Abstract
We previously established that Indole-3-Carbinol (I3C), a natural hydrolysis product of glucobrassicin in cruciferous vegetables, arrests the proliferation of estrogen-dependent human breast cancer cells and induces protein degradation of estrogen receptor-alpha (ERα). We demonstrate in human MCF-7 breast cancer cells that I3C ablates expression of Insulin-like Growth Factor Receptor-1 (IGF1R) and Insulin Receptor Substrate-1 (IRS1), downstream effectors of the IGF1 signaling pathway. Exogenous ERα reversed the I3C mediated loss of IGF1R and IRS1 gene expression demonstrating that down-regulation of ERα is functionally linked to I3C control of IGF1R and IRS1 expression. I3C disrupted binding of endogenous ERα, but not Sp1, to ERE-Sp1 composite elements within the IGF1R/IRS1 promoters. Exogenous ERα abrogated, and combined expression of IGF1R and IRS1 attenuated, the I3C mediated cell cycle arrest. Therefore, I3C inhibits proliferation of estrogen-sensitive breast cancer cells through disruption of ERα-mediated transcription of cell signaling components within the IGF1 cascade.
Keywords: I3C, Indole-3-carbinol; ERα, Estrogen receptor-alpha; IRS1, insulin receptor substrate-1; IGF1R, Insulin-like Growth Factor-1 Receptor, hormone sensitive breast cancer
1. Introduction
The Insulin-like Growth Factor-1 Receptor (IGF1R) is a transmembrane receptor tyrosine kinase that is activated upon binding to its ligand, Insulin-like Growth Factor-1 (IGF1), resulting in phosphorylation of tyrosine residues in the receptor itself and of intracellular protein substrates such as Insulin Receptor Substrate-1 (IRS1). Phosphorylated IGF1R and IRS1 recruit cellular signal transduction molecules that can activate down stream pathways involved in proliferation of hormone sensitive breast cancer cells (Chitnis et al., 2008). Aside from being a requisite for normal growth and development, the IGF1R signaling pathway is also associated with the genesis of breast cancer (Jones et al., 2009; Jones et al., 2007). High levels of serum IGF1 can increase breast cancer risk by up to 7-fold in pre-menopausal women, and is therefore often used as a prognostic indicator in these women. In this regard, elevated levels of IGF1R and IRS1 protein levels correlate extremely well with increased tumorigenicity, metastases, and invasion (Nagle et al., 2004). IGF1R expression increases by up to 14-fold in estrogen sensitive breast cancer cells compared to normal epithelial cells (Happerfield et al., 1997).
The pro-proliferative activities of estradiol (E2) in breast cancer cells are mediated by the Estrogen Receptor-alpha (ERα) dependent transcriptional regulation of target genes that trigger cell cycle progression and cell survival responses. E2 activated ERα interacts with its target gene promoters through binding as homodimers to Estrogen Response Elements (ERE) or binding as heterodimers with transcription factor binding partners to composite DNA elements that contain an ERE half site and the corresponding transcription factor binding site (Marino et al., 2006). The resulting transcription factor complex that is tethered to the estrogen receptor includes breast cancer cell expressed co-activators and chromatin remodeling factors that direct transcription of genes involved in cell proliferation (Shibata et al., 1997; Schultz-Norton et al., 2011). Several studies show that the expression of IGF1R and IRS1 is regulated by E2 in estrogen sensitive breast cancer cells (Clarke et al., 1997; Mauro et al., 2001; Stewart et al., 1990), whereas, ERα negative breast cancer cell lines express comparatively low levels of IGF1R and IRS1 (Surmacz and Bartucci, 2004).
Elucidation of the IGF1 receptor activated signaling cascade and its role in controlling proliferation of breast cancer cells has influenced the development of several therapeutic strategies that target this pathway. One approach has been to disrupt IGF1 binding to IGF1R, either by employing neutralizing monoclonal antibodies directed against IGF1, or by increasing levels of IGF Binding Proteins (IGFBP), which naturally bind and sequester IGF1 (Goya et al., 2004; Van den Berg et al., 1997). In a complementary manner, monoclonal antibodies can be directed toward IGF1R to induce receptor-mediated endocytosis, followed by proteolytic degradation (Lee et al., 2003; Sachdev et al., 2003; Wang et al., 2005). However, antibody binding can, in some cases, induce partial activation of the receptor and its proliferative pathway (Li et al., 2000).
An alternative strategy is to chemically disrupt IGF1R activity with membrane permeable tyrosine kinase inhibitors that block IGF1R phosphorylation and subsequent interaction with specific sets of cell signaling molecules (Carboni et al., 2005; Garcia-Echeverria, 2006; Wittman et al., 2005). The lack of specificity of currently used inhibitors, due to the homology between receptor tyrosine kinases, makes this strategy less promising because of the high probability of off-target effects (Riedemann J. and Macaulay V.M., 2006). Because IGF1R and IRS1 are expressed in the majority of normal tissues, and for example play essential roles in enhanced neuronal survival, maintenance of cardiac function, and survival of pancreatic beta cells, disruption of these activities can potentially lead to significant side effects (Da Silva Xavier et al., 2004; Liu et al., 2009). Therefore, an important issue is to develop new therapeutic strategies that target the IGF signaling axis with increased efficacy and reduced side effects.
Epidemiological and physiological studies have suggested that phytochemicals from vegetables and fruits represent intriguing natural sources to uncover new classes of potential anti-cancer molecules with minimal adverse side effects (Manson et al., 2005). One such phytochemical is Indole-3-Carbinol (I3C), a natural compound derived by hydrolysis from glucobrassicin produced in Brassica cruciferous vegetables such as cabbage, broccoli, and Brussels sprouts (Aggarwal and Ichikawa, 2005). There is compelling evidence in estrogen-sensitive human breast cancer cell lines, such as MCF-7 and T47D, that I3C treatment disrupts estrogen responsive gene expression and inhibits estrogen-dependent cell proliferation (Auborn et al., 2003; Cover et al., 1999; Wang et al., 2006; Sundar, et al., 2006; Firestone and Sundar, 2009). We now demonstrate that I3C blocks expression of both IGF1R and IRS1 transcript and protein levels in estrogen responsive human breast cancer cells through the targeted disruption of ERα expression and loss of endogenous ERα interactions with the promoters of both genes. We also show that the down regulation of IGF1R and IRS1 expression contributes to the I3C cell cycle arrest of human breast cancer cells that express functional ERα.
2. Materials & methods
2.1 Reagents
Indole-3-Carbinol (I3C), 17β-Estradiol (E2), and dimethylsulfoxide (DMSO) were obtained from Sigma Chemical Company (St. Louis, MO). Propyl pyrazole triol (PPT) was obtained from LC Laboratories (Woburn, MA). All other chemicals were of the highest quality available.
2.2 Cell Culture
MCF-7 human breast cancer cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in Dulbecco’s Modified Eagles Medium (DMEM) from BioWhittaker (Walkersville, MD), supplemented with 10% fetal bovine serum from Mediatech (Manassas, VA), 10 mg/ml insulin, 50 U/ml penicillin, 50 U/ml streptomycin, and 2 mM L-glutamine from Sigma (St. Louis, MO). Cells were grown to subconfluency in a humidified chamber at 37°C containing 5% CO2. Stock solutions of 200 mM I3C, 100 mM PPT and 10 mM E2 were prepared by dissolving each in DMSO. I3C, PPT, or E2 was then diluted 1:1000 in media prior to culture plate application. Phenol red-free media supplemented with 10% dextran charcoal-stripped media from Gemini Bio-Products (Sacramento, CA) was employed for all estrogen sensitivity assays.
2.3 Western Blotting
After the indicated treatments, western blots were performed as previously indicated (Sundar et al., 2006). Rabbit anti-IRS1 (CS-2382), and rabbit anti-IGF1R (CS-3027) were diluted 1:200 in Tris-Buffered Saline and Tween 20 (TBST) (Cell Signaling Technology, Danvers, MA). Mouse anti-ERα (sc-8005), was diluted 1:200 in TBST (Santa Cruz Biotechnology, Santa Cruz, CA). HSP90 (#610419 BD Transduction laboratories, Franklin Lakes, NJ), HSP60 (Cell Signaling Technology, Danvers, MA), and actin (#AAN01 Cytoskeleton, Inc. Denver, CO) were used as loading controls, and antibodies for these were diluted 1:2000 and 1:1000 respectively, in TBST. Immunoreactive proteins were detected after incubation with horseradish peroxidase-conjugated secondary antibodies diluted 3×10−4 in 1% Non-Fat Dried Milk (NFDM) in TBST. Blots were then treated with enhanced chemiluminescence reagents (Eastman Kodak, Rochester NY) visualization on film.
2.4 Expression Plasmid Transfection
Cells were grown and indicated treatments performed on 10 cm tissue culture plates from Nunc (Fisher Scientific, Rochester, NY). Human CMV-IRS1 expression plasmid was obtained from Addgene, “Addgene plasmid 11238” (Cambridge, MA). Human pBABE-IGFIR plasmid was obtained from Addgene “Addgene plasmid 11212” (Cambridge, MA). Human CMV-ERα was a kind gift from Dr. Benita Katzenellenbogen, University of Illinois at Urbana-Champagne. Transfection of expression vectors was performed using Polyfect transfection reagent from Qiagen (Valencia, California) per manufacturers’ recommended protocol.
2.5 RT-PCR
Total RNA from MCF-7 cells treated with indicated compounds was isolated with Trizol Reagent according to manufacturer’s protocol from Sigma (St. Louis, MO). Total RNA (4 μg) was used to synthesize cDNA using Moloney murine leukemia virus-reverse transcriptase from Promega Corp (Madison, WI) with random hexamers as primers. The cDNA reaction product (400 ng) was amplified with primers of the following sequences: ERα Forward: 5′-AGC ACC CAG TGA AGC TAC T-3′, ERα Reverse: 5′-TGA GGC ACA CAA ACT CCT-3′; IGF1R Forward: 5′-TGA GGA TCA GCG AGA ATG TG-3′, IGF1R Reverse 5′-GAC CCA TTC CCA GAG AGA GA-3′; PR Forward: 5′-CGA AAA CCT GGC AAT GAT TTA GAC-3′, PR Reverse 5′-GAA CCA GAT GTG ATC TAT GCA GGA-3′; IRS1 Forward: 5′-CAG AGG ACC GTC AGT AGC TCA A-3′, IRS1 Reverse 5′-GGA AGA TAT GAG GTC CTA GTT GTG AAT-3′; GAPDH Forward 5′-TGA AGG TCG GAG TCA ACG GAT TTG-3′, GAPDH Reverse: 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′. PCR products were analyzed on 1.2 % agarose gel along with 1-kb Plus DNA ladder from Invitrogen (Carlsbad, CA) and the products were visualized with GelRed from Biotium (Hayward, CA).
2.6 Chromatin Immunoprecipitation (ChIP) Assays
MCF-7 cells were grown to subconfluency and treated for 48 hours with 200 μM I3C or DMSO vehicle control. ChIP was performed as previously described (Sundar et al, 2008). Primers for ChIP experiments were as follows: pIRS1 Forward: 5′-ACA CCC ATT GAA CCA CCC TA-3′, Reverse: 5′-CGT TTG TTT GTG GGG AGA CT-3′; pIGF1R Forward: 5′-GGA GCC GCT CAT TCA TTT TGA C-3′, Reverse: 5′-CTA GGC GAG GAA AAA CAA GC-3′. Products were visualized on a 1.5 % agarose gel buffered with Tris-Boric Acid-EDTA.
2.7 Flow Cytometry
MCF7 cells were plated onto six-well tissue culture dishes and grown in phenol red-free media containing dextran charcoal-stripped 10 % FBS. Cells were treated with 10 nM E2 or 100 nM PPT in the presence or absence of 200 mM I3C. Cells were exposed to the indicated treatments for 48 hours and hypotonically lysed in 300 mL of DNA staining solution (0.5 mg/mL propidium iodide, 0.1 % sodium citrate, and 0.05 % Triton-X 100). Nuclear emitted fluorescence wavelength of more than 585 nm was measured with a Coulter Elite instrument with laser output adjusted to deliver 15 mW at 488 nm. Nuclei (10,000) were analyzed from each sample at a rate of 300-500 nuclei/second. The percentage of cells within the G1, S, and G2/M phases of the cell cycle were determined by analysis with the Multicycle computer program provided by Phoenix Flow Systems in the Cancer Research Laboratory Microchemical Facility of the University of California, Berkeley. This experiment was repeated twice to confirm results.
2.8 Statistical Analysis
Statistical Analysis was performed using Prism 5 Software. Dose responses, multi cell line treatment, and overexpression graphs were determined using a one-way repeated measures ANOVA with the Bonferroni multiple comparisons correction to all combinations. Post-tests for linear trend were also performed on dose response graphs and deemed significant. Time courses were analyzed using paired t-tests with bonferroni correction. P values denoted as follows, “ns” > 0.05, “*”<0.05, “**”<0.01, “***”<0.001.
3. Results
3.1 I3C down-regulates expression of Insulin-like Growth Factor Receptor-1 (IGF1R) and Insulin Receptor Substrate (IRS1) in MCF-7 human breast cancer cells
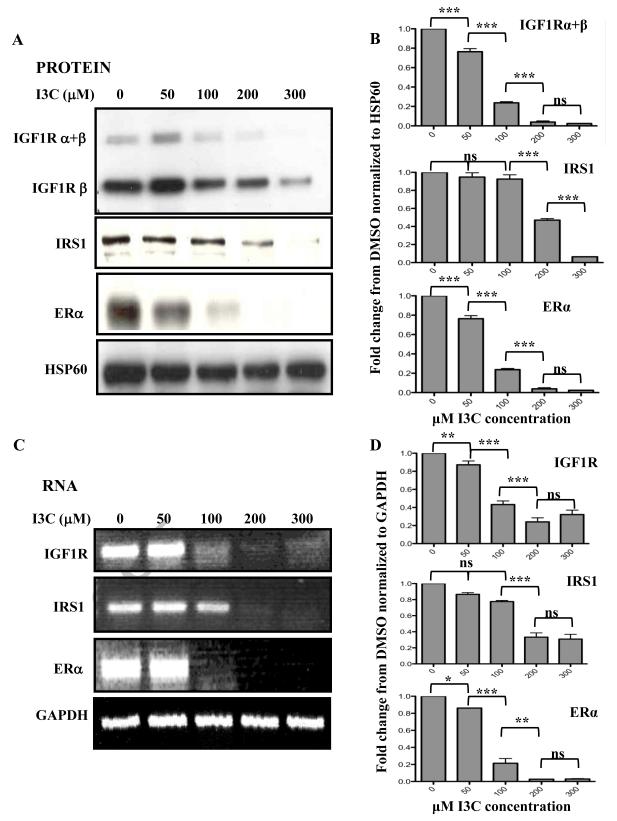
Because of the critical role of the IGF1 receptor signaling pathway in the proliferation of human breast cancer cells (Werner and Bruchim, 2009), we initially examined whether the I3C anti-proliferative response is associated with altered expression of key signaling components within this growth factor receptor pathway in human MCF-7 breast cancer cells. These cells are highly estrogen responsive and express functional ER. Initially, MCF-7 cells were treated with increasing concentrations of I3C for 48 hours, and the levels of Insulin-like growth factor-1 receptor (IGF1R) and insulin receptor substrate-1 (IRS-1) protein and transcripts determined in comparison to ERα. Western blot analysis of electrophoretically fractionated total cell extracts (Fig 1A and 1B) and RT-PCR analysis of total isolated cellular RNA (Fig 1C and 1D) revealed that I3C strongly down regulated the protein and transcripts of both IGF1R and IRS1. The half-maximal response for IGF1R was approximately at 75 μM I3C, which closely approximated that observed for ERα, consistent with previous observations showing that ER can be down-regulated by I3C in estrogen sensitive breast cancer cells (Marconett et al., 2010; Sundar et al., 2006; Wang et al., 2006). Hsp60 was used as a gel loading control for the western blots and GAPDH represented a gel loading control for the RT-PCR.
Fig. 1.
Dose-response of I3C down-regulation of IGF1R and IRS1 protein and transcript expression. MCF-7 breast cancer cells were treated with indicated concentrations of I3C for 48 hours. (A) Isolated cell lysates were fractionated by SDS polyarylamide gel electrophoresis and IGF1R ( and/or isoforms), IRS1 and ERα protein was monitored by western blot analysis in comparison the HSP90 gel loading control. Representative blots from four independent experiments are shown. (B) Densitometry quantification of the data shown in Fig 1A normalized to HSP60. Statistically significant differences are noted with asterisks. (C) Total cellular RNA was isolated, and IGF1R, IRS1 and ERα transcript expression was determined by RT-PCR in comparison to the GAPDH constitutively expressed control transcript. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide. Representative gels from four independent experiments are shown. (D) Densitometry quantification of the data shown in Fig 1C normalized to GAPDH. Statistically significant differences are noted with asterisks.
In contrast to the effects on IGF1R expression, slightly higher concentrations of I3C were needed to attain the half maximal down regulation of IRS1, which likely reflects the relative cellular stability of the IRS1 gene products (Cui et al., 2006). I3C regulation of IRS1 transcription was surprising given that IRS1 regulation typically occurs at the level of protein stability (Kang et al., 2006; Parathath S.R., 2008; Ruiz-Alcaraz A.J., et al., 2005). By 200 μM I3C, the maximal down-regulation of IGF1R and IRS1 were observed, which corresponded to the indolecarbinol concentration required for the maximal cell cycle arrest of MCF-7 breast cancer cells (Cover et al., 1999). This concentration of indole was used in the subsequent experiments.
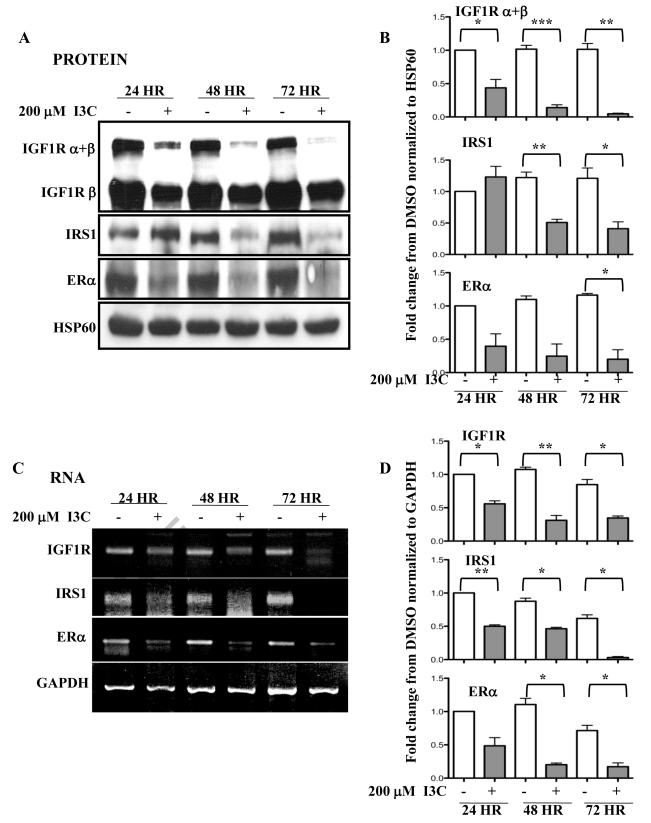
3.2 Time course of I3C mediated down-regulation of IGF1R and IRS1 expression
The kinetics of I3C down-regulation of IGF1R, IRS1 and ERα gene expression in MCF7 cells were compared over a 72 hour time course. With generally similar kinetics, I3C rapidly down-regulated the levels of IGF1R and ERα protein (Fig 2A and 2B) and transcripts (Fig 2C and 2D). At the 24 hour time point, I3C strongly inhibited the expression of both genes and by 72 hours treatment, expression of both IGF1R and ERα was barely detectable. The I3C down-regulation of IRS1 displayed a delayed kinetics in that only a minor effect was observed at 24 hours, while at 48 hours and beyond I3C significantly reduced IRS1 expression. Throughout this time course, no changes were detected in either of the gel loading controls, HSP60 (protein) or GAPDH (mRNA).
Fig. 2.
Kinetic analysis of the effects of I3C on the expression of IGF1R and IRS1 protein and transcripts. MCF-7 cells were treated with or without 200 μM I3C for the indicated times. (A) The levels of IGF1R ( and/or isoforms), IRS1 and ERα protein was monitored by western blot analysis in comparison the HSP90 gel loading control. Representative blots from four independent experiments are shown. (B) Densitometry quantification of the data from Fig 2A normalized to HSP60. Statistically significant differences are noted with asterisks. (C) IGF1R, IRS1 and ERα transcript expression was determined by RT-PCR in comparison to GAPDH constitutively expressed control transcript. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide. Representative gels from four independent experiments are shown. (D) Densitometry quantification of the data from Fig. 2C normalized to GAPDH. Statistically significant differences are noted with asterisks.
3.3 I3C disrupts IGF1R signaling via ERα in an estrogen dependent manner in ERa-expressing cell lines
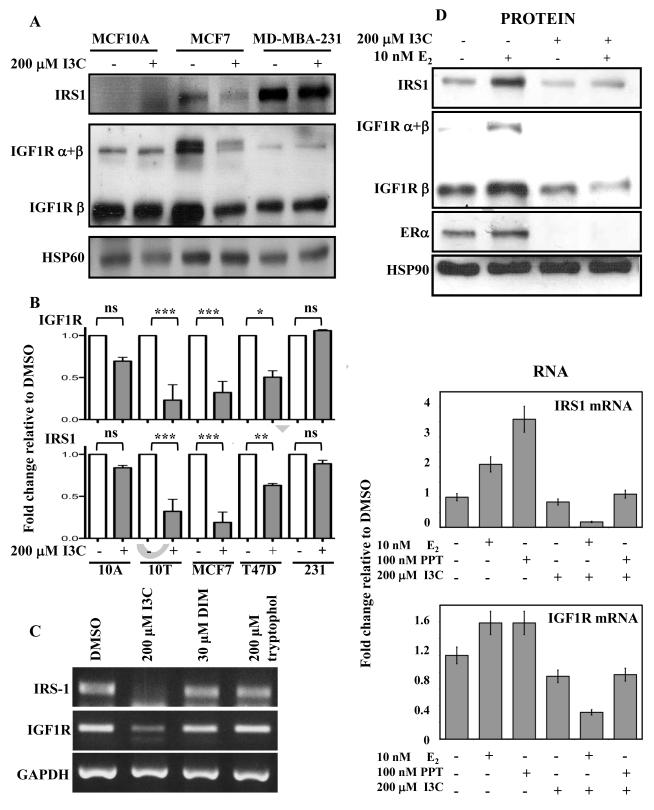
To initially determine whether there is any mechanistic link between the I3C down-regulation of ERα expression and that of IGF1R and IRS1 expression, the effects of 48 hour treatment of I3C on IGF1R and IRS1 expression was measured in multiple breast cancer cell lines of both hormone sensitive (ERα+) and hormone resistant (ERα−) phenotypes. MCF-7 cells express functional ERα, whereas, both MCF10A and MB-MDA-231 cells do not produce ERα (Zhou et al., 2007). Western blot analysis revealed that I3C strongly down regulated both IGF1R and IRS1 protein levels in the ERα+ MCF-7 cell line and failed to alter the expression levels of either gene product in the two ERα− cell lines (Fig 3A). Also, I3C downregulated IRS1 and IGF1R transcript levels in multiple estrogen sensitive cell lines, including MCF10T and T47D cells (Shekhar et al., 1998) in addition to the MCF7 cells, whereas, I3C was unable to affect transcript levels of these genes in the estrogen insensitive cell lines such as MDA-MB-231 and MCF10A cells (Fig 3B). Take together, the I3C down-regulation of IGF1R and IRS1 expression was consistently observed only in breast cancer cell lines with an ERα+ phenotype, indicating a correlation between IGF1R and IRS1 expression and ERα.
Fig. 3.
I3C down-regulation of IGF1R and IRS1 expression in estrogen responsive human breast cancer cell lines. (A) Estrogen responsive MCF-7 or estrogen insensitive MCF10A and MDA-MB-231 human breast cancer cells were treated with or without 200 μM I3C for 48 hours. Total cell lysates were fractionated by SDS-polyacrylamide electrophoreisis and the levels of IGF1R ( and/or isoforms) and IRS1 protein were monitored by western blot analysis in comparison the HSP90 gel loading control. Representative blots from three independent experiments are shown. (B) Densitometry quantification of fold changes in IGF1R and IRS1 expression in multiple breast cancer cell lines treated with or without 200 μM I3C for 48 hours. Bands were normalized to the GAPDH loading control and DMSO treatment for each cell line. Estrogen sensitive cell lines: MCF10T (denoted as 10T), MCF7, and T47D; Estrogen insensitive cell lines: MCF10A (denoted as 10A) and MD-MBA-231 (denoted as 231). (C) MCF-7 cells were treated with the indicated indole compounds or with the DMSO vehicle control for 48 hours, total RNA isolated and the levels of IGF1R and IRS1 transcript expression was determined by RT-PCR in comparison to the GAPDH constitutively expressed control transcript. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide. Representative gel results from three independent experiments are shown. (D) MCF-7 cells were grown in steroid deficient media for 24 hours, then treated with the indicated combinations of 200 μM I3C, 10 nM E2, 100 nM PPT or the DMSO vehicle control for 48 hours. IGF1R ( and/or isoforms), IRS1 and ERα protein expression was monitored by western blot analysis compared to an actin gel loading control (upper panel). Representative western blot from three independent experiments is shown. IGF1R, IRS1 and ERα transcript expression was determined by RT-PCR and the transcript levels were quantified and normalized to the constitutively expressed GAPDH (lower panel). The fold change in transcript expression relative to the DMSO vehicle control is shown as an average of three independent experiments.
We previously established that I3C blocks ERα expression by a process that is highly specific to the chemical structure of I3C, as the natural self-condensation product of I3C, 3,3′diindoylylmethane (DIM), is able to induce a G1 cell cycle arrest of MCF-7 cells but fails to alter ERα expression (Marconett et al., 2010; Sundar et al., 2006). In order to determine if the I3C mediated down-regulation of IGF1R and IRS1 was consistent with the effects of I3C on ERα, MCF-7 cells were treated for 48 hours with the DMSO vehicle control, 200 μM I3C, 30 μM DIM, or with 200 μM tryptophol, which is an indole with similar structure to I3C but with no observable affect on proliferation. Total RNA was harvested and subjected to RT-PCR. As shown in Fig 3C, I3C selectively blocked IGF1R and IRS1 transcript expression under conditions in which treatment with either DIM or tryptophol had no effect. This result shows that the down-regulation of IGF1R and IRS1 expression are specific to the mechanism by which I3C inhibits ERα expression, and not a generalized effect of overall growth arrest or due to any nonspecific indole response.
Estrogen has been shown to induce IGF1R promoter activity in a receptor dependent manner (Maor et al., 2006), and ligand-activated ERα can bind to regulatory regions of the IRS1 promoter and subsequently increase IRS1 transcription in mice (Mauro et al., 2001). Because I3C can stimulate the proteasome-mediated degradation of ERα that leads to the loss of ERα expression (Marconett et al., 2010), the ablation of ERα by I3C is predicted to disrupt estrogen dependent IGF1R and IRS1 gene expression. To directly test this concept, MCF-7 cells were grown in steroid deficient media in the presence or absence of 200 μM I3C and/or 10 nM 17-β-estradiol (E2) for 48 hours and levels of IGF1R and IRS1 protein examined. As shown in Fig 3D (upper panels), in the absence of I3C, treatment with 10 nM E2 strongly enhanced production of both IGF1R and IRS1 protein, whereas, in the presence of I3C the estrogen responsive production of both proteins was ablated. To assess whether ERα mediates this response, MCF7 cells cultured in steroid deficient medium were treated with 10 nM E2, which binds to both ER subtypes (ERα and ER ), or 10 nM propyl pyrazole triol (PPT), a relatively specific ERα ligand agonist, in the absence or presence of 200 μM I3C for 48 hours. As shown in Fig 3D (lower panels), quantification of RT-PCR analysis of total isolated RNA showed that both E2 and PPT significantly increased IRS1 and IGF1R expression, and that I3C ablated both increases in gene expression. Taken together, these results show that I3C blocks ERα dependent stimulation of IRS1 and IGF1R expression in estrogen sensitive MCF-7 breast cancer cells.
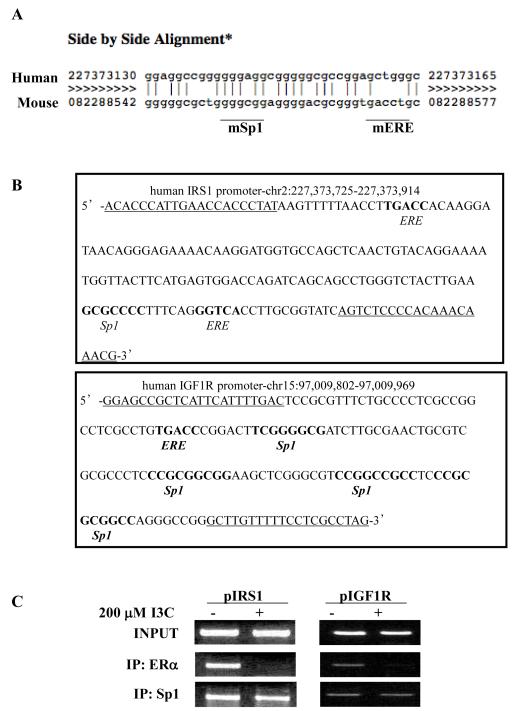
3.4 I3C disrupts ERα protein interactions with IGF1R and IRS1 promoters
The ability of I3C to disrupt estrogen induced expression of IGF1R and IRS1 suggests that the effects are mediated through I3C regulated interactions of ERα with each corresponding gene promoter. The mouse IGF1R and IRS1 promoter activities and transcripts have previously been shown to be stimulated by ligand-activated ERα (Maor et al., 2006; Mauro et al., 2001), however, relatively little is known about ERα interactions with these gene promoters in estrogen responsive human cancer cells. The sequence of the IRS1 promoter regulatory region diverges between mice and humans at the identified ERα binding site (Fig. 4A). Two potential ERα binding sites were identified in the human IGFR1 promoter within 1 kB upstream of the transcription start site; one located 222 bases upstream (−222 IGF1R) and the other 426 bases upstream (−426 IGF1R) of the transcription start site (Fig. 4B). Also, within the IRS1 gene promoter, sequence analysis revealed one potential ERα binding site at −1975 (−1975 IRS1) that is within 2 kB of the transcription start site.
Fig. 4.
I3C disrupts endogenous ERα protein binding to ERE-Sp1 composite elements in the IGF1R and IRS1 gene promoters. (A) Alignment of human and mouse genomic sequence revealed sequence divergence at the previously identified ERE site within the mouse IRS1 promoter. Human genomic sequence is on top, mouse genomic sequence is on the bottom. Matching bases denoted by vertical line. (B) Genomic sequences of the IRS1 and IGF1R promoters contain composite ERE −Sp1 sites. Primers used to amplify ERE site for chromatin immunoprecipitation are underlined. Sequence and chromosomal location were obtained from the UCSC Genome Browser. (C) Chromatin immunoprecipitation (ChIP) was employed to characterize endogenous ERα interactions with the ERE region of the IGF1R and IRS1 promoter regions. Chromatin was isolated from MCF-7 cells treated with or without 200 μM I3C for 24 hours. ERα or Sp1 were immunoprecipitated from total cell extracts using Sepharose G bound to either anti-ERα antibodies or anti-Sp1 antibodies. DNA released from ERα or Sp1 was amplified using the indicated oligonucleotide primers. Control primers directed at an alternate site in the IGF1R promoter (−426) showed no amplification in IP samples. Input samples represent total genomic DNA from each treatment (loading control). Representative gels from three independent experiments are shown.
Interestingly, the −426 IGF1R and −1975 IRS1 ER consensus binding sites are half-ERE elements located in close proximity to predicted Sp1 binding sites, potentially forming an Sp1-ERE composite element (Fig. 4B). The Sp1 transcription factor has been shown to be involved in target gene activation (Fry C.J. and Farnham P.J., 1999; Saville et al., 2000), and Sp1 activity can be selectively disrupted by I3C in a target gene specific manner (Cram et al., 2001; Marconett et al., 2011). Therefore, chromatin immunoprecipitation (ChIP) was used to determine whether I3C could affect endogenous ERα and/or Sp1 recruitment to the native IGF1R and IRS1 promoters in human breast cancer cells. MCF7 cells were treated with or without 200 M I3C for 24 hours. DNA derived from chromatin immunoprecipitated with anti-human ERα or anti-Sp1 antibodies was PCR amplified with primers specific to the Sp1-ERE composite element DNA binding sites contained within each promoter. ERα and Sp1 bound to both the −426 IGF1R and −1975 IRS1 sites in the absence of indole treatment, but not on the predicted −222 IGF1R site (Fig. 4C). Treatment with I3C ablated endogenous binding of ERα to each promoter, but had no effect on Sp1 interactions, thereby indicating that the I3C disruption of ERα binding to both the IFGR1 and IRS1 gene promoters directly leads to reduced transcription of both genes.
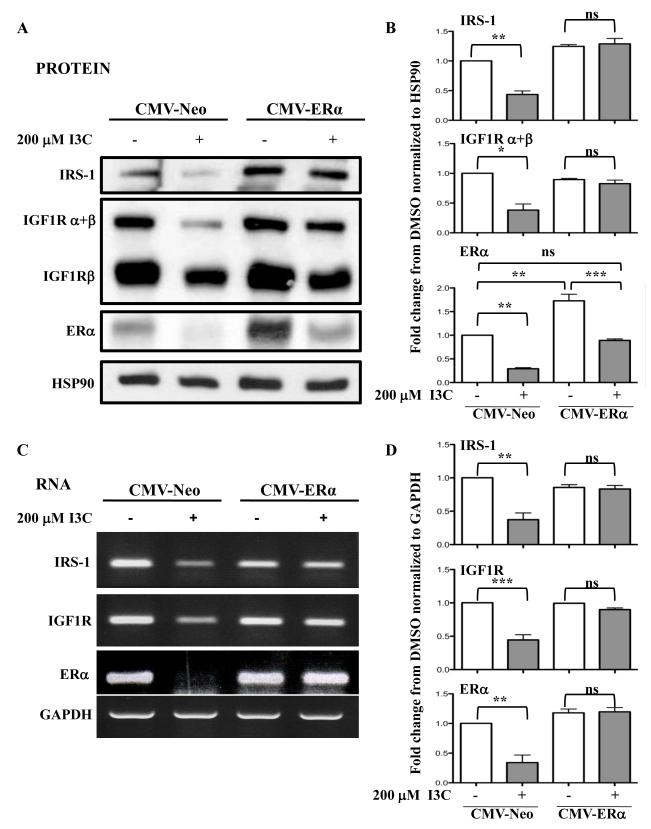
3.5 Expression of exogenous ERα rescues the I3C down-regulation of IGF-1R and IRS1 gene expression
A key prediction of whether the I3C dependent down-regulation of ERα is sufficient for loss of IGF1R and IRS1 gene expression is that transfected exogenous ERα should prevent the I3C inhibition of both gene products. To test this possibility, MCF-7 cells were stably transfected with either a CMV-ER expression vector or with a CMV-Neo empty vector control and examined for ER, IGF1R and IRS1 protein (Fig 5A and 5B) and transcript (Fig 5C and 5D) levels in the presence or absence of I3C. CMV-ERα transfected cells express higher levels of ERα than control CMV-Neo transfected cells (Fig. 5A and 5B). The exogenous ERα protein is down-regulated in I3C treated cells as predicted by the indole induced proteasomal degradation of ERα protein (Marconett et al., 2010). However, enough residual exogenous ERα protein remained that the overall ERα levels in I3C treated cells are not significantly different from endogenous levels in CMV-Neo transfected cells treated with the vehicle control. In the presence of exogenous ERα protein, the I3C-induced down-regulation of endogenous IGF1R and IRS1 transcripts and protein was effectively blocked (Fig 5). Because ectopic expression of ERα reversed the I3C down-regulation of IGF1R and IRS1 transcript expression, the I3C disruption of ERα interactions with the IGF1R and IRS1 promoters mediates the I3C-dependent loss of IGF1R and IRS1 gene expression, respectively.
Fig. 5.
Expression of exogenous ER reverses the I3C down-regulation of IGF1R and IRS1 gene expression. CMV-ER or CMV-neo transfected MCF-7 cells were treated with or without 200 μM I3C for 48 hours. (A) Total cell lysates were fractionated by SDS polyarylamide gel electrophoresis and IGF1R ( and/or isoforms), IRS1 and ERα protein was monitored by western blot analysis in comparison the HSP90 gel loading control. (B) Densitometry quantification of data from Fig 5A normalized to HSP90. Statistically significant differences are noted with asterisks. (C) Total cellular RNA was isolated, and IGF1R, IRS1 and ERα transcript expression was determined by RT-PCR in comparison to the GAPDH constitutively expressed control transcript. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide. Representative gels from 3 independent experiments shown. (D) Densitometry quantification of data from Fig 5C normalized to GAPDH. Statistically significant differences are noted with asterisks.
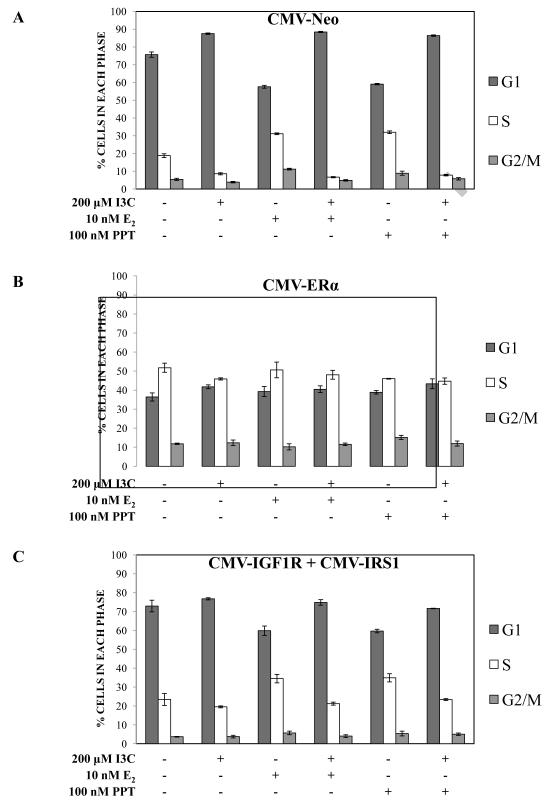
3.6 Ectopic expression of ERα or the combination of IGF1R and IRS1 disrupts I3C-mediated cell cycle arrest
To functionally test the role of IGF1R and IRS1 down-regulation in the I3C-mediated cell cycle arrest of estrogen treated breast cancer cells, MCF-7 cells were transfected with individual or combinations of IRS1 and IGF1R expression plasmids driven from constitutive viral promoters (CMV-IRS1 and/or CMV-IGF1R), or with an ERα expression plasmid (CMV-ERα). Cells were also transfected with an empty expression vector (CMV-Neo) as a control for any non-specific effects of the transfection on indole responsiveness. Verification of constitutive expression was performed by western blot analysis (data not shown). All transfected cell lines were then cultured in steroid-free medium and treated with the indicated combinations of 200 μM I3C, 10 nM E2, and 100 nM PPT, an ERα specific agonist, and subjected to flow cytometry of propidium iodide stained nuclei. As expected, E2 and PPT induced proliferation of control CMV-Neo transfected cells as measured by an increase of cell population in S phase, whereas, I3C treatment alone or in combination with either ERα agonist arrested the cells in the G1 phase of the cell cycle (Fig. 6A). In contrast, ectopic expression of ERα induced an increased proliferative capacity, regardless of whether the cells were treated with E2, PPT, or I3C (Fig. 6B). Also, it is important to point out that consistent with the known pro-proliferative effects of ERα, expression of exogenous ERα increased the number of S phase MCF7 cells observed in the absence of any hormone or I3C treatment (Fig 6B vs 6A, first set of bar graphs). Our results functionally demonstrate that the I3C down-regulation of ERα and its gene targets play an important role in the I3C-mediated cell cycle arrest in hormone sensitive breast cancer.
Fig. 6.
Exogenous expression of ERα, or the combination of IGF1R and IRS1, disrupts I3C-mediated cell cycle arrest. MCF-7 cells were transiently transfected with the empty vector CMV-Neo (A: upper panel), CMV-ERα (B: middle panel), or CMV-IGF1R and CMV-IRS1 (C: lower panel) plasmids. Cells were subsequently cultured in steroid deficient media supplemented with 10% dextran charcoal stripped phenol red-free medium for 16 hours, then treated for 48 hours with the indicated combinations of 200 μM I3C, with or without 10 nM E2, or with or without 100 nM PPT. Cells were harvested in PBS and stained with a hypotonic solution containing propidium iodide. Stained nuclei were subjected to flow cytometry analysis as described in the methods and materials section. Flow cytometry results from three independent experiments were quantified and the results averaged. The bar graphs indicate percent cells in G1, S and G2/M phases with S.E bars.
The potential effect of expressing combinations of exogenous IGF1R and IRS1 on the I3C-mediated cell cycle arrest was assessed by flow cytometry. Ectopic expression of either IGF1R or IRS1 alone was unable to block the I3C-mediated arrest (data not shown). However, co-expression of IGF1R and IRS1 in MCF-7 cells eliminated the I3C driven G1 cell cycle arrest (Fig. 6C, left two sets of bar graphs) in that there were no observed increased in G1 arrested cells or significant decrease in S phase cells. Furthermore, in the presence of the E2 and PPT estrogen agonists, I3C treatment only partially triggered a loss of S phase cells or increased the number of G1 phase cells compared to the effects of I3C observed in the control CMV-Neo transfected cells (Fig 6C vs Fig 6A, middle and right sets of bar graphs). Thus, exogenous expression of a combination of IGF1R and IRS1 were able to override the I3C-dependent cell cycle arrest in the absence of active ERα, and attenuate the cell cycle arrest in the presence of estrogen agonists. These results implicate a role of IGF1R and IRS1 triggered down-stream signaling in the I3C anti-proliferative response.
4. Discussion
Our results demonstrate that the I3C mediated down-regulation of ERα causes the loss of ERα binding to the human IGF1R and IRS1 gene promoters and ablation of estrogen dependent transcriptional induction of these two ERα target genes. For both gene promoters, I3C disrupted endogenous ERα interactions with an ERE-Sp1 composite DNA element in which Sp1 binding is not altered in indolecarbinol treated cells. We also observed that the I3C mediated loss of IGF1R and IRS1 expression significantly dampens the estrogen dependent proliferation of hormone sensitive MCF-7 human breast cancer cells. Consistent with a critical role for ERα in this process, exogenous expression of this steroid receptor ablated the I3C down-regulation of IGF1R and IRS1 gene expression and blocked the I3C induced cell cycle arrest. Moreover, I3C treatment prevented either estradiol, which binds to both ER isotypes, or PPT, an ERα selective agonist, from stimulating IGF1R and IRS1 gene expression and cell proliferation.
The cellular connections between ERα function and proliferation in breast cancer involve several distinct mechanisms of cross-talk between IGF1R and ERα signaling pathways (Santen et al., 2009). For example, the IGF1 induced intracellular phosphorylation of IGF1R and IRS1 triggers down stream cascades that can lead to phosphorylation and ligand independent activation of ERα (Strissel et al., 2008). Furthermore, E2 can stimulate the nuclear translocation of IRS-1 (Morelli et al, 2004) and that the direct binding of ERα to IRS-1 involved the activation function-1 and DNA binding domain region of ERα and the N-terminal pleckstrin homology domain and a region within the C-terminus of IRS-1 (Sisci et al., 2007). Knock down of IRS-1 can also reduce the IGF-1-dependent transcriptional activity of unliganded ERα (Sisci et al., 2007). In mice, ERα induces IRS1 transcription (Mauro et al., 2001), and E2 has been shown to stimulate IGF1R transcript levels (Stewart et al., 1990). Together with our previous report (Marconett et al., 2010), our results have defined the cellular cascade by which I3C disrupts the proliferation of ERα expressing human breast cancer cells through the loss of IGF1 signaling. We propose that I3C induces the degradation of ERα protein, which disrupts an ERα/GATA3 positive regulatory loop, resulting in the loss of ERα gene transcription (Marconett et al., 2010). The down regulation of ERα causes the steady state loss of estrogen dependent transcription of the IGF1R and IRS1 genes and thereby disrupts down stream signaling events involved in the IGF1R and IRS1 mediated proliferation of ERα-expressing human breast cancer cells.
In human cells, ERα-target genes regulate E2-dependent cascades that control cell proliferation, and through these regulated pathways this ER isotype has been proposed to play a role in the etiology of breast cancer. IGF1R and its ligand IGF1 have been implicated in metastatic breast cancer (Klinakis et al, 2009), and although the precise mechanism is unknown, it has been speculated that progression from estrogen-sensitive to estrogen-insensitive breast cancer is carried out by ERα-mediated increases in expression and/or activity of IGF1 and IGF1R (Fagan and Yee, 2008). This response causes an increase in growth factor signaling pathways that leads to growth and proliferation independent of hormone responsive ERα (Osborne and Schiff, 2011). Thus, growth factor signaling components represent important targets of therapeutic strategies for breast cancer (Ryan and Goss, 2008).
Current therapies targeting IGF1 receptor-dependent signaling in breast cancer directly inhibit receptor tyrosine kinase activation through the use of small molecule inhibitors, antibodies, or modulation of the growth hormone-IGF axis. However, these strategies can disrupt the functioning of IGF signaling in off-target tissues, such as pancreatic islet function. I3C is a strong potential therapeutic candidate for estrogen-sensitive breast cancers because this indolecarbinol molecule selectively decreases the levels or activities of several proteins involved in cell proliferation and deregulated in human breast cancer, including many ERα-regulated gene products (Wang et al., 2006; Sundar, et al., 2006; Firestone and Sundar, 2009). Our results directly implicate the expression of IGF1R and IRS1 as two critical ERα-regulated target genes that is disrupted in I3C treated cells. We are currently assessing the effects of more potent and stable indolecarbinol derivatives of I3C on IGF signaling as targeted therapeutic molecules for estrogen sensitive breast cancer.
Acknowledgements
We thank C. Ronald Kahn for generation of the CMV-IRS1 expression plasmid (Addgene plasmid #11238), Dr. Eva Surmacz for the pBABE-IGF1R plasmid (Addgene plasmid #11212), and Dr. Benita Katzenellenbogen for the CMV-ERα plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Ichikawa H. Molecular targets and anti-cancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1205. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, Chen D, Carter TH. Indole-3-carbinol is a negative regulator of estrogen. J Nutr. 2003;133:2470S–2475S. doi: 10.1093/jn/133.7.2470s. [DOI] [PubMed] [Google Scholar]
- Carboni JM, Lee AV, Hadsell DL, Rowley BR, Lee FY, Bol DK, Camuso AE, Gottardis M, Greer AF, Ho CP, Hurlburt W, Li A, Saulnier M, Velaparthi U, Wang C, Wen ML, Westhouse RA, Wittman M, Zimmermann K, Rupnow BA, Wong TW. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 2005;65:3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Anderson E. Type I insulin-like growth factor receptor gene expression in normal human breast tissue treated with oestrogen and progesterone. Br J Cancer. 1997;75:251–257. doi: 10.1038/bjc.1997.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover CM, Hsieh SJ, Cram EJ, Hong C, Riby JE, Bjeldanes LF, Firestone GL. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res. 1999;59:1244–1251. [PubMed] [Google Scholar]
- Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J Biol Chem. 2001;276:22332–22340. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- Cui X, Kim HJ, Kuiatse I, Kim H, Brown PH, Lee AV. Epidermal Growth Factor Induces Insulin Receptor Substrate-2 in Breast Cancer Cells via c-Jun NH2-Terminal Kinase/Activator Protein-1 Signaling to Regulate Cell Migration. Cancer Res. 2006;66:5304–5313. doi: 10.1158/0008-5472.CAN-05-2858. [DOI] [PubMed] [Google Scholar]
- Da Silva Xavier G, Qian Q, Cullen PJ, Rutter GA. Distinct roles for insulin and insulin-like growth factor-1 receptors in pancreatic beta-cell glucose sensing revealed by RNA silencing. Biochem J. 2004;377:149–158. doi: 10.1042/BJ20031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan DH, Yee D. Crosstalk between IGF1R and Estrogen receptor signaling in breast cancer. Jour. Mammary Gland Biol. Neoplasia. 2008;13:423–429. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- Firestone GL, Sundar SN. Minireview: modulation of hormone receptor signaling by dietary anticancer indoles. Molecular Endocrinology. 2009;23:1940–1947. doi: 10.1210/me.2009-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CJ, Farnham PJ. Context-dependent activation of transcription. J. Biol. Chem. 1999;274:29583–29586. doi: 10.1074/jbc.274.42.29583. [DOI] [PubMed] [Google Scholar]
- Garcia-Echeverria C. Medicinal chemistry approaches to target the kinase activity of IGF-1R. IDrugs. 2006;9:415–419. [PubMed] [Google Scholar]
- Goya M, Miyamoto S, Nagai K, Ohki Y, Nakamura K, Shitara K, Maeda H, Sangai T, Kodama K, Endoh Y, Ishii G, Hasebe T, Yonou H, Hatano T, Ogawa Y, Ochiai A. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res. 2004;64:6252–6258. doi: 10.1158/0008-5472.CAN-04-0919. [DOI] [PubMed] [Google Scholar]
- Happerfield LC, Miles DW, Barnes DM, Thomsen LL, Smith P, Hanby A. The localization of the insulin-like growth factor receptor 1 (IGFR-1) in benign and malignant breast tissue. J Pathol. 1997;183:412–417. doi: 10.1002/(SICI)1096-9896(199712)183:4<412::AID-PATH944>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26:1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Wood GA, Petrik JJ, Moorehead RA. Reversibility and recurrence of IGF-IR-induced mammary tumors. Oncogene. 2009;28:2152–2162. doi: 10.1038/onc.2009.79. [DOI] [PubMed] [Google Scholar]
- Kang SG, Brown AL, Chung JH. Oxygen Tension Regulates Stability of Insulin Receptor Substrate-1 (IRS-1) through Caspase-mediated Cleavage. J. Biol. Chem. 2006;282(9):6090–6097. doi: 10.1074/jbc.M610659200. [DOI] [PubMed] [Google Scholar]
- Klinakis A, Szabolcs G, Chen G, Xuan S, Hibshoosh H, Efstratiadis A. IGF1R as a therapeutic target in a mouse model of basal-like breast cancer. Proceedings of the Nat Acad. of Sciences. 2009;7:2359–2364. doi: 10.1073/pnas.0810221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AV, Schiff R, Cui X, Sachdev D, Yee D, Gilmore AP, Streuli CH, Oesterreich S, Hadsell DL. New mechanisms of signal transduction inhibitor action: receptor tyrosine kinase down-regulation and blockade of signal transactivation. Clin Cancer Res. 2003;9:516S–523S. [PubMed] [Google Scholar]
- Li SL, Liang SJ, Guo N, Wu AM, Fujita-Yamaguchi Y. Single-chain antibodies against human insulin-like growth factor I receptor: expression, purification, and effect on tumor growth. Cancer Immunol Immunother. 2000;49:243–252. doi: 10.1007/s002620000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ye P, O’Kusky JR, D’Ercole AJ. Type 1 insulin-like growth factor receptor signaling is essential for the development of the hippocampal formation and dentate gyrus. J Neurosci Res. 2009;87:2821–2832. doi: 10.1002/jnr.22129. [DOI] [PubMed] [Google Scholar]
- Manson MM, Farmer PB, Gescher A, Steward WP. Innovative agents in cancer prevention. Recent Results Cancer Res. 2005;166:257–275. doi: 10.1007/3-540-26980-0_17. [DOI] [PubMed] [Google Scholar]
- Maor S, Mayer D, Yarden RI, Lee AV, Sarfstein R, Werner H, Papa MZ. Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumor cells: involvement of transcription factor Sp1. J Endocrinol. 2006;191:605–612. doi: 10.1677/joe.1.07016. [DOI] [PubMed] [Google Scholar]
- Marconett CN, Sundar SN, Poindexter KM, Stueve TR, Bjeldanes LF, Firestone GL. Indole-3-carbinol triggers aryl hydrocarbon receptor-dependent estrogen receptor (ER)alpha protein degradation in breast cancer cells disrupting an ERalpha-GATA3 transcriptional cross-regulatory loop. Mol Biol Cell. 2010;21:1166–1177. doi: 10.1091/mbc.E09-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconett CN, Sundar SN, Tseng M, Tin AS, Tran KQ, Mahuron KM, Bjeldanes LF, Firestone GL. Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the G1 cell cycle arrest of human breast cancer cells. Carcinogenesis. 2011;32:1315–1323. doi: 10.1093/carcin/bgr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro L, Salerno M, Panno ML, Bellizzi D, Sisci D, Miglietta A, Surmacz E, Ando S. Estradiol increases IRS-1 gene expression and insulin signaling in breast cancer cells. Biochem Biophys Res Commun. 2001;288:685–689. doi: 10.1006/bbrc.2001.5815. [DOI] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, Vecchione A, Sauter ER, Miller WH, Jr., Surmacz E. Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene. 2004;23:7517–7526. doi: 10.1038/sj.onc.1208014. [DOI] [PubMed] [Google Scholar]
- Nagle JA, Ma Z, Byrne MA, White MF, Shaw LM. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol Cell Biol. 2004;24:9726–9735. doi: 10.1128/MCB.24.22.9726-9735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annual Rev. Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parathath SR, Mainwaring LA, Fernandez-L A, Campbell DO, Kenney AM. Insulin receptor substrate 1 is an effector of sonic hedgehog mitogenic signaling in cerebellar neural precursors. Development. 2008;135:3291–3300. doi: 10.1242/dev.022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann J, Macaulay VM. IGF1R signaling and its inhibition. Endocr Relat Cancer. 2006;13(Suppl 1):S33–S43. doi: 10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- Ruiz-Alcaraz AJ, Liu HK, Cuthbertson DJ, Mcmanus EJ, Akhtar S, Lipina C, Morris AD, Petrie JR, Hundal HS, Sutherland C. A novel regulation of IRS1 (insulin receptor substrate-1) expression following short term insulin administration. Biochem. J. 2005;392:345–352. doi: 10.1042/BJ20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Clin. Pharmaco. 2008;13:16–24. doi: 10.1634/theoncologist.2007-0199. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Li SL, Hartell JS, Fujita-Yamaguchi Y, Miller JS, Yee D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–635. [PubMed] [Google Scholar]
- Santen R, Cavalieri E, Rogan E, Russo J, Guttenplan J, Ingle J, Yue W. Estrogen mediation of breast tumor formation involves estrogen receptordependent, as well as independent, genotoxic effects. Ann N Y Acad Sci. 2009;1155:132–140. doi: 10.1111/j.1749-6632.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- Shekhar PV, Chen ML, Werdell J, Heppner GH, Miller FR, Christman JK. Transcriptional activation of functional endogenous estrogen receptor gene expression in MCF10AT cells: a model for early breast cancer. Int J Oncol. 1998;13:907–915. doi: 10.3892/ijo.13.5.907. [DOI] [PubMed] [Google Scholar]
- Shibata H, Spencer TE, Oñate SA, Jenster G, Tsai SY, Tsai MJ, O’Malley BW. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- Sisci D, Morelli C, Cascio S, Lanzino M, Garofalo C, Reiss K, Garcia M, Russo A, Ando S, Surmacz E. The estrogen receptor alpha:insulin receptor substrate 1 complex in breast cancer: structure-function relationships. Ann Oncol. 2007;18(Suppl 6):vi81–85. doi: 10.1093/annonc/mdm232. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Johnson MD, May FE, Westley BR. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in the estrogen stimulated proliferation of human breast cancer cells. J Biol Chem. 1990;265:21172–21178. [PubMed] [Google Scholar]
- Strissel PL, Ellmann S, Loprich E, Thiel F, Fasching PA, Stiegler E, Hartmann A, Beckmann MW, Strick R. Early aberrant insulin-like growth factor signaling in the progression to endometrial carcinoma is augmented by tamoxifen. Int J Cancer. 2008;123:2871–2879. doi: 10.1002/ijc.23900. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Ziegler YS, Nardulli AM. ERα-associated protein networks. Trends Endocrinol Metab. 2011;22:124–129. doi: 10.1016/j.tem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Sundar SN, Kerekatte V, Equinozio CN, Doan VB, Bjeldanes LF, Firestone GL. Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol. 2006;20:3070–3082. doi: 10.1210/me.2005-0263. [DOI] [PubMed] [Google Scholar]
- Sundar SN, Marconett CN, Doan VB, Willoughby JA, Sr., Firestone GL. Artemisinin selectively decreases functional levels of estrogen receptor-alpha and ablates estrogen-induced proliferation in human breast cancer cells. Carcinogenesis. 2008;29:252–2258. doi: 10.1093/carcin/bgn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacz E, Bartucci M. Role of estrogen receptor alpha in modulating IGF-I receptor signaling and function in breast cancer. J Exp Clin Cancer Res. 2004;23:385–394. [PubMed] [Google Scholar]
- Van den Berg CL, Cox GN, Stroh CA, Hilsenbeck SG, Weng CN, McDermott MJ, Pratt D, Osborne CK, Coronado-Heinsohn EB, Yee D. Polyethylene glycol conjugated insulin-like growth factor binding protein-1 (IGFBP-1) inhibits growth of breast cancer in athymic mice. Eur J Cancer. 1997;33:1108–1113. doi: 10.1016/s0959-8049(97)00071-3. [DOI] [PubMed] [Google Scholar]
- Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006;17:659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hailey J, Williams D, Lipari P, Malkowski M, Wang X, Xie L, Li G, Saha D, Ling WL, Cannon-Carlson S, Greenberg R, Ramos RA, Shields R, Presta L, Brams P, Bishop WR, Pachter JA. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol Cancer Ther. 2005;4:1214–1221. doi: 10.1158/1535-7163.MCT-05-0048. [DOI] [PubMed] [Google Scholar]
- Werner H, Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem. 2009;115:58–71. doi: 10.1080/13813450902783106. [DOI] [PubMed] [Google Scholar]
- Wittman M, Carboni J, Attar R, Balasubramanian B, Balimane P, Brassil P, Beaulieu F, Chang C, Clarke W, Dell J, Eummer J, Frennesson D, Gottardis M, Greer A, Hansel S, Hurlburt W, Jacobson B, Krishnananthan S, Lee FY, Li A, Lin TA, Liu P, Ouellet C, Sang X, Saulnier MG, Stoffan K, Sun Y, Velaparthi U, Wong H, Yang Z, Zimmermann K, Zoeckler M, Vyas D. Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem. 2005;48:5639–5643. doi: 10.1021/jm050392q. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol Ther. 2007;6:64–69. doi: 10.4161/cbt.6.1.3549. [DOI] [PubMed] [Google Scholar]