Abstract
Objective
Apnea is one of the most common problems in premature newborns. The present study aimed to determine the effect of olfactory stimulation by vanillin on prevention of apnea in premature newborns.
Methods
In this randomized controlled trial, 36 premature newborns with the postnatal age of 2 days and weight under 2500 grams referred to the hospitals affiliated to Shiraz University of Medical Sciences, were selected through simple random sampling and allocated into control and experimental groups. The experimental group received olfactory stimulation by saturated vanillin solution, while the control group received no interventions. The newborns of both groups were continuously monitored for presence/absence of apnea and number of episodes of apnea as well as arterial blood oxygen saturation and heart rate for 5 days. The data were analyzed by independent Student t-test and repeat measure ANCOVA.
Findings
The presence of apnea revealed to be significantly different between the two groups in the first, second, and fourth day of the study (P<0.05). The number of episodes of apnea during five days was also significantly different between the study groups (t=8.32, P<0.05). Using olfactory stimulation by vanillin caused a 3.1-fold decrease in apnea and the effect size was 0.72. Moreover, the two groups were significantly different regarding the arterial blood oxygen and heart rate during the study period (P<0.05).
Conclusion
This study indicated the beneficial effect of saturated vanillin solution on apnea; therefore, it may be used for prevention and treatment of apnea in premature infants. Further studies are needed to improve evidence-based practice in this regard.
Keywords: Vanillin, Apnea, Premature Infant, Newborn, Olfactory, Chemical Stimulation
Introduction
Apnea is cessation of airflow for 20 seconds or longer, or a shorter pause in the airflow which is accompanied by bradycardia or cyanosis[1]. The incidence of apnea increases with decrease of gestational age (GA) and newborn's weight. In general, apnea occurs in 80% of the newborns below 30 weeks of GA as well as 30% of those under 1500 grams[2, 3]. Idiopathic apnea of prematurity is a type of neonatal apnea which mostly occurs in the second up to the seventh day after birth[1, 4].
Apnea in premature infants is associated with many complications such as bradycardia, cyanosis, brain damage, hypotension, hypotonia, hydro-cephalus, neurologic complications, and even death[2, 3, 5, 6]. It is reported that there was a relationship between apnea and bradycardia and desaturation[7]. In addition, a correlation was found between low expiratory lung volume as well as hypoxemia and apnea[6].
Since 1970, apnea in premature newborns could only be treated by pharmacological methods through methylxanthines, such as theophylline and caffeine[2, 8–12]. Doxapram is a powerful respiratory stimulant and has been used for treatment of apnea unresponsive to methylxanthines[13–15]. However, most of these pharmacological methods are not completely effective; so that apnea attack might reappear in the treated newborns and may even necessitate mechanical ventilation as well as application of continuous positive airway pressure[2]. Also, the medicines used to treat apnea are usually accompanied by several side effects such as irritability, tachycardia, and sleeping disorders[2, 6]. In addition, caffeine decreases the cerebral blood flow, while doxapram increases the blood pressure and, consequently, enhances the risk of cerebral hemorrhage[16]. Thus, in order to reach an effective treatment with the least side effects, more studies are needed to be conducted on the issue.
A study which was performed on the ability of premature newborns in identifying and realizing the smells showed that the number of the newborns’ breaths tended to change based on evaluating the joy of the smells[2]. In fact, pleasant smells lead to an increase in the newborns’ respiratory efforts, while unpleasant ones result in a decrease in their respiratory effort during their active sleep[17]. One of the pleasant smells for the premature infants is that of vanillin. The odor of vanillin was reported to be accompanied by physiological as well as behavioral reactions in newborns in the previous studies. Vanillin has a mild odor and does not cause any harm for the newborns. Moreover, the newborns perceive the scent of vanillin as a pleasant smell; and an olfactory stimulant must be perceived as pleasant in order to be effective in treatment[18].
The effect of olfactory stimulation by vanillin on apnea in premature newborns was first investigated by Marlier et al, revealing that this method could be used in apnea non-responding to caffeine and doxapram[2]. It was reported that vanillin, as an olfactory stimulant, led to enhantment in orbito-frontal blood flow detectable by near-infrared spectroscopy in normal newborns[19]. Also, it seems that there is even an olfactory memory in premature newborns which is capable of having soothing effects upon pain stimulation[20]. Although some studies have confirmed the effectiveness of vanillin, Marom et al showed that olfactory stimulation by saturated solution of vanillin had no influence on increasing the resting energy expenditure in preterm infants[21].
Despite the possible effect of olfactory stimulation by vanillin on the treatment of apnea in premature infants, few studies have been conducted in this regard. Therefore, this study was undertaken to determine the effect of olfactory stimulation via vanillin on prevention of apnea in premature newborns.
Subjects and Methods
In the present randomized, double-blind, controlled trial, 36 premature infants were selected through simple random sampling and then allocated to experimental (n = 18) and control (n = 18) groups by block randomization. The eligible subjects in both groups were followed up for five days (the second up to the seventh day after birth). This period was selected due to the fact that neonatal apnea mostly occurs in these days[1, 4]. The study was performed from July 2010 to January 2011.
Setting
The current study was conducted at level two of neonatal intensive care unit (NICU) from three hospitals (Namazi, Hafez, and Zeynabeyah) affiliated to Shiraz University of Medical Sciences (SUMS). There were 8 beds in the level 2 of all the study hospitals.
Participants
This study was conducted on 36 premature infants. The inclusion criteria were being born before 36 weeks of gestation, postnatal age of 2 days, weight under 2500 grams, being inside the incubator, rate of hemoglobin being over 10 g/dl, obtaining written informed consent from the parents, not suffering from congenital heart diseases and acute infections, no need for ventilators, not receiving oxygen through continuous positive airway pressure, not having any background of addiction or using hypnotics as well as sedative drugs of mother. The premature infants who had hypocalcemia, hypoglycemia, evidence of intracranial hemorrhage during the intervention, need for ventilators during the study, acute infections, hemoglobin less that 10g/dl, or received hypnotics as well as sedative drugs and traditional treatments during the intervention, and those whose parents were unwilling to continue their cooperation with the researcher were excluded from the study. It should be noted that a neonatologist confirmed that the premature infants had no congenital heart diseases, acute infections, or intracranial hemorrhage.
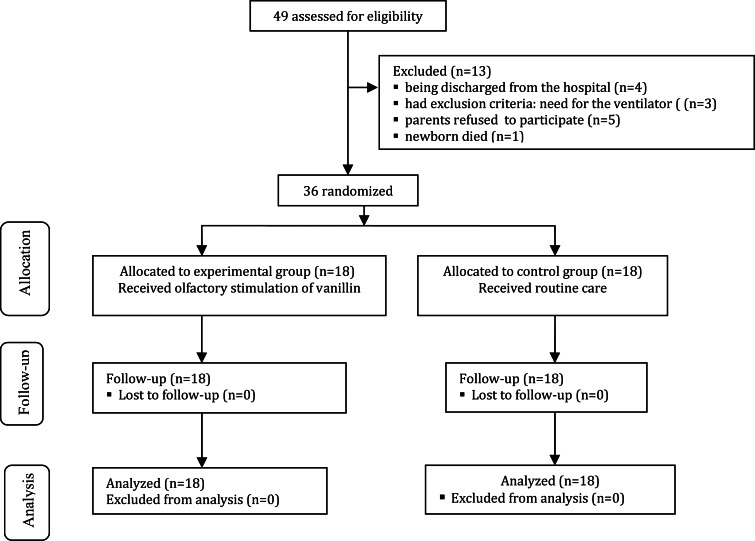
Considering the power of 90% and α = 0.05, a 36-subject sample size was selected for the study (18 subjects in each group). Overall, 49 premature newborns were assessed for eligibility. However, 13 newborns were excluded from the study due to being discharged from the hospital (n = 4), death (n = 1), need for the ventilator (n = 3), and parents’ refusal to his/her participation in the study (n = 5). Finally, 36 newborns were entered into the study and none were excluded during the follow up and analysis. Fig. 1 shows the diagram of participants in the study.
Fig. 1.
Diagram of enrollment, allocation, follow up, and data analysis of the premature infants
Procedure
The procedure was started since 12 A.M in the second day after birth in both study groups. In addition to the routine care, the experimental group received the following intervention: At 12 A.M in the second day after birth, while the newborn was in the incubator, 2 cc (0.04 g) of 2% saturated solution of vanillin (Merc, Germany) was dragged from the vial using a 5 cc syringe. Then, it was poured on a piece of cotton and placed inside the incubator (Tosan, Iran) 20 cm far from the newborn's head, while having no skin contact with the newborn. The intervention was performed every 12 hours (12 a.m. and 12 p.m.). It should be noted that in these two episodes, only saturated solution of vanillin was used and other interventions, such as using caffeine, was avoided. The newborns were still monitored from the second through the fifth day of the study; however, no other interventions were performed during this period.
On the other hand, the premature newborns of the control group did not receive any interventions except for the routine care, but were monitored continuously.
In case apnea occurred during the study period, the nurse would use gentle tactile stimulation in order to treat it. However, if apnea reoccurred, aminophylline or theophylline was prescribed for the newborn. Finally, if apnea was not cured, the newborn was treated by CPAP or IMV ventilator and, as a result, was excluded from the study.
Measures
Physiological parameters, such as the percentage of oxygen saturation (SpO2) and heart rate (HR), were measured using a digital and electronic pulse oximeter device (520A-Novametrix, USA) and continuously recorded on computer. During the data measurement, the probe of the pulse oximeter monitor was placed on the left sole of the newborn.
The newborns’ weight was measured at the beginning of the morning shift using baby scales (Seca 354, Germany). Moreover, Golbahar white cotton made in Iran was used in the study.
Measurement reliability
In this study, inter-rater reliability was measured for gathering the HR and SaO2 parameters. Besides, two individuals observed the same newborn's pulse oximeter on several occasions for enhancing the reliability of the observations (Kappa coefficient = 0.93).
Outcome measures
The outcome measures in this study included the presence/absence of apnea, SpO2, and HR during the five days of the study and number of episode of apnea during each day. According to the general acceptance, apnea is defined as cessation of respiration for >20 sec or cessation of respiration of any duration accompanied by bradycardia (HR <100/min) and/or cyanosis[4]. Any time the cessation of respiration occurred for >20 sec or pulse oximetry showed the blood oxygen saturation under 88% or bradycardia (HR 90 beats per minute or less)[2], it was reported as the presence of apnea by the assistants of the research.
Ethical consideration
The study was approved by the Ethic Committee of SUMS. The parents received explanation about the procedure of using saturated solution of vanillin for their premature infants. The parents were also ascertained that participation/non participation in the study would not affect the infants’ care and treatment and reassured about their anonymity and confidentiality, as well. It should be mentioned that the basic human rights to freedom from risk of injury, privacy, and autonomy were closely evaluated by the Ethic Committee of SUMS. Moreover, written informed consents had been obtained before the study.
Data collection
The demographic characteristics of all the eligible participants, including gender, birth type (vaginal or cesarean section), mother's age, birth weight, and GA were collected through face to face interview with parents of the premature infants. Other data, including presence/absence of apnea, SpO2, and HR were gathered during the first to the fifth day of the study in both the experimental and the control group. SpO2 and HR were measured in the morning (8 a.m.), in the evening (2 p.m.), and at night (10 p.m.). Then, mean of the three parameters were measured in each day. Moreover, number of episodes of apnea was measured by the research assistant during each day of the study. It should be noted that the research assistant who collected the study data, the nurse, and the physicians were blind to the intervention as well as the experimental and control groups. Data analysis was also performed by an individual who was blind to the study groups.
Statistical analysis
Data analysis was performed by SPSS statistical software (version 16). Chi-square and independent Student t-test were used for comparison of the demographic characteristics in both study groups. Moreover, Fisher's exact test was used in order to compare the two groups regarding apnea in the first up to the fifth day of the study. Also, the two groups were compared regarding the number of episodes of apnea during the five days of the study using independent Student t-test, and the GA was considered as a covariate variable. Finally, repeated measure ANCOVA was used in order to compare the two groups regarding the mean of HR and SpO2 changes in the first up to the fifth day of the study. P. value <0.05 was considered as statistically significant.
Findings
In this study, most of the premature infants were female (86.1%) and majority of births had occurred through cesarean section (52.8%). The mean age of the infants’ mothers was 20.44 [standard deviation (SD) = 7.50] years. In addition, the gestational age ranged from 29 to 33 weeks with a mean of 30.38 (SD = 1.31) weeks. Besides, the mean of the infants’ birth weight was 1397.50 g (SD = 291.24) ranging from 1230 to 2300 g. The demographic characteristics of the subjects in the two groups are shown in Table 1. No significant difference was found between the two groups regarding gender, birth type, mother's age, birth weight, and GA (Table 1). This study indicated a significant difference between the two study groups regarding the presence/absence of apnea in the first, second, and fourth day of the study (P<0.05) (Table 2). However, no significant difference was observed between the two groups concerning the presence/absence of apnea in the third and fifth day of the study (P>0.05) (Table 2).
Table 1.
Demographic characteristics of the participants in the experimental and control groups
| Demographic characteristics | Experimental group | Control group | P. value | |
|---|---|---|---|---|
| Gender, n (%) | Female | 6 (33.3) | 10 (55.6) | 0.2† |
| Male | 12 (66.7) | 8 (44.4) | ||
| Birth type | Vaginal | 6 (33.3) | 4 (22.2) | 0.45† |
| Cesarean section | 12 (66.7) | 14 (77.8) | ||
| Mother's age (years)[mean (SD)] | 28.0 (6.6) | 30.3 (9.3) | 0.4§ | |
| Gestational age | 33.2 (1.6) | 32.9 (1.8) | 0.6§ | |
| Birth weight (g) | 1936.7 (340.7) | 1848.9 (374.5) | 0.5§ | |
Chi-square statistics
Independent t-test; SD: Standard Deviation
Table 2.
Comparison of the presence/absence of apnea in the two groups from the first up to the fifth day of the study
| Day of the study | Experimental group | Control group | P. value | ||
|---|---|---|---|---|---|
| presence of Apnea | absence of Apnea | Presence of Apnea | absence of Apnea | ||
| 1st | 0 | 18 | 12 | 6 | <0.001 |
| 2nd | 1 | 17 | 7 | 11 | 0.04 |
| 3rd | 2 | 16 | 3 | 15 | 1.0 |
| 4th | 0 | 18 | 5 | 13 | 0.04 |
| 5th | 0 | 18 | 3 | 15 | 0.2 |
The result showed the mean of the number of apnea episodes during the five days of the study as 0.4 (SD = 1.4) and 3.5 (SD = 3.1) in the experimental and the control group, respectively. A significant difference was found between the two groups regarding the number of apnea episodes during the five days of the study after controlling GA as a covariate (t=8.32, P=0.001). Furthermore, using vanillin caused a 3.1-fold decrease in apnea and the effect size was 0.72.
The study findings also revealed a significant difference between the two groups regarding the mean of SpO2 changes from the first to the fifth day of the study (F = 11.59, P=0.002) (Table 3). In addition, a significant difference was found between the two groups regarding HR from the first to the fifth day of the study (F = 24.93, P<0.0001) (Table 4).
Table 3.
Comparison of SpO2 between the two groups from the first up to the fifth day of the study*
| SpO2 in day of the study | Experimental group Mean (SD) | Control group Mean (SD) |
|---|---|---|
| 1 st | 89.76 (3.11) | 89.72 (3.89) |
| 2 nd | 89.70 (2.01) | 90.36 (3.64) |
| 3 rd | 89.80 (3.12) | 91.33 (3.96) |
| 4 th | 91.10 (2.19) | 91.44 (3.59) |
| 5 th | 91.22 (4.25) | 93.50 (3.64) |
repeated measure ANCOVA: F= 11.59, P=0.002; SD: Standard Deviation
Table 4.
Comparison of HR between the two groups from the first up to the fifth day
| HR in day of the study | Experimental group Mean (SD) | Control group Mean (SD) |
|---|---|---|
| 1st | 130.02 (10.16) | 121.47 (19.57) |
| 2nd | 129.80 (11.26) | 125.69 (14.40) |
| 3rd | 128.15 (8.34) | 123.48 (17.98) |
| 4th | 127.72 (13.60) | 126.02 (20.48) |
| 5th | 131.67 (8.72) | 126.46 (17.13) |
repeated measure ANCOVA: F= 24.93, P<0.0001; SD: Standard Deviation
Discussion
The present study aimed to evaluate the preventive effect of vanillin on apnea in premature infants. The study results revealed a significant difference between the two groups regarding the presence/absence of apnea in the 1st, 2nd, and 4th day of the study. In addition, a significant difference was found between the two groups concerning the number of apnea episodes during the five days of the study. Besides, using vanillin resulted in a 3.1-fold decrease in apnea with an effect size of 0.72. Moreover, a significant difference was observed between the study groups regarding SpO2 and HR during the study period. This shows the effectiveness of saturated vanillin solution in preventing apnea. In the same line, Marlier et al demonstrated that using the mild and pleasant odor of the saturated vanillin solution inside the incubator significantly decreased apnea in the second day of the study compared to the first and the third days[4]. It was also reported that the longer exposure to the scent of vanillin decreased the newborns’ crying time and distress[18]. Other researchers have also shown that the newborns that had been exposed to the scent of vanillin before heel-stick had lower crying time in comparison to the other groups[20, 22]. There are three hypotheses regarding the mechanism of the vanillin effect. First, vanillin, directly or indirectly, affects the respiratory center in the brain. In fact, vanillin passes through the nasal mucosa and enters the brain through the blood flow. It can also reach the brain through the nerves existing in the newborns’ olfactory system[2]. Second, vanillin enhances orbito-frontal blood flow detectable by near-infrared spectroscopy[19]. Third, vanillin helps the newborns cope with stress[2]. It can be implied that vanillin decreases distress and, eventually, it might prevent apnea which is related to stress.
The finding of the study showed that in comparison to the control group, the changes in SpO2 were less in the experimental group. In other words, these changes had occurred within a consistent range, which is beneficial for the newborns. Based on the results of the study conducted by Bartocci et al, the capacity of HbO2 significantly increased in the newborns exposed to the odor of vanillin[19]. The findings of the study by Sadathosseini et al demonstrated that after arterial sampling from the newborns’ skin, the level of SpO2 revealed to be significantly higher in the newborns that had been exposed to the vanillin scent for 9 hours before taking the blood in comparison to the other two groups under study[22].
The findings of the present study indicated a significant difference between the control and experimental groups regarding the mean of HR during the five days of the study. In a study on adults, olfactory stimulation during sleep was shown to increase heart rate[23]. Moreover, it has been stated that odorization of the incubator by vanillin decreased the number of apnea events associated with severe bradycardia[2]. However, Sadathosseini et al showed no significant difference between the three groups under study (familiar smell; i.e., vanillin, unfamiliar smell, and control) regarding HR after entering the needle and bringing it out[22]. Based on the results of the above-mentioned studies, as a soothing sensory intervention continues for a longer period of time, the newborns’ experience of pain will decrease at the same time[24]. Therefore, the newborns will have more time in order to balance the changes in their physiological system[22].
One of the limitations of this study was the small number of the participants. Another limitation was the exclusion criteria of the study. In fact, the excluded infants might have benefitted from the procedure.
Furthermore, this study investigated the effect of vanillin on only one type of apnea, i.e. idiopathic apnea of prematurity in the newborns. Therefore, further studies are suggested to be performed in order to determine the effectiveness of this non-pharmacological method on different types of apnea and also for improving its evidence based practice.
Conclusion
The present study showed that smelling the scent of the saturated vanillin solution could significantly decrease apnea through the newborns’ olfactory stimulation. It was also highly effective in the newborns’ SpO2 as well as the mean of HR. Thus, it can be concluded that vanillin saturated solution might decrease the incidence of apnea in premature newborns and, at the same time, protect them against the dangerous complications of the disease.
Acknowledgements
The present article was extracted from the thesis written by Hajar Pourpulad, financially supported by Shiraz University of Medical Sciences, in November 2011 (Grant No. 90-5789). The authors would like to thank the research vice-chancellor and the neonatal research center of Shiraz University of Medical Sciences. They are also grateful for Research Improvement Center of Shiraz University of Medical Sciences and Ms. A. Keivanshekouh for improving English of the manuscript.
Conflict of Interest
None
References
- 1.Deacon J. Core curriculum for neonatal intensive care nursing. 2nd ed. Philadelphia: Saunders; 1999. Apnea of the newborn infant: Neonatal pulmonary disorders and management; pp. 152–3. [Google Scholar]
- 2.Marlier L, Gaugler C, Messer J. Olfactory stimulation prevents apnea in premature newborns. Pediatrics. 2005;115(1):83–8. doi: 10.1542/peds.2004-0865. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Shaweesh JM, Martin RJ. Apnea of prematurity: past, present and future. J Arab Neonatal Forum. 2005;2:63–73. [Google Scholar]
- 4.Mishra S, Agarwal R, Jeevasankar M, et al. Apnea in the newborn. Indian J Pediatr. 2008;75(1):57–61. doi: 10.1007/s12098-008-0008-7. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aif S, Alvaro R, Manfreda J, et al. Inhalation of low (0.5%-1.5%) CO2 as a potential treatment for apnea of prematurity. Semin Perinatol. 2001;25(2):100–6. doi: 10.1053/sper.2001.23199. [DOI] [PubMed] [Google Scholar]
- 6.Poets CF. Apnea of prematurity: What can observational studies tell us about pathophysiology? Sleep Med. 2010;11(7):701–7. doi: 10.1016/j.sleep.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Poets CF, Southall DP. Patterns of oxygenation during periodic breathing in preterm infants. Early Hum Dev. 1991;26(1):1–12. doi: 10.1016/0378-3782(91)90038-5. [DOI] [PubMed] [Google Scholar]
- 8.Bairam A, Boutroy MJ, Badonnel Y, et al. Theophylline versus caffeine: comparative effects in treatment of idiopathic apnea in the preterm infant. J Pediatr. 1987;110(4):636–9. doi: 10.1016/s0022-3476(87)80569-3. [DOI] [PubMed] [Google Scholar]
- 9.Muttitt SC, Tierney AJ, Finer NN. The dose response of theophylline in the treatment of apnea of prematurity. J Pediatr. 1988;112(1):115–21. doi: 10.1016/s0022-3476(88)80135-5. [DOI] [PubMed] [Google Scholar]
- 10.Murat I, Moriette G, Blin M, et al. The efficacy of caffeine in the treatment of recurrent idiopathic apnea in premature infants. J Pediatr. 1981;99(6):984–9. doi: 10.1016/s0022-3476(81)80038-8. [DOI] [PubMed] [Google Scholar]
- 11.Mathew O. Apnea of prematurity: pathogenesis and management strategies. J Perinatol. 2011;31(5):302–10. doi: 10.1038/jp.2010.126. [DOI] [PubMed] [Google Scholar]
- 12.Luckey JF. The xanthine treatment of apnea of prematurity. Pediatrics. 1975;55(5):584–6. [PubMed] [Google Scholar]
- 13.Alpan G, Eyal F, Sagi E, et al. Doxapram in the treatment of idiopathic apnea of prematurity unresponsive to aminophylline. J Pediatr. 1984;104(4):634–7. doi: 10.1016/s0022-3476(84)80568-5. [DOI] [PubMed] [Google Scholar]
- 14.Sagi E, Eyal F, Alpan G, et al. Idiopathic apnoea of prematurity treated with doxapram and aminophylline. Arch Dis Child. 1984;59(3):281–3. doi: 10.1136/adc.59.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrington KJ, Finer NN, Peters KL, et al. Physiologic effects of doxapram in idiopathic apnea of prematurity. J Pediatr. 1986;108(1):124–9. doi: 10.1016/s0022-3476(86)80786-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Gonzalez F, Mu D. Apnea of prematurity: from cause to treatment. Eur J pediatr. 2011;170(9):1097–105. doi: 10.1007/s00431-011-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arzi A, Sela L, Green A, et al. The influence of odorants on respiratory patterns in sleep. Chem Senses. 2010;35(1):31–40. doi: 10.1093/chemse/bjp079. [DOI] [PubMed] [Google Scholar]
- 18.Williams M. Soothe babies with familiar smells. J Dev Behav Pediatr. 2005;26(2):86–92. doi: 10.1097/00004703-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Bartocci M, Winberg J, Ruggiero C, et al. Activation of olfactory cortex in newborn infants after odor stimulation: a functional near-infrared spectroscopy study. Pediatr Res. 2000;48(1):18–23. doi: 10.1203/00006450-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Goubet N, Rattaz C, Pierrat V, et al. Olfactory experience mediates response to pain in preterm newborns. Dev Psychobiol. 2003;42(2):171–80. doi: 10.1002/dev.10085. [DOI] [PubMed] [Google Scholar]
- 21.Marom R, Shedlisker-Kening T, Mimouni FB, et al. The effect of olfactory stimulation on energy expenditure in growing preterm infants. Acta Paediatrica. 2012;101(1):11–4. doi: 10.1111/j.1651-2227.2011.02399.x. [DOI] [PubMed] [Google Scholar]
- 22.Sadathosseini A, Negarande R, Mehran A. Effect of familiar olfactory stimulus on responses to blood sampling pain in neonates. Sci J Hamdan Univ Med Sci. 2011;18(1):10–9. [In Persian] [Google Scholar]
- 23.Heuberger E, Hongratanaworakit T, Buchbauer G. East Indian Sandalwood and alpha-santalol odor increase physiological and self-rated arousal in humans. Planta Med. 2006;72(9):792–800. doi: 10.1055/s-2006-941544. [DOI] [PubMed] [Google Scholar]
- 24.Castral TC, Warnock F, Leite AM, et al. The effects of skin-to-skin contact during acute pain in preterm newborns. Eur J Pain. 2008;12(4):464–71. doi: 10.1016/j.ejpain.2007.07.012. [DOI] [PubMed] [Google Scholar]