon stimulation; B, net degranulation of non-desensitized cells upon stimulation after subtracting spontaneous degranulation; D=B-A, net degranulation of non-desensitized cells after subtracting degranulation during desensitization
To monitor degranulation during desensitization, accumulated release of β-hexosaminidase was measured after each step of antigen treatment. At doses ≤200 pg/ml, no significant degranulation occurs. However, from doses of 500 pg/ml to 5 ng/ml, the accumulated release of β-hexosaminidase became significantly higher than spontaneous release (Fig. 2b). The incremental net% release values for β-hexosaminidase ranged from 0.5 to 8.7 over these desensitization concentrations. A plateau in β-hexosaminidase release appeared to occur at an accumulated allergen dose of 5 ng/ml, probably indicating that desensitization had been achieved at this point in the protocol.
Sequential desensitization is also observed when human skin mast cells are sensitized with 100% NP-specific IgE (1 μg/ml) overnight and then incubated with incremental doses of NP-BSA. As shown in Fig. 2c, mast cells desensitized in this manner showed virtually no response to further stimulation with both 22E7 and NP-BSA, a pattern that is essentially identical to that observed with mast cells armed with 10% NP-specific IgE. Thus, desensitization is essentially complete for mast cells armed with 100% as well as with 10% NP-specific IgE.
The effectiveness of desensitization depends both upon the amount of degranulation that occurs during the desensitization process, and the amount of degranulation that can be elicited by stimulation of desensitized cells. Further, both of these measures relate to the optimal amounts of allergen-mediated degranulation that occur with non-desensitized cells. A rigorous assessment of desensitization should take into account both of these parameters. Accordingly, we define %degranulation during desensitization (%DegDD) as 100 times the net% release during desensitization divided by the net% release of non-desensitized cells, whereby the spontaneous release of non-desensitized cells is subtracted from both the numerator and denominator (because this material is not available to be released by allergen stimulation; Fig. 2d). A low % DegDD is desirable; 100 meaning degranulation during desensitization equals that of non-desensitized cells stimulated with an optimal activating concentration of allergen, and 0 meaning no degranulation during desensitization. The %DegDD values for NP sequential desensitization and NP challenge in Fig. 2a and c were 29 and 30, respectively. For NP sequential desensitization and 22E7 challenge in Fig. 2a, %DegDD was 29. The second parameter, % desensitization (%Des), is defined as 100 times the difference of 1 minus the ratio of net% release of desensitized cells after optimal stimulation over the net% release of optimally stimulated non-desensitized cells (whereby the amount released during desensitization is subtracted from both the numerator and denominator because this material is no longer available to be released by desensitized cells; Fig. 2d). A higher %Des is desirable; 100 being complete desensitization and 0 being no desensitization. The %Des values for NP sequential desensitization and NP challenge in Fig. 2a and c were 100 and 96, respectively. For NP sequential desensitization and 22E7 challenge in Fig. 2a, %Des was 96. Notably, % Des values for substance P, compound 48/80, C5a, and A23187 were <10.
Exposure of Human Skin Mast Cells to a Fixed Concentration of DNP-BSA Is Not Optimal for Desensitization
Whether exposure to a single dose of antigen, particularly one that is suboptimal for causing degranulation, could desensitize human skin mast cells was determined. Skin mast cells sensitized as above were incubated overnight with concentrations of NP-BSA ranging from 0.0006 to 10 ng/ml in parallel with a buffer control (desensitization phase). These cells were then stimulated with NP-BSA (10 ng/ml; activation phase). As shown in Fig. 3, mast cell degranulation was monitored during both the desensitization (black bars) and activation (grey bars) phases. Mast cells exposed to NP-BSA concentrations ≥2.5×10−3 ng/ml of NP-BSA (fourth bar from left) degranulated in total during desensitization and activation phases to a greater extent than non-desensitized activated cells (first bar on left). At the two antigen doses <5×10−3 ng/ml, no desensitization was detected, %Des being <5% in each case. Thus, no dose of NP-BSA used for fixed-dose desensitization compared favorably with the sequential allergen desensitization protocol described above.
Fig. 3.
Attempted desensitization of mast cells with fixed concentrations of NP-BSA. Mast cells sensitized with 100% NP-specific IgE (1 μg/ml) were incubated overnight with various fixed concentrations of NP-BSA (“Methods” section), and then stimulated with 10 ng/ml of NP-BSA (n=3). Asterisk, p=0.01 comparing each value to the buffer control. Bars: black, degranulation during desensitization; grey, degranulation during activation
To assess whether a fixed dose of 22E7 might desensitize mast cells more effectively than a fixed dose of NP-BSA, 22E7 was incubated overnight with human skin mast cells at concentrations ranging from 0.0006 to 100 ng/ml (desensitization phase). Mast cells were then exposed to 100 ng/ml of 22E7 for 30 min (activation phase; data not shown). At desensitizing concentrations below those causing significant levels of degranulation (≤0.31 ng/ml), the total level of degranulation to the activating concentration of 22E7 was comparable to cells that had not been desensitized. As the desensitizing dose of 22E7 increased from 0.625 to 100 ng/ml, the less percent of release is noticed during the activation phase. At no fixed dose of 22E7 was the total degranulation (desensitization phase + activation phase) significantly lower than full strength activation by an optimal dose of 22E7. Thus, a fixed dose of neither 22E7 nor antigen led to desensitization of skin mast cells.
Mechanism of Desensitization
Surface expression of CD63 on mast cells was examined as an additional indicator of mast cell activation. As shown in Fig. 4a, CD63 expression is high on only 2±0.3% of the unstimulated mast cells, whereas stimulation of these non-desensitized cells with 22E7 results in high CD63 expression on 54±3% of the cells, a significant increase. In contrast, NP-BSA-desensitized mast cells exhibit high CD63 expression on 18±7% of the mast cells; but after stimulation of desensitized mast cells with 22E7, high CD63 expression is found on 19±8% of the cells, an insignificant difference. Thus, CD63 surface expression corresponds to degranulation of desensitized compared to non-desensitized skin mast cells.
Fig. 4.
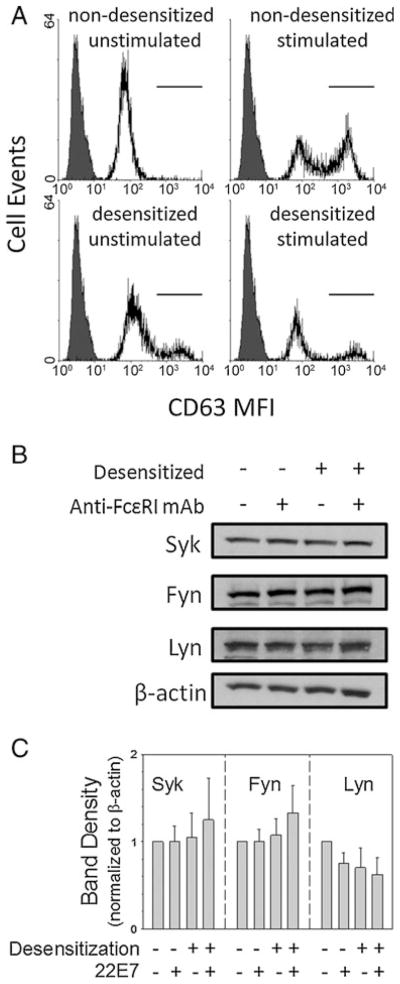
Effect of sequential desensitization on surface CD63 and on Syk, Fyn, and Lyn. Mast cells sensitized with 10% NP-specific IgE (100 μg/ml total IgE) were either not desensitized or sequentially desensitized with NP-BSA. a Surface CD63 (n=3) after stimulation with NP-BSA (10 ng/ml). Filled, isotype control; unfilled, anti-CD63. b Syk, Fyn, and Lyn Western blot 5 min after stimulation with 22E7. c Analysis of Western blot band densities (n=4)
Src family kinases are involved in proximate signal transduction events following FcεRI stimulation. As shown in Fig. 4b, c, the total protein expression levels (normalized to those of β-actin) of Syk, Fyn, and Lyn by Western blotting in activated, in desensitized, and in desensitized and activated mast cells are not significantly different from the levels in unstimulated and non-desensitized mast cells p=0.92, 0.60, and 0.42, respectively). Thus, neither Lyn, Fyn, nor Syk are consumed during desensitization to an extent that would significantly lower their levels in these cells.
The cytoplasmic calcium concentration rises with stimulation of both FcεRI and certain G protein-coupled receptors. To further explore the underlying mechanism of desensitization, intracellular calcium levels were measured. As shown in Fig. 5, non-desensitized cells demonstrated a robust calcium flux when activated with either 22E7 or with C5a. However, desensitized cells fail to increase their intracellular calcium levels when stimulated with 22E7. In contrast, the calcium flux in response to C5a stimulation of the G protein-coupled receptor CD88 is not diminished by desensitization. Thus, allergen-desensitized cells are capable of raising intracellular calcium levels after C5a stimulation, but fail to do so when stimulated with allergen.
Fig. 5.
Sequential desensitization and calcium flux. Mast cells sensitized with 10% NP-specific IgE (1 μg/ml total IgE) were either not desensitized (a, c) or desensitized (as in Fig. 2; b, d) and were then stimulated with 22E7 (100 ng/ml, a, b) or C5a (100 ng/ml, c, d) as indicated by arrows. Representative real-time OD 340/380 ratios from one of three independent experiments are shown
Cross-Desensitization Between NP-BSA and Der p2 Antigen
Based on the results above that NP-BSA desensitization of mast cells armed with IgE composed of 10% NP-specific IgE desensitized these cells not only to NP-BSA but also to 22E7, it would appear likely that antigen cross-desensitization would occur between different antigens. To test this prediction directly, human skin mast cells were sensitized overnight with 10% NP-specific IgE together with 10% Der p2-specific IgE. Armed mast cells were then washed and desensitized with sequential doses of NP-BSA. However, when these desensitized cells were stimulated with three different FcεRI-dependent stimuli, 22E7, NP-BSA, and aggregated Der p2, additional release of β-hexosaminidase was negligible, mean values being 1%, 0.6%, and 0.3%, respectively (Fig. 6). In contrast, non-desensitized cells had respective mean net% release values of 34, 32, and 9. Thus, desensitization with one antigen cross-desensitizes these mast cells to other antigens recognized by distinct IgE:FcεRI complexes.
Fig. 6.
Allergen cross-desensitization. Skin mast cells sensitized with 10% NP-specific and 10% Der p2-specific IgE (1 μg/ml total IgE) were sequentially desensitized with NP-BSA (%Degranulation=17± 5%), and then challenged with NP-BSA, conjugated Der p2, or 22E7, and net% degranulation was calculated (n=3). Asterisk, p<0.05
Upregulation of FcεRI with IgE Does Not Impair Incremental Allergen Desensitization
Total IgE is often elevated in atopic patients, which in turn increases FcεRI surface levels on mast cells. To test whether such upregulation affects desensitization in vitro, human skin mast cells were incubated with 1 μg/ml of IgE (10% NP-specific IgE) for 1 week prior to sequential desensitization. As expected, surface FcεRI levels rose from a net MFI of 45±4.7 before IgE to 431±109 after IgE (Fig. 7). These cells released 6.7% of their β-hexosaminidase during desensitization (%DegDD=20). However, neither NP-BSA (net% release=1.4) nor 22E7 (net% release=0.2) stimulated further degranulation, i.e., % Des was 95 for NP-BSA and 99 for 22E7. Thus, mast cells can be desensitized in vitro by sequential allergen administration regardless whether surface levels of FcεRI and allergen-specific IgE on skin mast cells are high or low.
Fig. 7.
Sequential desensitization after FcεRI upregulation. Mast cells were incubated with 10% NP-specific IgE (1 μg/ml) for 1 week and analyzed. a Surface expression of FcεRI by flow cytometry with 22E7 (n=3). Grey fill-in, isotype control; black line, baseline; grey line, upregulated. b Sequential desensitization of mast cells with NP-BSA as in Fig. 2. Mast cells were then stimulated with NP-BSA (10 ng/ml) or 22E7 (100 ng/ml) and assessed for degranulation (n=3). Dagger, p< 0.001 comparing desensitized to non-desensitized cells
Functional Recovery of Mast Cells after Desensitization
To determine whether desensitization by sequential antigen exposure in vitro is reversible, mast cells desensitized with NP-BSA as in Fig. 2a were washed and placed into fresh medium lacking IgE or allergen. At different time points afterward, the cells were challenged with NP-BSA or 22E7. Desensitized cells regained a portion of their degranulation response to 22E7 by 3 h and became fully activatable by 72 h (Fig. 8a). However, these mast cell remained unresponsive to NP-BSA throughout the 3-day time period. To determine whether this difference between 22E7 and NP-BSA responsiveness following desensitization reflected the unavailability of allergen-specific IgE, fresh NP-IgE was added to cells after desensitized cells were washed. As shown in Fig. 8b, such cells recovered about 40% of their degranulation response by 24 h, and most of their degranulation response by 48–72 h. Thus, desensitization is essentially a reversible process if the free allergen used for desensitization is removed.
Fig. 8.
Recovery of mast cells after sequential desensitization. a Mast cells, sensitized with 10% IgE anti-NP (100 μg/ml total IgE), were desensitized as in Fig. 2a, washed, placed in fresh medium for 1–72 h, and then stimulated with 22E7 (100 ng/ml) or NP-BSA (10 ng/ml; n=3). Asterisk, p<0.01; dagger, p<0.001 comparing each time point and the IgE− (only stimulated with 22E7) to the corresponding non-desensitized IgE+ cells. b Mast cells, sensitized and desensitized as above, were washed, incubated in fresh medium with NP-IgE for 24–72 h, and stimulated with NP-BSA (10 ng/ml). Asterisk, p<0.05; dagger, p<0.001 comparing each time point to the IgE+ cells (n=3)
Discussion
The current study demonstrates an in vitro model for desensitization of human skin mast cells, which may provide insights into desensitization in vivo. IgE antibodies with specificities to multiple different allergens are present in atopic patients and proportionately decorate the surfaces of their mast cells and basophils. Sensitivity to a specific allergen in vivo is in part attributed to the portion of total IgE targeted to that allergen. The current study demonstrates this to be the case with primary skin mast cells in vitro. The composition of IgE used to arm these mast cells requires at least 1% NP-specific IgE for degranulation to occur following NP-BSA stimulation, with maximal degranulation occurring when ≥10% of the IgE is NP-specific.
Allergen desensitization of patients, typically to a drug, occurs over hours and is transient after discontinuation of the allergen. In some patients, signs or symptoms attributed to mast cell activation occur during desensitization. To study desensitization of mast cells in vitro, skin mast cells were exposed to increasing concentrations of antigen. Under these conditions, a modest amount of degranulation occurs during desensitization, but the greatest amount of degranulation occurring after any one desensitization dose is less than 10%. It is likely that small amounts of degranulation also occur in some patients during desensitization. However, small amounts of histamine released in vivo would be rapidly metabolized, perhaps minimizing the clinical response, while in vitro released β-hexosaminidase accumulates.
Importantly, upon completion of mast cell desensitization with sequential NP-BSA in vitro, mast cells no longer degranulate to NP-BSA or to anti-FcεRI. If mast cells are sensitized with NP and Der p2 IgE antibodies, desensitization with NP-BSA cross-desensitizes them to Der p2. If cross-allergen desensitization occurs in vivo, one could consider clinical scenarios whereby desensitization to one allergen might usefully reduce sensitivity to another allergen, e.g., someone with multiple drug allergies desensitized to one drug might tolerate others.
The current study also demonstrates that in vitro desensitization of mast cells with NP-BSA is reversible after free NP-BSA is removed (Fig. 8). Such mast cells regain partial sensitivity 1 day later and full sensitivity to anti-FcεRI antibody after 2–3 days. However, sensitivity to NP-BSA did not return during this time interval unless fresh NP-specific IgE were added to the washed and desensitized cells, presumably because the NP-specific IgE that had previously been used to arm these cells was no longer available to bind to fresh antigen. Whether this is because the NP-BSA:IgE:FcεRI complexes are internalized or simply occupied was not determined. A study in which human foreskin-derived mast cells had been sensitized with atopic serum and desensitized with increasing doses of goat anti-IgE Ab showed that apparent desensitization could be rapidly reversed (≤2 h) by re-incubating such mast cells with atopic serum [20], indicating that unresponsiveness was due to removal or neutralization of surface IgE rather than desensitization to FcεRI-mediated signaling as in the current study. One important difference between the desensitization protocols of this previous study and the current one is that mast cells were washed after each incubation step in the former, but not in the current study. When skin mast cells were washed after each incubation step (data not shown), the cumulative degranulation during desensitization was comparable to desensitization with fixed doses of antigen as shown in Fig. 3. In vivo, unlike in vitro, the desensitizing drug accumulates during desensitization because it cannot be easily washed away. Each drug has a characteristic elimination time course with respect to free drug. Much less well-understood is the time course for removal of proteins that are haptenized with a drug, which might be considerably longer than for free drug, thereby prolonging the interval of clinical tolerance post-desensitization. On the other hand, newly synthesized antigen-specific IgE might serve to diminish the tolerant interval.
One question of interest regarding desensitization is whether tolerance is the result of blocking antigen-specific IgE aggregation by antigen rather than of tolerizing the mast cell itself. Against this possibility is that neither anti-FcεRI antibody (which cross-links all surface FcεRI molecules) nor aggregated Der p2 (which should aggregate only IgE anti-Der p2) activate mast cells that had been armed with 10% NP-specific IgE and 10% Der p2-specific IgE and desensitized with NP-BSA (Fig. 6). Thus, desensitization by the sequential antigen protocol likely affects an event distal to allergen-mediated aggregation of FcεRI.
Regarding the cellular and molecular mechanism(s) of desensitization, early studies revealed that desensitization of basophils [21, 22] and mast cells [23–25] could be achieved in calcium-free medium, a non-physiologic condition. Later, peripheral blood basophils were desensitized to a fixed dose of allergen or of anti-IgE antibody in calcium-replete medium [26]. A potential clue to the mechanism of desensitization emerged, as discussed in the “Introduction” section, related to Syk deficiency [6, 9–11], an essential molecule for FcεRI-mediated mast cell degranulation. However, in the current study, no consumption of Syk was observed either with sequential antigen desensitization (Fig. 4) or with fixed-dose antigen desensitization (not shown). Further, neither Fyn nor Lyn was consumed during desensitization. Although this does not rule out a more subtle involvement of these tyrosine kinases in desensitization, gross changes in cellular levels did not occur.
More recently, desensitization studies on IL-3-dependent bone marrow-derived murine mast cells indicated sequential was more effective than fixed-dose antigen exposure [27], in agreement with the current study using human mast cells. Estimating from the murine β-hexosaminidase release data (Fig. 1c [25, 27]), the %DegDD was 25 while the % Des was 58, values that appear to be less optimal than those in the current study. Another difference was that in these murine mast cells, when armed with IgE against two distinct antigens (DNP-human serum albumin and ovalbumin) and desensitized with only one antigen, cross-antigen desensitization was not observed. Whether these differences relate to human versus mouse, type or maturity of the mast cells or experimental conditions are uncertain. Another study of desensitization used rat peritoneal mast cells that had been sensitized with mouse IgE anti-DNP and then exposed to sequential tenfold increments of DNP-human serum albumin [25], washing the cells between each step. In this study, the cumulative release of histamine during desensitization was about two-thirds of that released by an optimal fixed dose of antigen on non-desensitized cells.
Proximal impairment of FcεRI-mediated signal transduction in allergen-desensitized human skin mast cells seems likely because allergen-desensitized mast cells are activated by C5a and substance P through G protein-coupled receptors and A23187, a calcium ionophore (Fig. 2). Also, C5a induces a calcium flux in both non-desensitized and desensitized cells, suggesting CRAC channels and Ca2+ storage are functional in desensitized mast cells. Of possible clinical relevance is that non-IgE-dependent mast cell activation in patients, e.g., by vancomycin, opioids, or radio-contrast media, would likely be unaffected by allergen-mediated desensitization.
Atopic patients often have elevated IgE levels [28]. IgE is known to increase levels of FcεRI on the surface of skin mast cells in vitro [29], thereby lowering the threshold for anti-FcεRI antibody to activate these cells. However, in the current study, antigen-induced desensitization also occurred with mast cells, on which FcεRI had been upregulated by IgE (Fig. 7). Thus, desensitization appears to be effective under both high and low IgE:FcεRI conditions.
In summary, by using an in vitro model, the current study demonstrates that primary human skin mast cells can be made tolerant to antigen by desensitization with sequential doses of antigen. Such desensitization affects IgE:FcεRI-dependent activation of mast cells, causing antigen cross-desensitization, but does not affect activation through G protein-coupled receptors and calcium ionophore. Further, in vitro desensitization is reversible over 2–3 days. Finally, the current study utilized purified mast cells and does not exclude the participation of other cell types such as T cells, dendritic cells, or keratinocytes in vivo. A better understanding of desensitization could facilitate immunotherapy of patients by using desensitization to neutralize mast cells and perhaps basophils such that safer and more effective immunotherapy targeting B and T cells could be administered with a reduced risk for immediate hypersensitivity reactions.
Acknowledgments
This study was supported in part by the National Institutes of Health grants U19AI077435 (LBS) and K08AI057357 (WZ). We would like to thank Shao-Hua Yu for her excellent technical assistance.
Abbreviations
- NP
4-hydroxy-3-nitrophenylacetyl
- %DegDD
%degranulation during desensitization
- %Des
%desensitization
Contributor Information
Wei Zhao, Department of Pediatrics, Virginia Commonwealth University, P.O. Box 980225, Richmond, VA 23298-0225, USA.
Gregorio Gomez, Department of Internal Medicine, Virginia Commonwealth University, P.O. Box 980263, Richmond, VA 23298-0263, USA.
Matthew Macey, Department of Pediatrics, Virginia Commonwealth University, P.O. Box 980225, Richmond, VA 23298-0225, USA.
Christopher L. Kepley, Joint School of Nanoscience and Nanoengineering, Greensboro, NC 27401, USA
Lawrence B. Schwartz, Email: lbschwar@vcu.edu, Department of Internal Medicine, Virginia Commonwealth University, P.O. Box 980263, Richmond, VA 23298-0263, USA
References
- 1.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 2.Golden DB. Insect sting allergy and venom immunotherapy. Ann Allergy Asthma Immunol. 2006;96:S16–21. doi: 10.1016/s1081-1206(10)60897-6. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan TJ, Yecies LD, Shatz GS, Parker CW, Wedner HJ. Desensitization of patients allergic to penicillin using orally administered beta-lactam antibiotics. J Allergy Clin Immunol. 1982;69:275–82. doi: 10.1016/s0091-6749(82)80004-3. [DOI] [PubMed] [Google Scholar]
- 4.Stark BJ, Earl HS, Gross GN, Lumry WR, Goodman EL, Sullivan TJ. Acute and chronic desensitization of penicillin-allergic patients using oral penicillin. J Allergy Clin Immunol. 1987;79:523–32. doi: 10.1016/0091-6749(87)90371-x. [DOI] [PubMed] [Google Scholar]
- 5.Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al. General considerations on rapid desensitization for drug hypersensitivity—a consensus statement. Allergy. 2010;65:1357–66. doi: 10.1111/j.1398-9995.2010.02441.x. [DOI] [PubMed] [Google Scholar]
- 6.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Syk deficiency in nonreleaser basophils. J Allergy Clin Immunol. 1999;104:279–84. doi: 10.1016/s0091-6749(99)70367-2. [DOI] [PubMed] [Google Scholar]
- 7.Black KM, Lussier AM, Gion WR, Kasaian MT. Cytokine priming of human basophils: description of allergen ‘nonreleasers’. Int Arch Allergy Immunol. 1996;111:142–51. doi: 10.1159/000237359. [DOI] [PubMed] [Google Scholar]
- 8.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Multiple defects in Fc epsilon RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol. 2000;165:5913–20. doi: 10.4049/jimmunol.165.10.5913. [DOI] [PubMed] [Google Scholar]
- 9.Kepley CL, Cohen N. Evidence for human mast cell nonreleaser phenotype. J Allergy Clin Immunol. 2003;112:457–9. doi: 10.1067/mai.2003.1671. [DOI] [PubMed] [Google Scholar]
- 10.MacGlashan D, Miura K. Loss of syk kinase during IgE-mediated stimulation of human basophils. J Allergy Clin Immunol. 2004;114:1317–24. doi: 10.1016/j.jaci.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005;138:29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 12.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–8. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–8. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matin R, Tam EK, Nadel JA, Caughey GH. Distribution of chymase-containing mast cells in human bronchi. J Histochem Cytochem. 1992;40:781–6. doi: 10.1177/40.6.1588024. [DOI] [PubMed] [Google Scholar]
- 15.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 16.Riske F, Hakimi J, Mallamaci M, Griffin M, Pilson B, Tobkes N, et al. High affinity human IgE receptor (Fc epsilon RI). Analysis of functional domains of the alpha-subunit with monoclonal antibodies. J Biol Chem. 1991;266:11245–51. [PubMed] [Google Scholar]
- 17.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–52. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Gomez G, Yu SH, Ryan JJ, Schwartz LB. TGF-beta1 attenuates mediator release and de novo Kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–72. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz LB, Austen KF. Acid hydrolases and other enzymes of rat and human mast cell secretory granules. Kroc Found Ser. 1981;14:103–21. [PubMed] [Google Scholar]
- 20.Rubinchik E, Shalit M, Levi-Schaffer F. Responsiveness of human skin mast cells to repeated activation: an in vitro study. Allergy. 1998;53:14–9. doi: 10.1111/j.1398-9995.1998.tb03768.x. [DOI] [PubMed] [Google Scholar]
- 21.MacGlashan DW, Jr, Lichtenstein LM. The transition from specific to nonspecific desensitization in human basophils. J Immunol. 1981;127:2410–4. [PubMed] [Google Scholar]
- 22.Pruzansky JJ, Patterson R. Desensitization of human basophils with suboptimal concentrations of agonist. Evidence for reversible and irreversible desensitization. Immunology. 1988;65:443–7. [PMC free article] [PubMed] [Google Scholar]
- 23.MacGlashan D, Jr, Lichtenstein LM. Basic characteristics of human lung mast cell desensitization. J Immunol. 1987;139:501–5. [PubMed] [Google Scholar]
- 24.Ishizaka T, Sterk AR, Daeron M, Becker EL, Ishizaka K. Biochemical analysis of desensitization of mouse mast cells. J Immunol. 1985;135:492–501. [PubMed] [Google Scholar]
- 25.Shalit M, Levi-Schaffer F. Challenge of mast cells with increasing amounts of antigen induces desensitization. Clin Exp Allergy. 1995;25:896–902. doi: 10.1111/j.1365-2222.1995.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 26.Komiya A, Hirai K, Iikura M, Nagase H, Yamada H, Miyamasu M, et al. Induction of basophil desensitization in physiological medium: enhancement after IgE-dependent upregulation of surface IgE binding on basophils. Int Arch Allergy Immunol. 2003;130:40–50. doi: 10.1159/000068374. [DOI] [PubMed] [Google Scholar]
- 27.Del Carmen Sancho-Serra M, Simarro M, Castells M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcepsilonRI internalization. Eur J Immunol. 2011 doi: 10.1002/eji.201040810. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Fritscher L, Chapman KR. Omalizumab for asthma: pharmacology and clinical profile. Expert Rev Respir Med. 2009;3:119–27. doi: 10.1586/ers.09.7. [DOI] [PubMed] [Google Scholar]
- 29.Gomez G, Jogie-Brahim S, Shima M, Schwartz LB. Omalizumab reverses the phenotypic and functional effects of IgE-enhanced Fc epsilonRI on human skin mast cells. J Immunol. 2007;179:1353–61. doi: 10.4049/jimmunol.179.2.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]









