Abstract
Glomerular involvement occurs as a rare form of renal manifestation in Plasmodium falciparum malaria. Here, we report a rare case of falciparum malaria-associated IgA nephropathy. A 28-year-old man was admitted because of fever and abdominal pain. Ultrasound and computed tomography (CT) showed right kidney pyonenphrosis. Despite placing a nephrostomy tube, fever continued. Repeated CT was in favor of focal pyelonephritis. In addition, peripheral blood smear suggested malaria. Anti-malarial drugs were initiated and right nephrectomy was performed. One year after recovery from malaria, a persistent rise in serum creatinine was detected. A left kidney biopsy showed mesangial proliferation and dominant IgA deposits in immunofluorescence study while C1q was not deposited. The impression was IgA nephropathy with M1E0S0T0 of Oxford classification. The patient was prescribed a combination of low dose prednisolone and angiotensin converting enzyme inhibitor. Six months after treatment serum creatinine decreased from 1.6 mg/dL to 1.3mg/dL and urine abnormalities were disappeared. Our findings suggest that malaria infection might be associated with IgA nephropathy.
Keywords: IgA nephropathy, Malaria, Plasmodium falciparum
Introduction
Malaria is an endemic disease caused by one of the several Plasmodium species (1). It is one of the most common parasitic infections in tropical regions (2). Acute renal failure as a complication of falciparum malaria has been observed in endemic areas (2, 3). This entity is mostly caused by acute tubular necrosis or interstitial nephritis. A less common form of kidney involvement in falciparum malaria is glomerulopathy, characterized by mesangial proliferation and widening of mesangial region (3,4). In glomerulopathy of malaria, IgM, IgG, and C3 deposits within the mesangium have been detected (3–8), however very few reports regaring the link between IgA nephropathy (IgAN) and falciparum malaria has been published. Here, we present a case of falciparum malaria-associated IgA nephropathy accompanied with renal failure that followed a right kidney pyonephrosis and nephrectomy.
Case
A 28-year-old man visited the Al-Zahra Hospital in Isfahan, Iran on September 2011, because of persistent fever for one month despite repeated use of antipyretics. The patient was an engineer who had come back from a work mission in Sudan. After 30 days of working in Sudan, he developed fever, chills, malaise and abdominal pain. In his past medical history, he had an open right ureterolithotomy 4 years earlier. The patient had, hematemesis and dysuria, not responding to medical therapy there.
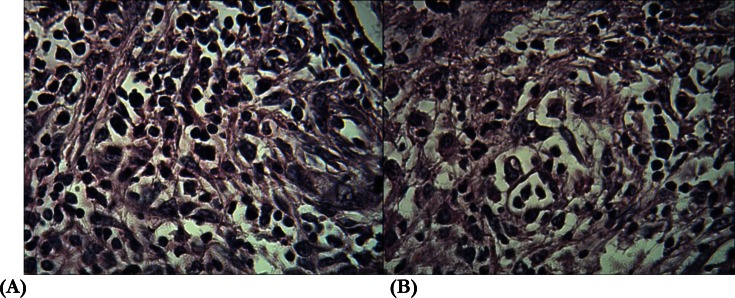
Upon admission, the patient was dehydrated and lethargic and had fever. He also had hematuria. Initial laboratory tests showed the following values: hemoglobin: 9.0 g/dl and serum creatinine: 1.9 mg/dl. Urine dipstick examination showed hematuria (2+) and proteinuria (2+). In kidney sonography, right kidney dilation and pus accumulation in the collecting system (pyonephrosis) was detected that was confirmed by CT. Based on his past history, we assumed that a ureteral stricture had occurred after the stone surgery, culminating in hydronephrosis and then, pyonephrosis. Despite the administration of wide spectrum antibiotics (meropenem 1g IV Tid, plus vancomycin) and right kidney nephrostomy, fever was not subsided and general condition was not improved. Upon placing the nephrostomy, 200 mL of pus was drained that in culture, showed the growth of pseudomas aeroginosa, and antibiotic changed to meropenem and amikacin. A repeat CT after nephrostomy showed focal pyelonephritis (lobar nephronia); therefore, the patient underwent nephrectomy. Examination of the tissue revealed significant inflammatory infiltration consisting of mononuclear and polynuclear cells were indentified, consistent with pyonephrosis. Also renal structures were destructed profoundly (Fig. 1). Before contemplating nephrectomy, a peripheral blood smear had been performed as a part of the sepsis work-up that showed hyperparasitemia with Plasmodium falciparum.
Fig. 1:
Significant inflammatory cells infiltration and destruction of renal structures of the right kidney (A&B)
Anti-malarial treatment on the base of national protocol including aresunate and fansidar was initiated immediately. Fever and other symptoms subsided and general condition became better, and serial peripheral blood smears showed low and negative parasite at third and seventh days after treatment. However, the patient had a serum creatinine up to 1.6 mg/dl at the time of discharge. Around one year after discharge, the patient was symptom free, and for further evaluation of mild renal failure, patient was referred to the clinic of nephrology (in November 2012). Primary work up revealed that the patient had a serum creatinine of 1.7mg/dl (eGFR# 75cc/min). Urine examination showed dysmorphic hematuria. In addition, there was a 500mg/day proteinuria. In further history taking, we noticed to serum creatinine of 0.8mg/dl with normal urine analysis prior to trip to Sudan, one year ago.
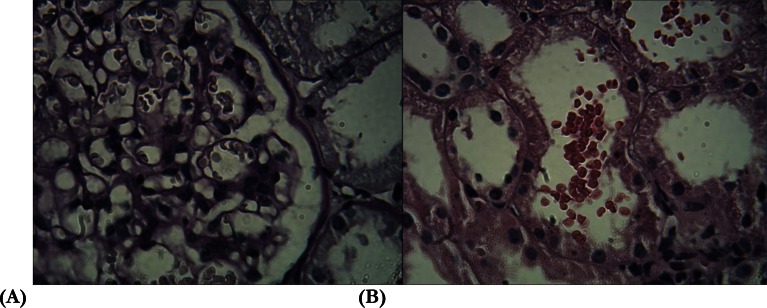
To find out the etiology of renal failure with proteinuria, a left kidney biopsy was conducted. In immunofluorescence (IF) study, prominent deposit of IgA antibody (3+ on a score of 0 to 3+) was detected .There was also 2+ deposit of C3. However, there was not C1q deposits. In light microscopy, mesangial proliferation in around 50% of the glomeruli was observed. In addition, there was a slight mesangial area thickness (Fig. 2). Tubules were closely packed and the interstitium was normal. However, RBC in tubules was very popular. Endocapillary or extracapillary proliferation was absent. Prominent deposit of IgA antibody along with C3 deposits and absence of C1q in IF study was interpreted as IgA nephropathy and according to the Oxford classification for this nephropathy, and biopsy was classified as M1E0S0T0(9–11). The patient was prescribed by a combination of low dose prednisolon and angiotensin converting enzyme inhibitor. Six months after treatment, serum creatinine decreased to 1.3mg/dl and urine abnormalities were disappeared.
Fig. 2:
mesangial widening and mesangial cell proliferation (A), and RBC in the tubular lumen (B) of the left kidney
Discussion
Since its first description in 1968 by Berger, IgAN has remained the most common form of idiopathic glomerulonephritis leading to chronic renal failure in developed countries (10,11). The exact pathogenesis of IgA nephropathy is still not well understood, however some infectious organisms have been reported to be associated with IgAN (10,11). These include Mycoplasma pneumonia, Staphylococcus spp., Haemophilus parainfluenzae, and contamination with Hepatitis B virus (10–14). Previously George et al., in a preclinical study on mice which was infected by Plasmodium berghei yoelii parasites intraperitoneally, described a glomerulonephritis associates with predominantly mesangial deposits of C3, IgG1, IgM and some IgA always developed after 7 days and persisted for up to 6 months (15).
To our best of knowledge, the only case suggesting the link between IgAN and P. falciparum infection was recently reported by Yoo et al. (16). They described a 49-year-old male who was diagnosed with P. falciparum malaria. Microhematuria and proteinuria in association with acute renal failure developed during the course of the disease. Renal biopsy revealed mesangial proliferation with dominant IgA deposits. Laboratory tests after recovery from malaria showed disappearance of urinary abnormalities and normalization of kidney function. They suggested that malaria infection might be associated with IgA nephropathy (16). In line with the previous studies, and the dominant IgA deposits with negative C1q deposition in IF study was mostly consistent with IgA nephropathy, which clearly explains our patient’s clinical features. In this patient, notably, IgAN developed after P. falciparum infection.
However, one might question whether the patient had nephropathy of IgA prior to the infection. Normal renal function (creatinine: 0.8mg/dl) and normal urine analysis one month before trip to Sudan make it unlikely. Thus findings taken together, suggest that P. falciparum may have been associated with IgA nephropathy in our patient. One important question is how malaria infection is involved in the development of IgAN. In general, undergalactosylated IgA1 is observed to play a role in the pathogenesis of IgAN(17–21), hence in our case, it is possible that P. falciparum might have induced formation of aberrantly glycosylated polymeric IgA1(9–11).
Conclusion
Our findings support that P. falciparum may be involved in the development of IgAN. However, very few studies published regarding the association of IgA with P. falciparum and the exact mechanism responsible remains speculative. Further studies to find the underlying pathogenesis is required.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
The authors would like to thank Dr. Baradaran Laboratory, Isfahan, Iran, for processing of renal biopsy. The authors declare that there is no conflict of interest.
References
- 1.Kissou SA, Cessouma R, Barro M, Traoré H, Nacro B. Acute renal failure and Plasmodium falciparum malaria: a case report. Arch Pediatr. 2012;19(1):34–7. doi: 10.1016/j.arcped.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Mueller I, Moorthy VS, Brown GV, Smith PG, Alonso P, Genton B, et al. Guidance on the evaluation of Plasmodium vivax vaccines in populations exposed to natural infection. Vaccine. 2009;18(27(41)):5633–43. doi: 10.1016/j.vaccine.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsoum RS. Malarial nephropathies. Nephrol Dial Transplant. 1998;13:1588–1597. doi: 10.1093/ndt/13.6.1588. [DOI] [PubMed] [Google Scholar]
- 4.Weber MW, Boker K, Horstmann RD, Ehrich JH. Renal failure is a common complcation in non-immune Europeans with Plasmodium falciparum malaria. Trop Med Parasitol. 1991;42(2):115–118. [PubMed] [Google Scholar]
- 5.Assadi F. The epidemic of pediatric chronic kidney disease: the danger of skepticism. J Nephropathology. 2012;1(2):61–64. doi: 10.5812/nephropathol.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheissari A, Hemmatzadeh S, Merrikhi A, Fadaei Tehrani S, Madihi Y. Chronic Kidney Disease in Children: A report from a tertiary care center over 11 years. J Nephropathology. 2012;1(3):177–182. doi: 10.5812/nephropathol.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kari J. Epidemiology of chronic kidney disease in children. J Nephropathology. 2012;1(3):162–163. doi: 10.5812/nephropathol.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheissari A, Mehrasa P, Merrikhi A, Madihi Y. Acute kidney injury: A pediatric experience over 10 years at a tertiary care center. J Nephropathology. 2012;1(2):101–108. doi: 10.5812/nephropathol.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mubarak M. Oxford classification of IgA nephropathy: Broadening the scope of the classification. J Nephropathology. 2012;1(1):13–16. doi: 10.5812/jnp.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasri H, Mortazavi M, Ghorbani A, Shahbazian H, Kheiri S, Baradaran A, et al. Oxford-MEST classification in IgA nephropathy patients: A report from Iran. J Nephropathology. 2012;1(1):31–42. doi: 10.5812/jnp.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mubarak M, Kazi JI, Kulsoom U, Ishaque M. Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescencetechnique. J Nephropathology. 2012;1(2):91–100. doi: 10.5812/nephropathol.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang NS, Wu ZL, Zhang YE, Guo MY, Liao LT. Role of hepatitis B virus infection in pathogenesis of IgA nephropathy. World J Gastroenterol. 2003;9:2004–2008. doi: 10.3748/wjg.v9.i9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunaga H, Oh M, Takahashi N, Fujieda S. Infection of Haemophilusparainfluenzae in tonsils is associated with IgA nephropathy. ActaOtolaryngol. 2004;(Suppl):15–19. [PubMed] [Google Scholar]
- 14.Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, Nadasdy T. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006;1:1179–1186. doi: 10.2215/CJN.01030306. [DOI] [PubMed] [Google Scholar]
- 15.George CR, Parbtani A, Cameron JS. Mouse malaria nephropathy. J Pathol. 1976;20:235–249. doi: 10.1002/path.1711200407. [DOI] [PubMed] [Google Scholar]
- 16.Yoo DE, Kim JH, Kie JH, Park Y, Chang TI, Oh HJ, et al. Immunoglobulin A nephropathy associated with Plasmodium falciparum malaria. J Korean Med Sci. 2012;27(4):446–9. doi: 10.3346/jkms.2012.27.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seif EI, Ibrahim EA, El hefnawy NG, Salman MI. Histological patterns of idiopathic steroid resistant nephrotic syndrome in Egyptian children: A single centre study. J Nephropathology. 2013;2(1):53–60. doi: 10.5812/nephropathol.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakeel Sh, Mubarak M, Kazi JI, Jafry N, Ahmed E. Frequency and clinicopathological characteristics of variants of primary focal segmental glomerulosclerosis in adults presenting with nephrotic syndrome. J Nephropathology. 2013;2(1):28–35. doi: 10.5812/nephropathol.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mubarak M. Collapsing focal segmental glomerulosclerosis: Increasing the awareness. J Nephropathology. 2012;1(2):77–80. doi: 10.5812/nephropathol.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi Torbati P. Focal segmental glomerulosclerosis; collapsing variant. J Nephropathology. 2012;1(2):87–90. doi: 10.5812/nephropathol.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kam-Tao Li PK, Burdmann EA, Mehta RL. Acute kidney injury: global health alert. J Nephropathology. 2013;2(2):90–97. doi: 10.12860/JNP.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasri H, Sajjadieh S, Mardani S, momeni A, Merikhi A, Madihi Y, et al. Correlation of immunostaining findings with demographic data and variables of Oxford classification in IgA nephropathy. J Nephropathology. 2013;2(3):190–195. doi: 10.12860/JNP.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mubarak M. Significance of immunohistochemical findings in Oxford classification of IgA nephropathy: The need for more validation studies. J Nephropathology. 2013;2(3):210–21. doi: 10.12860/JNP.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]