Abstract
Objective
Adiponectin is secreted from adipose tissue. This hormone has a fundamental role in pathogenesis of insulin resistance, and has anti-inflammatory and anti-atherogenic effects. The objectives of this study were to compare serum adiponectin level between type 1 diabetics and healthy people and to assess its related factors, and also to determine the relationship between adiponectin and metabolic state.
Methods
This was a case control study involving 60 diabetics (25 good and 35 poor metabolic controlled) and 28 healthy persons (younger than 18 years old). The data about demographic (age and sex), clinical and paraclinical characteristics [body mass index (BMI), duration of disease, puberty state, and glycosylated hemoglobin (HbA1c) and adiponectin level in serum] were collected. Determinants of adiponectin were assessed using univariate and multiple linear regression analyses.
Findings
Mean (±SD) serum adiponectin level in healthy persons, good-controlled and poor-controlled type 1 diabetes mellitus patients were 9.16 (±4.2) µg/cc, 10.89 (±4.48)µg/cc, and 15.92 (±8.26)µg/cc, respectively. Post hoc analysis revealed that differences of adiponectin between poor- and good-controlled type 1 diabetes mellitus patients (P=0.01) and between healthy persons and poor controlled type 1 diabetes mellitus (P<0.0001) were statistically significant. Adiponectin level was associated with puberty state and BMI in healthy persons. It was associated with puberty state and HbA1c in type 1 diabetic persons.
Conclusion
Serum level of adiponectin was higher in type 1 diabetics than in healthy persons and it can be used as a good marker for metabolic control state among diabetics.
Keywords: Cytokines, Adiponectin, Type 1 Diabetes Mellitus, Glycosylated Hemoglobin, Puberty
Introduction
Adiponectin is a protein secreted solely from adipose tissue and like other adipocytokines makes connection between different organs[1]. This hormone has a fundamental role in pathogenesis of insulin resistance and type 2 diabetes mellitus (DM). In studies with human samples it has been observed that adiponectin level is negatively correlated with body mass index (BMI), waist circumference, systolic blood pressure, plasma glucose and glycosylated hemoglobin (HbA1c) in type 2 DM but it has been positively related to high-density lipoprotein cholesterol (HDL)[2]. Adiponectin has anti-inflammatory and anti-atherogenic effects[3].
Adiponectin level varies among adults with type 1 DM and it is higher in this population compared to healthy persons[4]. Although the level of adiponectin increases in poor controlled type 1 DM, the physiological insulinopenic catabolic states such as fasting does not augment adiponectin level[1]. Several studies have shown that there is no difference in adiponectin levels between patients with initiated stages of type 1 DM and healthy persons[4]. The level of adiponectin is different among children and adult patients who are living two or three decades after disease initiation[5]. Although there was not enough information about the effects of puberty on serum level of adiponectin, it has been seen that adiponectin level is decreased in boys with type 1 DM as well as healthy boys[5]. The effects of genetic and ethnic factors on serum adiponectin level have been reported in many studies[5].
There are a limited number of studies assessing the effects of serum adiponectin level on children with type 1 DM; the results of those studies are very inconsistent too. While many studies did not report any difference in serum adiponectin level between type 1 DM and healthy children (or even they found higher serum levels in type 1 DM group), other studies have showed that serum adiponectin level in children with type 1 DM is lower than in healthy children[1, 5, 6].
Most previous studies about the relation between serum adiponectin level and its related factors with type 1 DM did not include a non-diabetic control group. Therefore the goals of this study were 1) to confirm that serum adiponectin level is higher among type 1 DM patients than in non-diabetic control group and 2) to delineate the determinants of adiponectin (including demographic and clinical factors) and to decide whether the serum adiponectin level is a valid factor for clinical diagnosis.
Subjects and Methods
In this case-control study, diabetic patients (60 patients, case group) were recruited through convenience sampling method among patients attending outpatient clinic and pediatric endocrine department of Nemazi Hospital, Shiraz University of Medical Sciences, Shiraz, Iran. Eligibility criteria for inclusion were: age <18 years, type 1 DM as defined by The American Diabetes Association[7], passing at least 6 months of diagnosis, and no evidence of diabetic ketoacidosis (DKA). From those children attending the emergency ward of Nemazi hospital, 28 healthy people (without DM and younger than 18 years of age) were selected as control group. The control group children were matched according to age and sex with the case group via group (frequency) matching procedure. Eligibility criteria for control group were not having any endocrine-metabolic disorder (including DM) or coronary artery disease and BMI percentile of less than 95. Clinical data like weight, height, BMI, and duration of disease were documented. All subjects were divided into three groups (prepuberty, early and late puberty) according to their pubertal stage. Prepubertal subjects were defined as having sexual maturity rating (SMR) 1. Early pubertal subjects were defined as having SMR 2 or 3. Late pubertal subjects were defined as having SMR 4 or 5. These data were collected by two expert interviewers.
Fasting venous blood was collected in case and control groups. Until the time of adiponectin analysis, serums were stored at -70°C. Laboratory personnel were blinded to the study hypothesis. The Ethics Committee of vice chancellor for research affairs, Shiraz University of Medical Sciences approved this study. Informed consents were obtained from the participants’ parents prior to gathering information and blood sampling.
HbA1c levels were measured by a commercial kit using a column chromatography (Biosystem, Spain). Serum adiponectin levels were measured by a commercial kit (DRG, Germany) using an enzyme-linked immunoassay (enzyme linked immunosorbent assay, ELISA).
The data related to continuous variables are presented as arithmetic mean and standard deviation and for categorical variables they are in form of percentages (Table 1). Normal distribution was calculated via Kolmogrov-Smirnov test. Normal distribution was met for age, duration of disease, HbA1c level and serum level of adiponectin. The people with type 1 DM were divided into two groups (HbA1c ≤8.5 as good-controlled, HbA1c >8.5 as poor-controlled). HbA1c level and disease duration in good- and poor-controlled groups were compared via the t-test. Age and serum adiponectin level in healthy people and two subgroups of type 1 DM (good- and poor-controlled) were compared by the ANOVA test. Spearman's rho correlation between serum adiponectin level and each of covariates were calculated for healthy children and two subgroups of type 1 DM (Table 2). Multiple linear regression analysis was used to determine the predictors of serum level of adiponectin. Backward elimination method was used in multiple regression model (Table 3). The statistical analyses were performed using SPSS (ver. 16.0) software for windows (Chicago, IL, USA). A P value <0.05 was considered as significant.
Table 1.
Demographic, clinical and preclinical characteristics of the Type 1 diabetics patients (n= 60)
| Parameter | Healthy persons (n= 28) | Good glycemic controlled (n= 25) | Poor glycemic controlled (n= 35) | P. value | |
|---|---|---|---|---|---|
| Age [Mean (SD)] | 11.5 (3.84) | 11.28 (3.2) | 11.78 (4.39) | 0.7* | |
| Sex (female/male) | 13/15 | 12/13 | 12/23 | 0.5† | |
| < 85 th | 25 (89.3) | 19 (76) | 30 (85.7) | ||
| BMI percentile | Between 85 th and 95 th | 3 (10.7) | 5 (20) | 5 (14.3) | Not Applicable |
| > 95 th | 0 (0) | 1 (4) | 0 (0) | ||
| Duration of disease | ----------- | 3.68 (3.1) | 3.84 (2.89) | 0.8‡ | |
| Prepubertal | 2 (28.6) | 9 (36) | 15 (42.9) | ||
| Pubertal state | Early pubertal | 9 (32.1) | 10 (40) | 11 (31.4) | 0.6† |
| Late pubertal | 11 (39.3) | 6 (24) | 9 (25.7) | ||
| HbA1c level | ----------- | 7.59 (0.73) | 11.92 (3.03) | 0.0001‡ |
ANOVA
chi square test
t-test
HBA1c: glycosylated hemoglobin
Table 2.
Association between serum level of adiponectin and study variables in different groups (correlation coefficient and statistical significance)
| Parameter | Healthy persons (n= 28) | Good glycemic controlled (n= 25) | Poor glycemic controlled (n= 35) | |||
|---|---|---|---|---|---|---|
| R | P. value | R | P. value | R | P. value | |
| Age | -0.31 | 0.1 | -0.512 | 0.009 | -0.157 | 0.4 |
| Sex | 0.04 | 0.8 | 0.267 | 0.2 | 0.083 | 0.6 |
| Duration of diabetes | -------- | ------- | 0.091 | 0.7 | 0.004 | 0.9 |
| Puberty state | -0.431 | 0.02 | -0.58 | 0.002 | -0.144 | 0.4 |
| Body Mass Index | -0.386 | 0.04 | -0.168 | 0.4 | 0.036 | 0.8 |
| HbA1c | -------- | -------- | -0.102 | 0.6 | 0.234 | 0.2 |
R: Regression coefficient / HBA1c: glycosylated hemoglobin
Table 3.
Determinants of serum level of adiponectin in final model of multiple regression analysis in different groups of the study
| Parameter | Total persons (n= 88) | Healthy persons (n=28) | Type 1 diabetics (n=60) | ||||
|---|---|---|---|---|---|---|---|
| P. value | r | P. value | r | P. value | r | ||
| HbA1c | 0.962 | 0.0001 | ---------- | ------- | 0.973 | 0.0001 | |
| Puberty | Early pubertal state | -0.274 | 0.855 | -0.817 | 0.649 | -0.061 | 0.976 |
| state | late pubertal state | -4.321 | 0.007 | -3.473 | 0.019 | -4.779 | 0.023 |
| BMI | Between 85 th and 95 th | --------- | -------- | -5.493 | 0.019 | ---------- | ------- |
| percentile | > 95 th | ---------- | -------- | --------- | ------- | ---------- | ------- |
R: Regression coefficient / HBA1c: glycosylated hemoglobin
Findings
Demographic and baseline characteristics are shown in Table 1. As shown, the differences between groups regarding age, sex, BMI, duration of disease, and puberty state were not statistically significant (P>0.05).
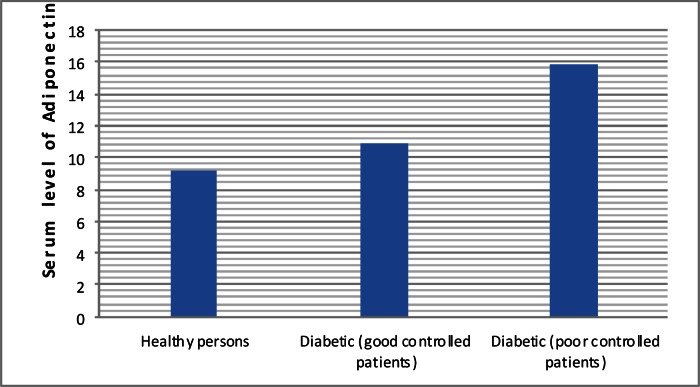
Fig. 1 delineates serum level of adiponectin in the studied groups. Mean and SD of adiponectin level in healthy persons, good-controlled and poor-controlled type 1 DM patients were 9.16 (±4.2) µg/cc, 10.89 (±4.48)µg/cc, and 15.92 (±8.26) µg/cc, respectively. Post hoc analysis revealed that differences of adiponectin between poor- and good-controlled type 1 DM patients (P=0.01) and between healthy persons and poor controlled type 1 DM (P<0.0001) were statistically significant. Although adiponectin level in good-controlled group of type 1 DM was higher than healthy in persons, this difference was not statistically significant (P=0.3). In univariate analysis the association between serum adiponectin level and demographic and baseline characteristics were evaluated separately in healthy and type 1 DM persons (Table 2). As shown, in healthy persons serum adiponectin level was negatively associated with puberty state (r=-0.386, P=0.02) and BMI (r=-0.386, P=0.04). In type 1 DM group serum adiponectin level was negatively associated with age (r=-0.264, P=0.041), puberty state (r=-0.314, P=0.014) and it was positively associated with level of HbA1c (r=0.364, P=0.004).
Fig. 1.
Serum level of adiponectin in healthy and type 1 diabetics (good- and poor-controlled patients)
Multivariate analysis showed that adiponectin level in healthy persons was associated with puberty state and BMI (Table 3). After adjusting other variables listed in the table, the adiponectin in non-pubertal healthy persons was averagely 3.472 µg/cc more than in pubertal persons. The association between serum adiponectin level and puberty state and between serum adiponectin level and BMI did not differ in boys and girls (interaction terms excluded from final model).
Besides according to the results of multivariate analysis in type 1 DM group, the serum adiponectin level was associated with HbA1c and puberty state (Table 3). Hence, with every one-unit increase in HbA1c, serum adiponectin level increased 0.973 units too and after adjusting other variables listed in the table, adiponectin (per ml) in non-pubertal persons was averagely 4.779 µg more than in pubertal persons. The association between adiponectin serum level and puberty state and between serum adiponectin level and HbA1c did not differ in boys and girls (interaction terms excluded from the final model).
Discussion
Our data showed that although serum adiponectin level in poor-controlled subgroup was higher than in good-controlled subgroup and healthy persons, there was no difference regarding serum adiponectin level between good-controlled subgroup and healthy children. Higher level of adiponectin in type 1 DM has been reported by previous reports[4, 8, 9]. Although there is no clear reason for this finding, it might be due to compensatory response of vessel displeasure[10], reduction of clearance due to renal failure[11], posttranslational modification of secreted adiponectin (e.g. glycosylation)[12], and subcutaneous injection of insulin[13].
The difference between serum adiponectinlevels in good- and poor-controlled subgroups revealed that the metabolic state affects serum adiponectin level. This study represents the effect of HbA1c level (as surrogate of metabolic state in type 1 diabetic patient) on adiponectin serum level in multiple logistic regression model after controlling age, sex, duration of disease, puberty state and BMI effects. Other reports showed a similar finding too[8, 14]. On the other hand, follow-up studies that were done in type 1 DM showed that serum adiponectin level was more affected by baseline level of adiponectin and BMI; however they did not show any association between serum adiponectin level and glycemic control state[5, 15].
Glycosylation of several conserved lysine in the collagenous domain of adiponectin is the important mechanism for posttranslational modification of secreted adiponectin[16]. Exposure to high level of glucose increases HbA1c level and affects the process of glycosylation and raises serum adiponectin level in type 1 DM persons too[16, 17]. Increase of blood glucose causes known complications of vessels, renal and other organs and it seems that body secretes further adiponectin as a compensatory mechanism[17]. Moreover high level of blood glucose is a marker for low level of insulin; such low levels of insulin cause future expression of adiponectin gene and more adiponectin secretion[18].
This study also found that while adiponectin level in pubertal adolescents was lower than in non-pubertal children, there was a negative association between serum adiponectin level and pubertal state after controlling other variables. Several studies have shown this finding too[5, 19]. This association might be due to androgen level since with the increase of testosterone and dehydroepiandrostenedione sulphate (DHEAS), serum adiponectin level decreases[20]. Considering that mechanism, it is expected to see more reduced levels of adiponectin in boys than in girls since the increase of post-pubertal level of androgens occurs more in boys than in girls[5, 8]. However, in our study decrease of post pubertal level of adiponectin was not different between boys and girls. A similar finding has been reported by previous authors. It seems that differences in decrease of adiponectin level might be more pronounced due to androgen level in pubertal stages rather than gender[21] but the exact mechanism requires further investigation[22]. However we did not assess androgen level and therefore we cannot show the relationship between them exactly.
We showed that there is an inversely association between adiponectin serum level and BMI only in healthy persons but not in type 1 DM persons. Several studies have shown the same finding[5, 23] and some other studies showed such a relation in type 1 DM persons too[24]. Undoubtedly, reservation of fat is a main criterion for serum level of adiponectin. It is not clear why adiponectin secreted from fat cells decreases when fat cells in obesity increase. It is clear that low level of adiponectin causes low rate of fatty acid oxidation and high level of fatty acid will accompany insulin resistance. Adiponectin mainly acts through liver and muscle receptor of adiponectin[6, 25]. Recent studies showed that aggregation of visceral and subcutaneous fat cells in fact might be due to disorder of cell action in adipose subjects and finally decrement of serum adiponectin level and cardio-metabolic disorders; this mechanism is not associated directly with BMI[24]. In our study just five percent of total samples lie in upper 85th percentile of BMI. It is not unlikely that our findings were affected by this rare sample of children with high BMI.
The present study had several limitations. The data of the present study were collected in a cross-sectional manner and therefore do not permit causal interference. Furthermore, BMI samples in upper percentiles were sparse and it was not feasible to precisely evaluate the association between adiponectin and BMI. We did not assess androgen level and therefore the relationship between testosterone and adiponectin was obscured. Although the power for detecting the difference between poor-controlled patients of case group and healthy persons, poor- and good-controlled patients were high (85% and 80% respectively), there are sparse persons in some subgroups. This poses a limitation to the results obtained from this research and will not be advisable to generalize the results to all type 1 DM patients.
The study also had several important strengths. It is unique because it evaluates determinants of serumadipo nectin level among type 1 DM patients younger than 18 years in Iran. This study had a control group; furthermore, the case group (i.e. type 1 DM group) consisted of various samples regardless of their metabolic state. This study helped to clarify an important hypothesis about determinants of adiponectin in one population of Asian ethnic.
Conclusion
Serum adiponectin level in poor-controlled type 1 DM persons is higher than in healthy and good-controlled type 1 DM persons. This hormone has direct association with HbA1c in type 1 diabetics. According to this study adiponectin has a protective role in type 1 DM persons and it can be used as a good marker for metabolic state among these patients.
Acknowledgement
This study was financially supported by vice chancellor for research affairs, Shiraz University of Medical Sciences; through contract no. 90-2145 as thesis of Dr. Narges Habibian. Authors would like to acknowledge cooperation of vice chancellor for research affairs and head of endocrine research center of Shiraz University of Medical Sciences and his colleagues.
Conflict of Interest
None
References
- 1.Semple RK, Halberg NH, Burling KO, et al. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes. 2007;56(6):1712–7. doi: 10.2337/db06-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kettaneh A, Heude B, Oppert JM, et al. Serum adiponectin is related to plasma high-density lipoprotein cholesterol but not to plasma insulin-concentration in healthy children: the FLVS II study. Metabolism. 2006;55(9):1171–6. doi: 10.1016/j.metabol.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MT, Babaahmadi-Rezaei H, Kadkhodaei-Eliaderani M, et al. Relationship of serum adiponectin with blood lipids, HbA(1)c, and hs-CRP in type II diabetic postmenopausal women. J Clin Lab Anal. 2007;21(3):197–200. doi: 10.1002/jcla.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maahs DM, Hamman RF, D'Agostino R, et al. The association between adiponectin/leptin ratio and diabetes type: the SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155(1):133–5. doi: 10.1016/j.jpeds.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galler A, Gelbrich G, Kratzsch J, et al. Elevated serum levels of adiponectin in children, adolescents and young adults with type 1 diabetes and the impact of age, gender, body mass index and metabolic control: a longitudinal study. Eur J Endocrinol. 2007;157(4):481–9. doi: 10.1530/EJE-07-0250. [DOI] [PubMed] [Google Scholar]
- 6.Zou CC, Liang L, Hong F. Relationship between insulin resistance and serum levels of adiponectin and resistin with childhood obesity. Indian Pediatr. 2007;44(4):275–9. [PubMed] [Google Scholar]
- 7.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 8.Habeeb N, Youssef O, Saab A, et al. Adiponectin as a Marker of Complications in Children with Type I Diabetes. Indian Pediatr. 2012;49(4):277–80. doi: 10.1007/s13312-012-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Meral C, Tascilar E, Karademir F, et al. Elevated plasma levels of apelin in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2010;23(5):497–502. doi: 10.1515/jpem.2010.081. [DOI] [PubMed] [Google Scholar]
- 10.Schalkwijk CG, Chaturvedi N, Schram MT, et al. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab. 2006;91(1):129–35. doi: 10.1210/jc.2005-1117. [DOI] [PubMed] [Google Scholar]
- 11.Zoccali C, Mallamaci F, Panuccio V, et al. Adiponectin is markedly increased in patients with nephrotic syndrome and is related to metabolic risk factors. Kidney Int Suppl. 2003;(84):98–102. doi: 10.1046/j.1523-1755.63.s84.49.x. [DOI] [PubMed] [Google Scholar]
- 12.Richards AA, Stephens T, Charlton HK, et al. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20(7):1673–87. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 13.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–51. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 14.Barnes MM, Curran-Everett D, Hamman RF, et al. Determinants of adiponectin levels in young people with Type 1 diabetes. Diabet Med. 2008;25(3):365–9. doi: 10.1111/j.1464-5491.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 15.Abi Khalil C, Mohammedi K, Aubert R, et al. Intensifying glycaemic control with insulin reduces adiponectin and its HMW isoform moderately in type 2, but not in type 1, diabetes. Diabetes Metab. 2011;37(3):259–61. doi: 10.1016/j.diabet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2009;425(1):41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- 17.Prior SL, Tang TS, Gill GV, et al. Adiponectin, total antioxidant status, and urine albumin excretion in the low-risk “Golden Years” type 1 diabetes mellitus cohort. Metabolism. 2011;60(2):173–9. doi: 10.1016/j.metabol.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Faraj M, Beauregard G, Tardif A, et al. Regulation of leptin, adiponectin and acylation-stimulating protein by hyperinsulinaemia and hyperglycaemia in vivo in healthy lean young men. Diabetes Metab. 2008;34(4 Pt 1):334–42. doi: 10.1016/j.diabet.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Lo MM, Salisbury S, Scherer PE, et al. Serum adiponectin complexes and cardiovascular risk in children with chronic kidney disease. Pediatr Nephrol. 2011;26(11):2009–17. doi: 10.1007/s00467-011-1906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gannage-Yared MH, Chedid R, Abs L. Relation between androgens and cardiovascular risk factors in a young population. Clin Endocrinol (Oxf) 2011;74(6):720–5. doi: 10.1111/j.1365-2265.2011.03987.x. [DOI] [PubMed] [Google Scholar]
- 21.Tasci E, Ozbek M, Onenli-Mungan N, et al. Low Serum Adiponectin Levels in Children and Adolescents with Diabetic Retinopathy. EAJM. 2011;43:18–22. doi: 10.5152/eajm.2011.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen KK, Frystyk J, Wolthers OD, et al. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007;92(5):1857–62. doi: 10.1210/jc.2006-2310. [DOI] [PubMed] [Google Scholar]
- 23.Margoni A, Perrea DN, Vlachos I, et al. Serum leptin, adiponectin and tumor necrosis factor-alpha in hyperlipidemic rats with/without concomitant diabetes mellitus. Mol Med. 2011;17(1-2):36–40. doi: 10.2119/molmed.2010.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishida K, Kim KK, Funahashi T, et al. Relationships between circulating adiponectin levels and fat distribution in obese subjects. J Atheroscler Thromb. 2011;18(7):592–5. doi: 10.5551/jat.7625. [DOI] [PubMed] [Google Scholar]
- 25.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]