Abstract
Background
This paper describes the development of a new quality of life instrument in advanced cancer patients receiving palliative care.
Methods
The Palliative Care Quality of Life Instrument incorporates six multi-item and one single-item scale. The questionnaire was completed at baseline and one-week after. The final sample consisted of 120 patients.
Results
The average time required to complete the questionnaire, in both time points, was approximately 8 minutes. All multi-item scales met the minimal standards for reliability (Cronbach's alpha coefficient ≥.70) either before or during palliative treatment. Test-retest reliability in terms of Spearman-rho coefficient was also satisfactory (p < 0.05). Validity was demonstrated by inter-item correlations, comparisons with ECOG performance status, factor analysis, criterion-related validation, and correlations with the Assessment of Quality of Life in Palliative Care Instrument (AQEL), and the European Organisation for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30, version 3.0).
Conclusion
The PQLI is a reliable and valid measure for the assessment of quality of life in patients with advanced stage cancer.
Background
Recently, the health care community has recognised the importance of using QoL measurement as an essential component of a treatment modality's efficacy [1]. At every stage of disease, treatment choices may involve modalities that differ in side effects and impact upon QoL. Comprehensive, yet efficient, questionnaires are needed to measure QoL in cancer patients. Quality-of-life assessment can be helpful in weighting the risks and benefits of treatment options, particularly when differences in survival among the options are small or non-existent [2].
Quality of life is subjective in nature, therefore there is a wide agreement that health-related quality of life should be conceptualised as a multidimensional construct [3]. Physical functioning, disease-and treatment-related symptoms, psychological/emotional well being, and social interactions are critical domains that are included in most efforts to measure overall quality of life. When considering quality of life in advanced cancer patients one of the treatment choices is a palliative one, therefore we consider of great importance the inclusion of a new dimension when measuring quality of life in such a population, that of "choice of treatment". Recent studies and new articles clearly indicate that physicians must be educated to routinely ask patients about their wishes for medical care and to recognise that they are legally and morally bound to honour those requests [4]. Such communication is especially pressing in the context of advanced illness, when the achievement of a peaceful death assumes priority over inappropriate prolongation of dying.
Many valid assessment instruments have been developed that measure QoL such as EORTC [5], The Functional Assessment of Cancer Treatment (FACT) [6]. In 1986, the European Organisation for Research and Treatment initiated a research program to develop an integrated, modular approach for evaluating the quality of life of patients participating in international clinical trials [7]. EORTC with its clinical focus and its multicultural orientation provides a rather unique context for developing and testing quality of life questionnaires [8].
The aim of our study was to assess the psychometric properties of a new quality of life instrument on a Hellenic sample of terminally ill cancer patients receiving only palliative treatment, which is called PQLI (Palliative Care Quality of Life Instrument). It was found to be concise, quantitative and easily used; it has been designed primarily for use by the patients themselves; it was based on the existing literature [7,9-11] and the items that the patients consider as most important to what they perceive as "quality of life", the latter was elicited by means of qualitative research. It became evident from the qualitative assessment on the patients' description on their QoL that there is a need to participate in the treatment process; this would give them a sense of control over their fatal disease [12]. Patients want a voice in their end-of-life care, and participation in treatment choices encompasses the psychosocial outcomes that these may have in their lives [13]. When considering quality of life in advanced cancer patients the treatment choice is a palliative one, therefore we consider of importance the inclusion of a new dimension when measuring quality of life in such a population, that of "choice of treatment". The lack of a questionnaire that examines the quality of life specifically in a palliative care setting, the individuals that form their support system, as well as the unique needs that these patients have, was the driving force for a measurement like this to be developed.
Methods
Patients with symptomatic incurable cancer disease were selected for study by means of palliative treatment modality. No restrictions were placed with regard to histologic type of cancer, age, or performance status. All patients attended the "Pain Relief and Palliative Care Unit" of Areteion Hospital where the study took place, between January 2002 and October 2002. Criteria for inclusion were: age > 18 years, no cerebral metastases, no known psychiatric disorder, to be cognitively capable of filling in the questionnaire, fluent in the Hellenic language, and off anticancer treatment for ≥3 months. From 630 cancer and non-cancer patients that were treated in the unit that period, 144 advanced cancer patients were drawn using stratified random sampling, according to the performance status, and were judged eligible to enter the study. From them, 24 patients (16.7%) were excluded due to refusal to participate in the study. The hospital's ethics committee also approved the study. The final sample was consecutive and consisted of 120 responding patients from whom written informed consent was obtained. The demographical data of the sample is shown in table 1.
Table 1.
Demographic and clinical data of sample
| Mean age | 61.17 (range:19–88) |
| Male to female ratio | 58/62 |
| N(%) | |
| Marital status | |
| Married or living with partner | 74(62%) |
| Widowed but living with their children | 43(36%) |
| Illiterate | 3(2%) |
| Education | |
| Elementary school | 40(30%) |
| High school | 42(35%) |
| University | 38(32%) |
| Types of cancer | |
| Lung | 32(27%) |
| Gastrointestinal | 25(21%) |
| Breast | 17(14%) |
| Urinary track | 14(12%) |
| Prostate | 10(8%) |
| Head and neck | 4(3%) |
| Multiple myeloma | 2(2%) |
| Bone-Sarcoma | 2(2%) |
| Soft tissue sarcoma | 2(2%) |
| Glioblastoma Multiform | 2(2%) |
| Astrocyttoma | 2(2%) |
| Non-Hodgkin Lymphoma | 2(2%) |
| Melanoma | 2(2%) |
| Cervical uteri | 2(2%) |
| Vulva | 1(1%) |
| Vagina | 1(1%) |
| ECOG status | |
| 0 | - |
| 1 | - |
| 2 | 39(32%) |
| 3 | 81(68%) |
Instrument development and procedures
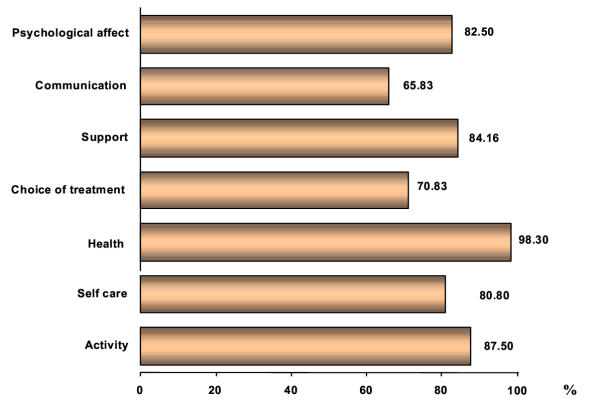
The PQLI is a 28-item questionnaire, composed of six multi-item scales (2 functional scales, 1 symptom scale, 1 choice of treatment scale, 1 psychological scale) and a single item scale (overall quality of life). Each scale was accompanied by a title. The questionnaire development involved the following phases: first, literature search that identified the relevant QoL issues, and the existing questionnaires, second, a provisional list of items was presented to 3 experts for feedback on appropriateness of content and breadth of coverage, third, the list was administered to patients from the target group to determine the extent to which they have experienced these problems, while they were asked to choose a number of issues that troubled them the most (Figure 1). Next, the resulting list of items was reviewed for clarity and overlap by other experts, and finally, the questionnaire was pretested by administering it to some patients (N = 20) from the target population, and through structured interviews with each patient individually after the completion of the questionnaire. In these interviews, the patients rated the questionnaire scales within a range of "1" (i.e. first choice) to "7" (i.e. seventh choice). The researchers, then, evaluated whether scores from the resulting PQLI corresponded well with those independently obtained ratings [14].
Figure 1.
Variables, which -according to patients -affect their Quality of Life.
In the final questionnaire format six of the scales were presented into three optional statements to be scored 1, 2 and 3 respectively. In "Choice of Treatment" scale the patients were asked to choose the item that is "most" important in the choice of treatment and rate it 1; then choose the item that is "next" important and rate it 2; and so on, the last item which is the "least" important, the patients were asked to rate it as 5. The single item scale "Overall Quality of Life" has the form of a Bi-Polar Numerical scale.
The patients were asked to complete the questionnaire twice, with 1-week interval. This rather short interval was chosen because of the imminent risk of sudden changes in their health status. The questionnaires were collected immediately after completion. The instrument was designed primarily to be a self-assessment but where the patient's condition would not permit it the researcher assisted him/her.
{In the Appendix - see additional file 1] is presented the questionnaire in the English language. Two independent translators translated the PQLI in English and then another two independent translators translated it back to Greek. A matching of these translations was then conducted. The same translation method has already been used in the validity and reliability of the EORTC QLQ C-30 (v.3) in Greek [8].
Statistical Analysis
A range of analyses was carried out to establish scale reliability, and to evaluate empirically the validity of the questionnaire scales. The average of the items that contribute to the scale is estimated. Higher mean scores from 0 to 100 represent a better level of functioning and QoL on the scales of "Activity", "Self-care", "Support", "Communication", "Psychological Affect", and "Overall Quality of Life", and higher mean values on the health status scale, represent more symptomatology and worse quality of life [7]. The current procedures for scoring the PQLI reflect the multidimensionality of the quality of life domain.
Internal consistency
internal consistency of the questionnaire before and during palliative treatment was assessed by Cronbach's alpha and was considered acceptable for group comparisons if the coefficient exceeded 0.70, as recommended by Nunnally [15]. Cronbach's alpha tests whether the items in a questionnaire have a homogeneous content with respect to the construct of interest.
Reliability
Test-retest reliability of patients' (N = 120) responses was evaluated by comparing the scores recorded on two occasions, an average of seven days apart (Spearman-rho test [16]). The patient's clinical stage did not change between the first and the second completion, and the status of the patients was stable between test and retest. Due to its hierarchical nature, the intertest reliability of the ranking statements (Choice of Treatment) was established by using the "Kendall's-W" test [15].
Validity
Five indirect methods to evaluate validity were adopted:
First, by comparing the scale scores with patients with poorer and better Eastern Cooperative Oncology Group (ECOG-the clinical variable assessed) [5] performance status using the Mann-Whitney U non-parametric test [15]. Second, by assessing the statistical difference of the questionnaire-scales before and during treatment in terms of Wilcoxon rank test between scales for related subjects. Third, by Exploratory factor analysis, using principal components with non-orthogonal (direct oblimin) rotation [17], was used to assess the validity of the PQLI. As a fourth process, correlations were calculated between PQLI items and those of two others instruments, the Assessment of Quality of Life in Palliative Care (AQEL) [18], and the European Organisation for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30, version 3.0) [5]. The AQEL is focusing in patients undergoing palliative treatment, includes 22 items in seven scales, from physical, psychological, social and existential domains, while it has proven to be reliable and valid. In addition, the EORTC QLQ-C30 is a widely used second-generation questionnaire designed to measure cancer patients' physical, psychological and social functions. It is a psychometrically established 30-item questionnaire, incorporated in nine multi-item, and several single-item scales [19].
Finally, criterion-related validation was also conducted. At first step, concurrent related validity was performed with correlations among the scales of PQLI (inter-scale correlations). Accordingly, the seven factors obtained from the interview from the 120 patients were rated and coded from 1 to 7 according to the patients' choice: 1st, 2nd, 3rd, 4th to 7th choice. The closeness of the hypothetical model of PQLI to the empirical data of interviewing rating scores is evaluated statistically through gamma test [20]. To evaluate whether scores from the PQLI instrument corresponded well with those independently obtained ratings, we first performed a factor analysis in which the seven latent variables from the PQLI form were intercorrelated with the seven forms from the interview. We then tested a predictive model to observe whether constructs from the PQLI instrument could predict analogous measured constructs from the interview measurements. Initially, all possible predictive paths were included simultaneously and non-significant paths were dropped gradually. This procedure was a test of both the convergent and discriminative validity of the PQLI instrument (i.e., variables on the PQLI should be related to corresponding variables on the interview and not to non-corresponding variables) and the criterion-related validity of the PQLI form (i.e., the ability of the PQLI to predict an independent criterion variable).
The whole statistical analysis was conducted using the SPSS version 8.0 statistical package (SPSS Inc, Chicago, IL).
Results
The 28 items were all acceptable to the participants. They encompassed physical, social, health, and psychological aspects of life. Each item exhibited distributions reflecting sensitivity to variations in the attributes measured. Only 3 patients (2.5%) needed assistance because they were illiterate. The 61.7% of the participants regarded as most important the variable of long-term quality of life, while the 76.7% of the respondents regarded as least important the variable "effects on sexual life". The distributions of the respondents in each category, for example, were 51.7% of the respondents are not working, a 40% can fully care for themselves, a 66.7% reported pain, 66.7% of the patients reported support from their friends and relatives, the 64.2% stated that they do not discuss their family problems with their doctor, and a 49.2% answered that they do not feel fear of death. From this figure the clinical profile of patients can be seen. Although restricted to a limited cultural setting, this data was considered quite interesting for clinicians.
Descriptive statistics and scale reliability (multitrait scaling analysis)
The reliability of the PQLI with the approach of internal consistency was evaluated. Internal consistency was calculated by Cronbach's standardised item alpha. Table 2 shows the means and standard deviations for the multi-item measures, before and during treatment. From the descriptive statistics matrix, the Cronbach's alpha for each scale was found to be greater than the critical value of 0.70, while the overall Cronbach's alpha was 0.787. The test-retest reliability (Table 3) of scales and items as well showed that all the coefficients of agreement were greater than 0.82 (P < 0.001 in all cases). Due to the nature of the "Choice of Treatment" scale the reliability was calculated by performing the Kendall's Coefficient of Concordance and was found 0.353 with a P-value < 0.0001.
Table 2.
Descriptive Statistics and scale reliability before and during treatment.
| Before treatment | During treatment | ||||||
| SCALES | Mean score | S. D. | Cronbach's alpha coefficient | Mean score | S. D. | Cronbach's alpha coefficient | P* |
| AC (activity) | 63.44 | 36.74 | 0.86 | 82.71 | 37.59 | 0.91 | <0.001 |
| SC (self care) | 47.29 | 41.10 | 0.90 | 57.93 | 42.13 | 0.89 | <0.001 |
| HE (health) | 57.92 | 31.30 | 0.89 | 68.16 | 31.93 | 0.88 | <0.001 |
| CT (choice of treatment) | 48.75 | 44.42 | 0.92 | 51.25 | 43.34 | 0.91 | 0.018 |
| SU (support) | 42.50 | 19.80 | 0.87 | 54.72 | 41.74 | 0.90 | 0.007 |
| CO (communication) | 74.58 | 31.01 | 0.81 | 79.02 | 32.03 | 0.77 | 0.021 |
| PA (psychological affect) | 51.44 | 36.24 | 0.92 | 57.50 | 41.95 | 0.83 | 0.009 |
* Wilcoxon test.
Table 3.
Test retest correlations for all PQLI scales and items.
| SCALES | Test-retest correlation | Items | Test-retest correlation |
| AC (activity) | 0.93 | Keep working | 0.97 |
| House chores | 0.89 | ||
| Enjoyment | 0.90 | ||
| Hobbies | 0.94 | ||
| SC (self care) | 0.98 | Driving or transportation | 0.98 |
| Self care | 0.99 | ||
| HE (health) | 0.87 | Pain | 0.82 |
| Nausea/vomiting | 0.98 | ||
| Lack of appetite | 0.76 | ||
| Weak/tired | 0.89 | ||
| Dyspnoea | 0.85 | ||
| Stool disturbances | 0.77 | ||
| Sleep disturbances | 0.98 | ||
| CT (choice of treatment) | 0.96 | Like to choose therapeutic schema | 0.96 |
| Able to choose therapeutic schema | 0.97 | ||
| SU (support) | 0.89 | Satisfactory support of relatives/friends | 0.95 |
| Satisfactory support of health care team | 0.83 | ||
| Satisfactory support of nursing stuff | 0.87 | ||
| CO (communication) | 0.90 | Discussion with the doctor on my social relationships | 0.91 |
| Discussion with the doctor on economic/professional problems | 0.92 | ||
| Discussion with the doctor on my family problems | 0.89 | ||
| PA (psychological affect) | 0.84 | Calm | 0.73 |
| Optimistic | 0.89 | ||
| Blue | 0.84 | ||
| Control of the situation | 0.94 | ||
| Fears of death | 0.74 | ||
| Overall Quality of life | 0.91 | ||
Validity
The correlation matrix of the scales within the PQLI-pre-and-on-treatment is displayed in Table 4. The agreements are strong, consistent and statistically significant at the 0.005 or 0.001 levels. As expected, the strongest correlations were observed between the "Activity", "Self-care", "Health Status", and "Choice of Treatment". However, they also correlated highly to "Psychological Affect". The "Overall Quality of Life" (OQoL), correlated substantially with "Activity", "Health Status", "Self-care", "Choice of Treatment", "Communication", "Support", and "Psychological Affect".
Table 4.
Correlations among the PQLI scales.
| AC | SC | HE | CT | SU | CO | PA | OQOL | |
| Activity (AC) | .89a | .90a | .81a | .80a | .88a | .96a | .93a | |
| Self Care (SC) | .87a | .92a | .85a | .87a | .82a | .93a | .92a | |
| Health (HE) | .89a | .90a | .95a | .80a | .63b | .96a | .90a | |
| Choice of Treatment (CT) | .78a | .84a | .95a | .77a | .69b | .78a | .88a | |
| Support (SU) | .81a | .84a | .76a | .78a | .62b | .79a | .82a | |
| Communication (CO) | .86a | .78a | .79a | .61b | .87a | .81a | .83a | |
| Psychological Affect (PA) | .96a | .91a | .94a | .82a | .86a | .75a | .94a | |
| Overall Quality of Life (OQoL) | .91a | .89a | .85a | .87a | .85a | .81a | .89a |
* Before treatment under the diagonal; during treatment above the diagonal. Values represent the Spearman-rho coefficient. a: correlation is significant at the .01 level (2-tailed). b: correlation is significant at the .05 level (2-tailed).
Factor analysis
Exploratory non-orthogonal factor analysis (Principal Axis Factoring extraction with Direct Oblimin rotation) was carried out to further explore the validity of the PQLI instrument. The correlations between the variables are high. The Bartlett Test of Sphericity was 3042.7 and it was significant (p < 0.0001). The Kaiser-Meyer-Olkin Measure of Sampling Adequacy was equal to 0.81 showing that the data is suitable for factor analysis. Principal axis factoring extraction was used to analyse the underlying structure of the questionnaire, yielding seven independent factors accounting for 79.7 % of the variance. This seven-factor solution was deemed appropriate by examining the magnitude and rate of change in eigenvalues. Based on the rule that meaningful factors should be associated with eigenvalues greater than 1.0 and a marginal change occur after seven factors (scree test), the seven-factor solution is appropriate [21,22]. For the interpretation of the factor solution direct oblimin rotation was performed (delta=-0.1). The results of the rotation are shown in Table 5. The variables constituting the seven factors are marked in bold fonts. By performing an orthogonal rotation using varimax, the same 7 factors were identified without any material difference confirming the results from the non-orthogonal rotation. Factor 1: Activity (keep working, house chores, enjoyment, hobbies), factor 2: Self-care (driving or transportation, self-sufficient), factor 3: Health Status (pain, nausea/vomiting, lack of appetite, weak/tired, dyspnoea, stool disturbances, sleep disturbances), factor 4: Choice of Treatment (Like to choose, able to choose), factor 5: Support (relatives/friends, health care team, nursing stuff), factor 6: Communication (social relationships, economic/professional problems, family problems), and factor 7: Psychological Affect (calm, optimistic, blue, control of the situation, fears of death). The scale of Overall Quality of Life is not included in any factor.
Table 5.
Loadings of variables on factors emerging from PQLI rotated factor matrix.
| Items | Factors | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Keep working | 0.93 | ||||||
| House chores | 0.56 | 0.43 | 0.48 | 0.46 | |||
| Enjoyment | 0.92 | 0.46 | |||||
| Hobbies | 0.93 | 0.43 | 0.42 | ||||
| Driving or transportation | 0.43 | 0.54 | 0.76 | ||||
| Self care | 0.93 | ||||||
| Pain | 0.52 | 0.69 | 0.44 | ||||
| Nausea/vomiting | 0.41 | 0.90 | |||||
| Lack of appetite | 0.80 | ||||||
| Weak/tired | 0.56 | 0.57 | 0.54 | ||||
| Dyspnoea | 0.80 | ||||||
| Stool disturbances | 0.41 | 0.72 | |||||
| Sleep disturbances | 0.51 | 0.76 | |||||
| Like to choose therapeutic schema | 0.88 | ||||||
| Able to choose therapeutic schema | 0.95 | ||||||
| Satisfactory support of relatives/friends | 0.45 | 0.88 | |||||
| Satisfactory support of health care team | 0.91 | ||||||
| Satisfactory support of nursing stuff | 0.47 | 0.90 | |||||
| Discussion with the doctor on my social relationships | 0.44 | 0.80 | |||||
| Discussion with the doctor on economic/professional problems | 0.47 | 0.75 | |||||
| Discussion with the doctor on my family problems | 0.88 | ||||||
| Calm | 0.71 | 0.45 | |||||
| Optimistic | 0.45 | 0.58 | 0.57 | 0.56 | |||
| Blue | 0.77 | 0.43 | 0.57 | ||||
| Control of the situation | 0.44 | 0.67 | 0.61 | 0.41 | |||
| Fears of death | 0.73 | 0.70 | |||||
Criterion-related validation
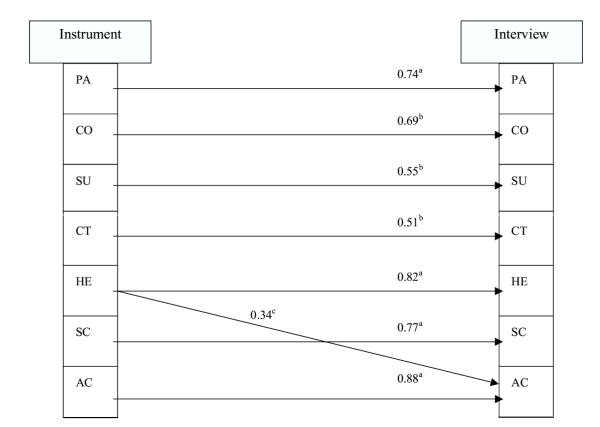
As shown in table 3, the correlations under the diagonal among the scales of PQLI were significantly associated, giving evidence of concurrent related validity. Following, the PQLI model was significantly associated with the empirical model deriving from the patients' interview (gamma = 0.78, SE = 0.11, p < 0.001). Correlations between the ratings derived from the interview and the PQLI factors are reported in Table 6. Interview ratings are arranged in columns. The highest correlation in each column coincides with the analogous PQLI latent construct. We then used the PQLI latent factors as predictors of the interview ratings. All factors were used as predictors of all constructs simultaneously. We allowed covariances (correlations) among the predictor variables and significant covariances among the error residuals of the outcome variables. We gradually dropped paths if they were nonsignificant until only significant paths remained. The fit indices for this final path model reflected an excellent fit (p < 0.001, chi2 test). Results of the predictive model are reported in Figure 2. We found that the PQLI constructs significantly predicted analogous interviewing scores by the patients. In most cases, there was considerable discriminative validity between similar observed and reported variables, except that PQLI "health" also predicted "activity" from the interview rating. To refine these results, we needed to determine whether the path from the PQLI "health" factor to the interviewing "health" rating was significantly larger than the path from PQLI "health" to the "activity" interviewing rating variable. Therefore, we ran a model that constrained these paths to equivalence and then examined the chi2-difference test between these nested models. The difference test revealed that the paths were significantly different in magnitude (p < 0.01), thereby providing additional evidence of the discriminant validity of the PQLI.
Table 6.
Correlations between PQLI factors and interview scores for the 120 patients (criterion related validity).
| PA | CO | SU | CT | HE | SC | AC | |
| Psychological affect (PA) | 0.89a | 0.58a | 0.49a | 0.51a | 0.45a | 0.37b | 0.41b |
| Communication (CO) | 0.60b | 0.75a | 0.67a | 0.74a | 0.38a | 0.32b | 0.32b |
| Support (SU) | 0.58a | 0.61a | 0.79a | 0.48a | 0.41b | 0.44b | 0.37b |
| Choice of treatment (CT) | 0.64a | 0.69a | 0.77a | 0.75a | 0.33b | 0.52a | 0.31b |
| Health (HE) | 0.41b | 0.58a | 0.62a | 0.49a | 0.82a | 0.58a | 0.82a |
| Self care (SC) | 0.53a | 0.49a | 0.65a | 0.38a | 0.43b | 0.68a | 0.64a |
| Activity (AC) | 0.41a | 0.37a | 0.54b | 0.46b | 0.74a | 0.66a | 0.89a |
Figure 2.
Significant regression paths among latent variables PQLI model predicting interviewing ratings (N = 120). Regression coefficients are standardized (ap < 0.001, bp < 0.01, cp #60; 0.05)
Clinical validity, comparative assessments
Between the PQLI and the Assessment of Quality of Life in Palliative Care instrument (AQEL) the correlations were, generally, strong in all the scales, ranging from 0.44 to 0.94. The strongest correlations were found between the items of "Insomnia" and "Sleep Disturbances" (0.94) and also in the items of "Pain" (0.93) (Table 7). Moreover, there were significant correlations between the EORTC QLQ C30 and the relevant items of PQLI, ranging from 0.79 to 0.97, especially between the items of "Pain" (0.97), and "Lack of Appetite" (0.97) (table 8). There were significant correlations between the scales of PQLI and the relevant scales of AQEL and EORTC QLQ C30, as shown in table 9. The distinction between patients with low or high ECOG performance status showed significant relationship between ECOG scores and instrument scale scores. As we see in Table 10, patients with a better ECOG performance status reported significantly higher scores in all the scales of the instrument.
Table 7.
Correlations between AQEL items and corresponding PQLI items (n = 28).
| PQLI | AQEL | Correlation |
| Keep working | Bodily strength | 0.87 |
| House chores | ||
| Enjoyment | Make you happy | 0.79 |
| Hobbies | ||
| Driving or transportation | - | |
| Self care | Help needed with hygiene/dressing | 0.72 |
| Pain | Pain | 0.93 |
| Nausea/vomiting | Nausea | 0.93 |
| Lack of appetite | - | |
| Weak/tired | Hours recumbent during day | 0.81 |
| Dyspnoea | - | |
| Stool disturbances | Troubled bowel | 0.93 |
| Sleep disturbances | Insomnia | 0.94 |
| Like to choose therapeutic schema | - | |
| Able to choose therapeutic schema | - | |
| Satisfactory support of relatives/friends | Sharing worries with any member of family | 0.55 |
| Regarded as usual by friends | 0.75 | |
| Satisfactory support of health care team | Ability to reach stuff | 0.74 |
| Receive appropriate care | 0.58 | |
| Satisfactory support of nursing stuff | Ability to reach stuff | 0.44 |
| Receive appropriate care | 0.49 | |
| Discussion with the doctor on my social relationships | - | |
| Discussion with the doctor on economic/professional problems | - | |
| Discussion with the doctor on my family problems | - | |
| Calm | Anxiety | 0.85 |
| Optimistic | Meaningfulness | 0.81 |
| Blue | Depression | 0.88 |
| Control of the situation | - | |
| Fears of death | - | |
| Overall QoL | Global quality of life | 0.79 |
Table 8.
Correlations between the European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30, version 3.0) and PQLI items.
| PQLI | EORTC QLQ-C30 | QLQ C30 Item | Correlation |
| Keep working | Keep working | 6 | 0.91 |
| House chores | Stay in bed or a chair | 4 | 0.83 |
| Enjoyment | Interfere with social activities | 27 | 0.79 |
| Hobbies | Hobbies | 7 | 0.88 |
| Driving or transportation | - | ||
| Self care | Help with eating, dressing... | 5 | 0.82 |
| Pain | Pain | 9 | 0.97 |
| Nausea/vomiting | Nausea Vomiting | 14 15 | 0.85 0.89 |
| Lack of appetite | Lack of appetite | 13 | 0.97 |
| Weak/tired | Weak | 12 | 0.85 |
| Dyspnoea | Short of breath | 0.92 | |
| Diarrhea or Constipation | Constipated Diarrhea | 16 17 | 0.91 0.90 |
| Sleep disturbances | Trouble sleeping | 11 | 0.95 |
| Like to choose therapeutic schema | - | ||
| Able to choose therapeutic schema | - | ||
| Satisfactory support of relatives/friends | |||
| Satisfactory support of health care team | |||
| Satisfactory support of nursing stuff | - | ||
| Discussion with the doctor on my social relationships | - | ||
| Discussion with the doctor on economic/professional problems | - | ||
| Discussion with the doctor on my family problems | - | ||
| Calm | Tense | 21 | 0.83 |
| Optimistic | Worry | 22 | 0.88 |
| Blue | Depressed | 24 | 0.90 |
| Control of the situation | - | ||
| Fears of death | - | ||
| Overall Q o L | Overall Quality of Life | 30 | 0.84 |
Table 9.
Correlations between relevant scales of the PQLI and the scales of AQEL as well as the European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30, version 3.0).
| PQLI | AQEL | Correlation | EORTC | Correlation |
| Self-Care | _ | Physical Functioning (1–5) | 0.86 | |
| Activity | Existential (7, 15, 16) | 0.78 | Role Functioning (20, 25) | 0.83 |
| Psychological Affect | Psychological (8–12) | 0.91 | Emotional Functioning (21–24) | 0.80 |
| Health Status | Physical (1–6) | 0.90 | Symptom Scales (8–19) | 0.89 |
| Overall Q of Life | Global (19) | 0.89 | Global Quality of Life (29, 30) | 0.88 |
| Cognitive (20, 25) | ||||
| Emotional (21–24) | ||||
| Social (26, 27) | ||||
| Financial impact (28) | ||||
| Choice of Treatment | _ | _ | ||
| Support | Social (13, 14) | _ | ||
| Communication | Medical (17, 18) | _ |
Table 10.
Scores by ECOG status, combined validity samples (n = 120).
| SCALES | ECOG status >2 | ECOG status ≤2 | |||
| Mean score | S. D. | Mean score | S. D. | P* | |
| Activity (AC) | 51.29 | 35.04 | 85.17 | 29.16 | <0.001 |
| Self Care (SC) | 37.98 | 30.17 | 63.95 | 41.29 | 0.001 |
| Health (HE) | 52.51 | 31.41 | 67.61 | 29.00 | 0.011 |
| Choice of Treatment (CT) | 42.86 | 41.89 | 66.28 | 40.42 | 0.004 |
| Support (SU) | 45.67 | 39.41 | 71.71 | 40.26 | 0.001 |
| Communication (CO) | 69.05 | 32.29 | 84.49 | 26.07 | 0.008 |
| Psychological Affect (PA) | 47.12 | 37.62 | 65.97 | 37.82 | 0.009 |
| Overall Quality of Life (OQoL) | 48.37 | 11.11 | 64.54 | 11.98 | <0.001 |
* Mann-Whitney U test
Discussion
The purpose of this study was to design and evaluate a method of collecting information about the quality of life of advanced ill cancer patients receiving palliative care treatment. Although the primary intent of this project was to establish the basic reliability and validity of the PQLI measurement system, we also hoped to demonstrate the sensitivity to change of this instrument by incorporating it into a clinical trial using repeated measures design.
The questionnaire was simple to administer and score, and was well accepted by the responding patients. In average, it required 8 minutes to complete and, in most cases, could be filled with little or no assistance. This is a further proof that the proposed instrument is appropriate for this patient population, bearing in mind that one of the reasons for developing QoL tools for palliative care is to meet the needs of patients who cannot use traditional measures because they are frail and very ill. Patients seemed to be genuinely pleased that an interest in their quality of life was a component of their overall care. Two aspects of this instrument development are worth noting: the opportunity given to the patients to actively participate in this process, and the incorporation, for the first time, of the patients' beliefs in their involvement in treatment decision and what this encompasses. The latter consists a major advantage of the PQLI over the already existing ones in Quality of Life.
PQLI showed acceptable to very good reliability and validity. The internal consistency coefficients for the PQLI subscales are all beyond the acceptable level required for making group comparisons when evaluating changes in scores over time. The scales that showed too significant associations were those of "Activity", "Self-care", and "Health status". The careful construction of the test deriving from the patients' interview as well as the setting of pass/fail scores through the exploratory factor analysis enhance the content and construct validity of the instrument. The correlation between the latent factors of PQLI and the empirical factors of patients' interview (Table 6) provide empirical evidence for concurrent criterion-related validity. Additionally, the latter was strongly indicated by the significant regression coefficients as shown in figure 2. Although the PQLI was related to both "health" and "activity" item of the interviewing rating score, this was not unexpected. Indeed, the high intercorrelation between the "health" and "activity" items supports the clinical observation that a regressive health status often occurs in the context/of regressive activity. Moreover, the significant correlation among the variables of PQLI indicates that performance is related across test components as might be expected. Thus the concurrent related validity had also strong evidence. Furthermore, the factor analysis showed that the various components do assess unique and independent domains, confirming the discriminative validity of the measures [19].
An important aspect that arises from this study concerns the doctor-patient communication and the relations of patients with their families and the important role of the latter. Concealing a diagnosis of cancer from patients themselves, unfortunately, is still common practice in Greece [23]. In "Psychological Affect", and specifically in the item concerning the "fear of death", 56% of the patients said that they don't feel fear of death, while reporting that many have been kept in the dark about the details of their illness diagnosis and prognosis. While most Greek doctors nowadays favour disclosing a cancer diagnosis and prognosis to the patient directly, the relatives often veto this decision, since the role of the family is very powerful in the Greek culture [24]. Nevertheless, they reported a high percentage (84.2%) on the item of support. Regarding the nature of their communication with their physicians on issues like social relationships, financial, and family relationships were very low (21.7%, 5.0%, and 12.5% respectively).
Quality of life research can provide the researcher and the clinician with a clearer view of the impact that a cancer treatment has on a patient's life. This is clearly shown on this instrument from the scale that examines the attitude of patients' toward treatment selection. The responding patients consider as most important their long-term quality of life. The participant patients were receiving palliative treatment, which aims are psychological support and symptom relief both in short and long term, so the findings are consistent with the nature of the treatment they are receiving. Moreover, another important aspect that arises from this study is the participation of the patients in the treatment choice. Half of the responding population (50%) reported that they want to choose the treatment they are going to receive, while a 47.5% said that they actually do choose their treatment. The latter is quite a large percentage considering they had no information on their diagnosis and prognosis.
Conclusions
The psychometric testing of the PQLI provided evidence that the elements of the measurement appropriately reflect quality of life in terminal cancer patients since the tests of validity and reliability yielded consistent results. Indeed, research initiatives must rely on established quality of life instruments with proved records of statistical reliability and validity administered by objective parties.
These results must be confirmed in larger multicenter trials to decrease any possible selection bias. Moreover, when conducting a study in quality of life, since it is subjective in nature, we must remain cautious in our findings, since numbers may be inappropriate to adequately describe all aspects of patients' lives. Inclusion of health related quality of life measures in current and future prospective studies would be necessary to provide a database that is richer and more useful to patients and physicians.
Taken together, these results lend considerable support to the clinical validity of the PQLI, and could be used as an audit in clinical studies involving patients with advanced stage cancer. In future studies we will continue to examine the validity of quality of life measurements obtained with the PQLI using a variety of research strategies including corroboration of additional sources of independent evidence.
Supplementary Material
Appendix - PALLIATIVE CARE QUALITY OF LIFE QUESTIONNAIRE.
Contributor Information
Kyriaki Mystakidou, Email: mistakidou@yahoo.com.
Eleni Tsilika, Email: eltsilika@yahoo.com.
Vassilios Kouloulias, Email: vkouloul@cc.ece.ntua.gr.
Efi Parpa, Email: parpae@hotmail.com.
Emmanuela Katsouda, Email: mistakidou@yahoo.com.
John Kouvaris, Email: vkouloul@cc.ece.ntua.gr.
Lambros Vlahos, Email: lampvla@aretaieio.uoa.gr.
References
- Walsh D. Palliative care: management of the patient with advanced cancer. Seminars in Oncology. 1994;21:100–106. [PubMed] [Google Scholar]
- Mystakidou K, Tsilika E, Befon S, Kululias V, Vlahos L. Quality of Life as a parameter determining therapeutic choices in cancer care. Palliat Med. 1999;13:385–392. doi: 10.1191/026921699669663451. [DOI] [PubMed] [Google Scholar]
- Cella D, Tulsky D. Measuring quality of life today: Methodological aspects. Oncology. 1990;4:29–38. [PubMed] [Google Scholar]
- Lewin T. Ignoring "Right to die" directives. Medical Community is being Sued. The New York Times. p. A1. 2 June 1996. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30; A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Aaranson N, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: A quality-of-Life instrument for use in International clinical trials. J Natl Cancer Inst. 1993;85:365–375. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Tsilika E, Parpa E, Kalaidopoulou O, Smyrniotis V, Vlahos L. The EORTC Core Quality of Life Questionnaire (QLQ-C30, Version 3.0) in terminally ill cancer patients under palliative care: validity and reliability in a Hellenic sample. Int J Cancer. 2001;94:135–139. doi: 10.1002/ijc.1439. [DOI] [PubMed] [Google Scholar]
- Winer EP. Quality-of-life research in patients with breast cancer. Cancer. 1994;74:410–15. doi: 10.1002/cncr.2820741328. [DOI] [PubMed] [Google Scholar]
- Krongrad A, Mark L, Lai H, Lai S. Dimensions of quality of life in prostate cancer. Journal of Urology. 1998;160:807–810. doi: 10.1097/00005392-199809010-00048. [DOI] [PubMed] [Google Scholar]
- Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta K. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- Rodin J. Health, control, and ageing. In: Baltes MM, Baltes PB, editor. The Psychology of control and ageing. Hillsdale, NJ; Eribaum; [Google Scholar]
- Singer PA, Martin DK, Kelner M. Quality End-of-life care: Patients' perspectives. Journal of the American Journal Association. 1999;281:163–168. doi: 10.1001/jama.281.2.163. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and Validation of a brief screening version of the Chidhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Nunnaly JC. Psychometric Theory. 2. New York: McGraw Hill; 1978. [Google Scholar]
- Siegel S. Nonparametric Methods for the Behavioural Sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- Zubrod CG, Schneiderman M, Frei E, et al. Cancer appraisal of methods for the study of chemotherapy of cancer in men: Thiophosphoramide. Journal of Chronic Disorders. 1960;11:7–33. [Google Scholar]
- Gorsuch RL. Factor analysis. Philadelphia: Saunders; 1974. [Google Scholar]
- Axelsson B, Sjöden P. Assessment of Quality of Life in Palliative Care (Psychometric properties of a short questionnaire) Acta Oncol. 1999;38:229–237. doi: 10.1080/028418699431663. [DOI] [PubMed] [Google Scholar]
- Armitage P, Berry G. Statistical Methods in Medical Research. 3. Oxford: Blackwell Scientific Publications; 1994. [Google Scholar]
- Harman HH. Modern Factor Analysis. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Gorgush RL. Psychometric Theory. New York: McGraw-Hill; 1978. [Google Scholar]
- Georgaki S, Kalaidopoulou O, Liarmakopoulos I, Mystakidou K. Nurse's attitudes towards truthful communication with patients with cancer. Cancer Nurs. 2002;25:436–441. doi: 10.1097/00002820-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Parpa E, Tsilika E, Kalaidopoulou O, Vlahos L. The families evaluation on management care, and disclosure for terminal stage cancer patients. BMC Palliat Care. p. 3. April 10 2002. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix - PALLIATIVE CARE QUALITY OF LIFE QUESTIONNAIRE.